ALDH1A1 Deficiency in Gorlin Syndrome Suggests a Central Role for Retinoic Acid and ATM Deficits in Radiation Carcinogenesis
Abstract
:1. Introduction
2. Experimental
2.1. Cell Culture
2.2. Irradiation
2.3. Clonogenic Survival
2.4. IncubATR
2.5. Principle Component Analysis
2.6. Western Blot Analysis
2.7. Statistics
3. Results and Discussion
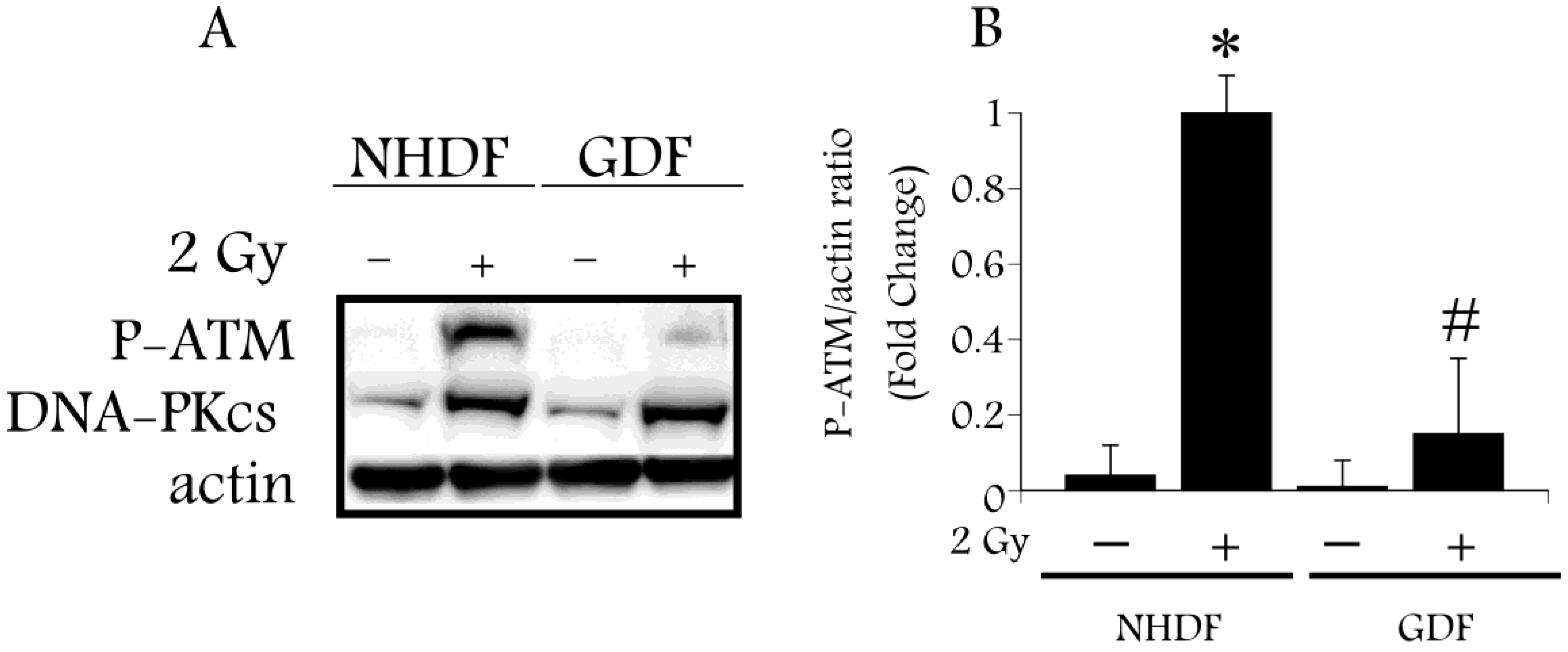
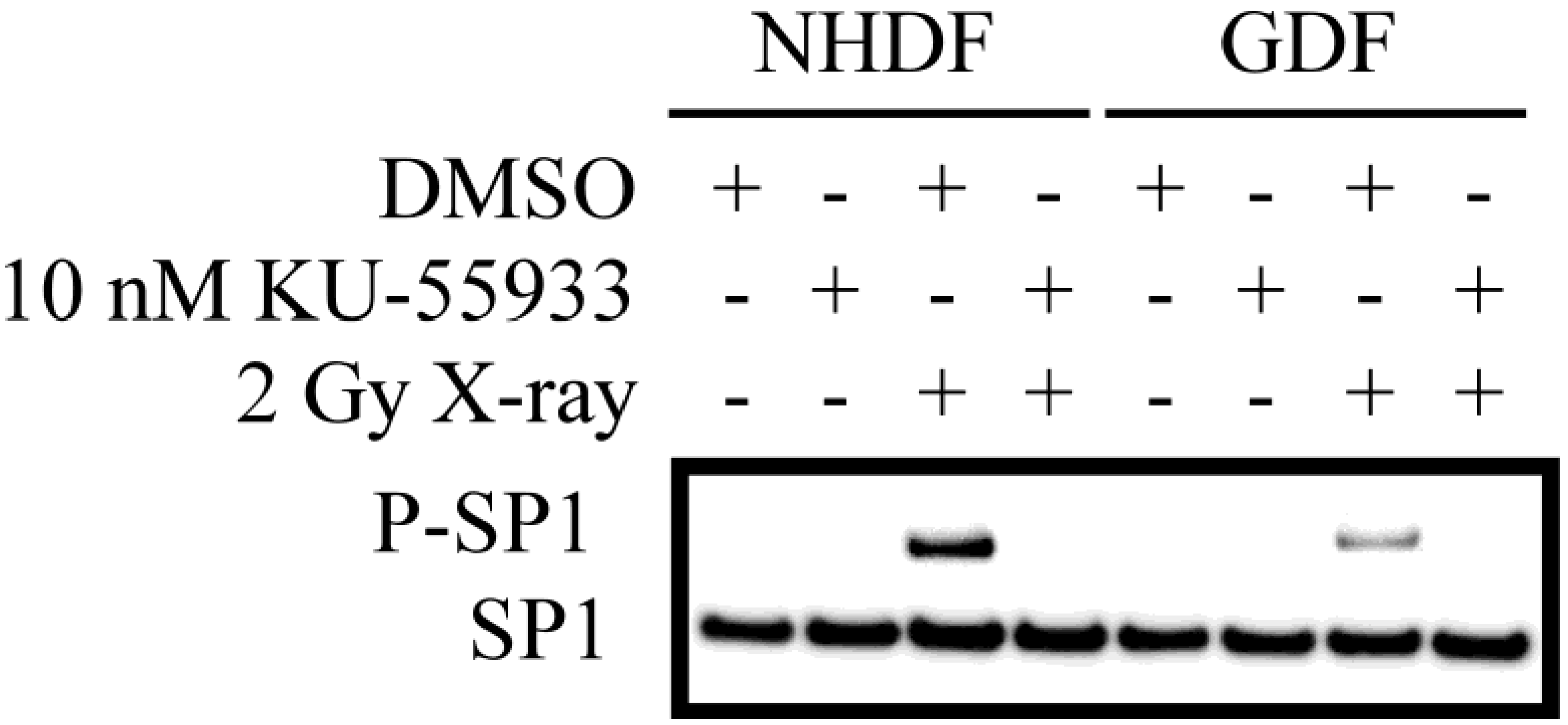
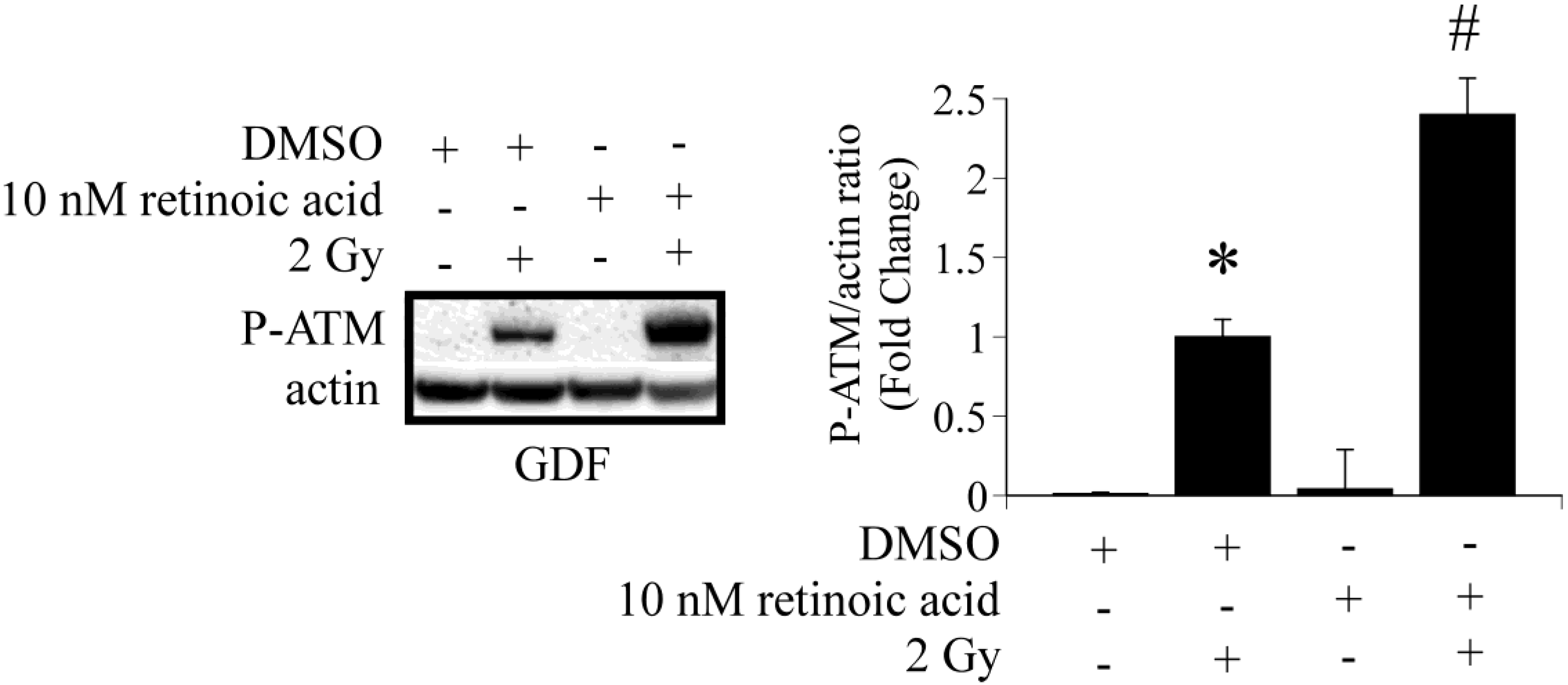
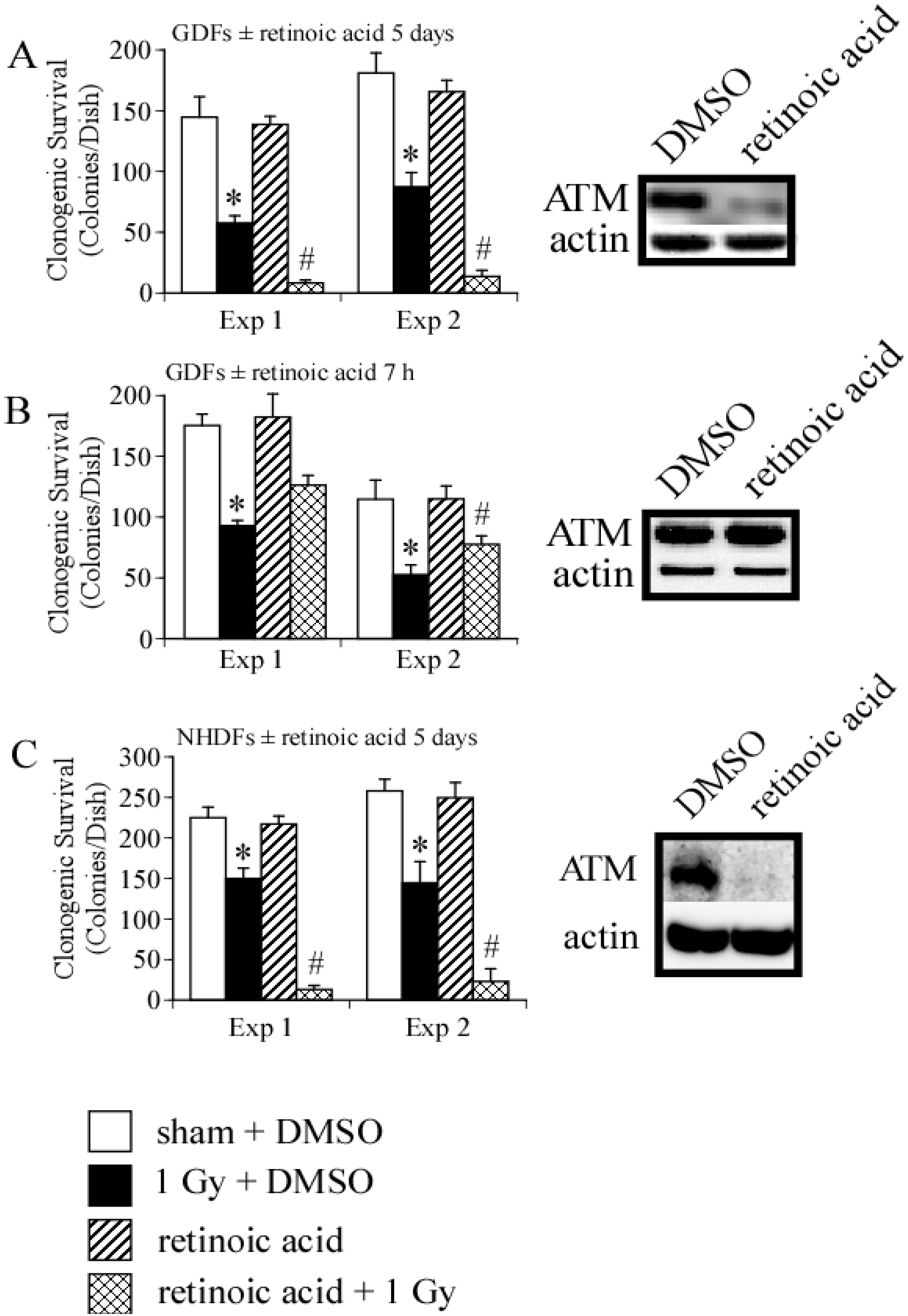
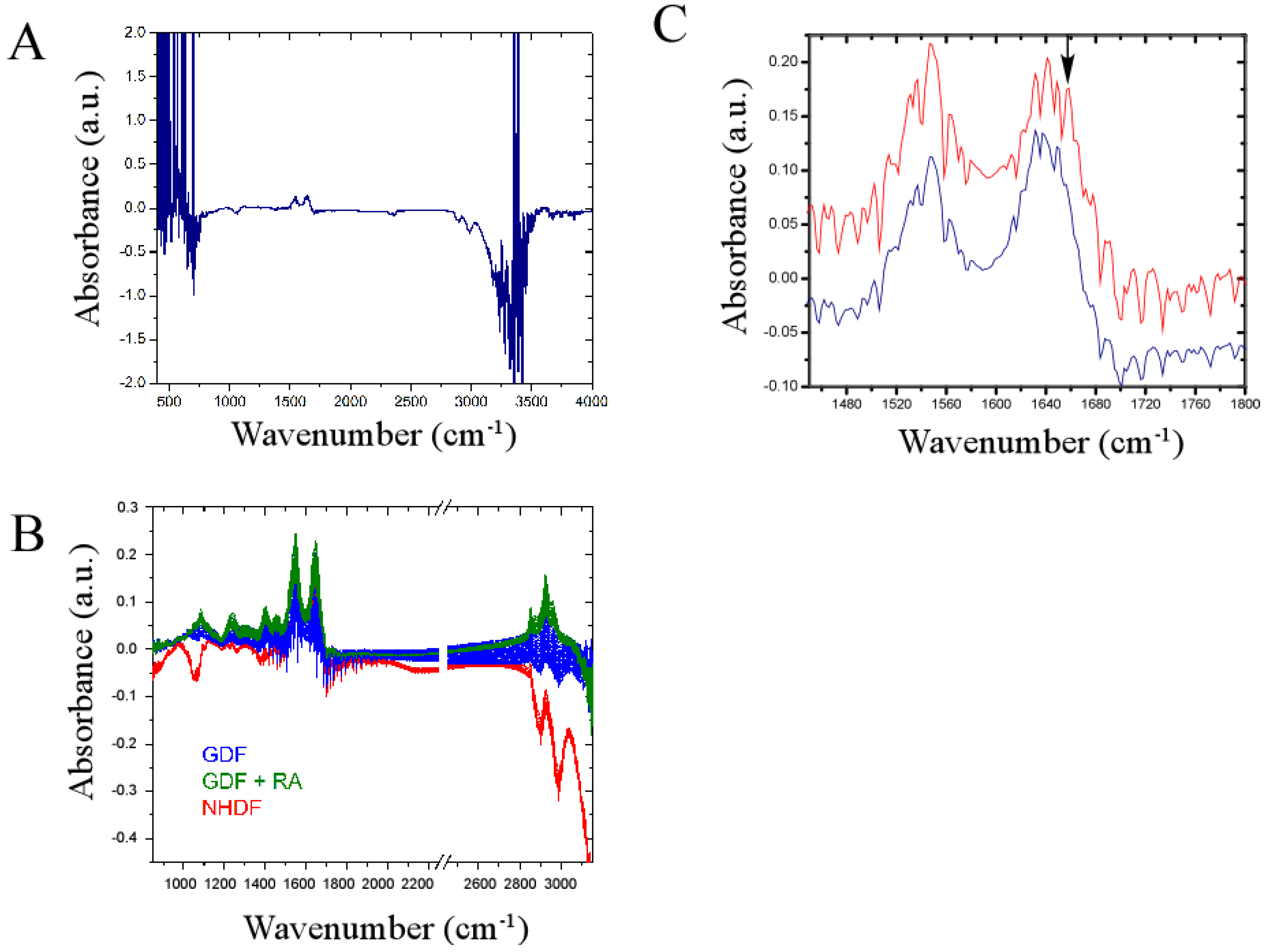
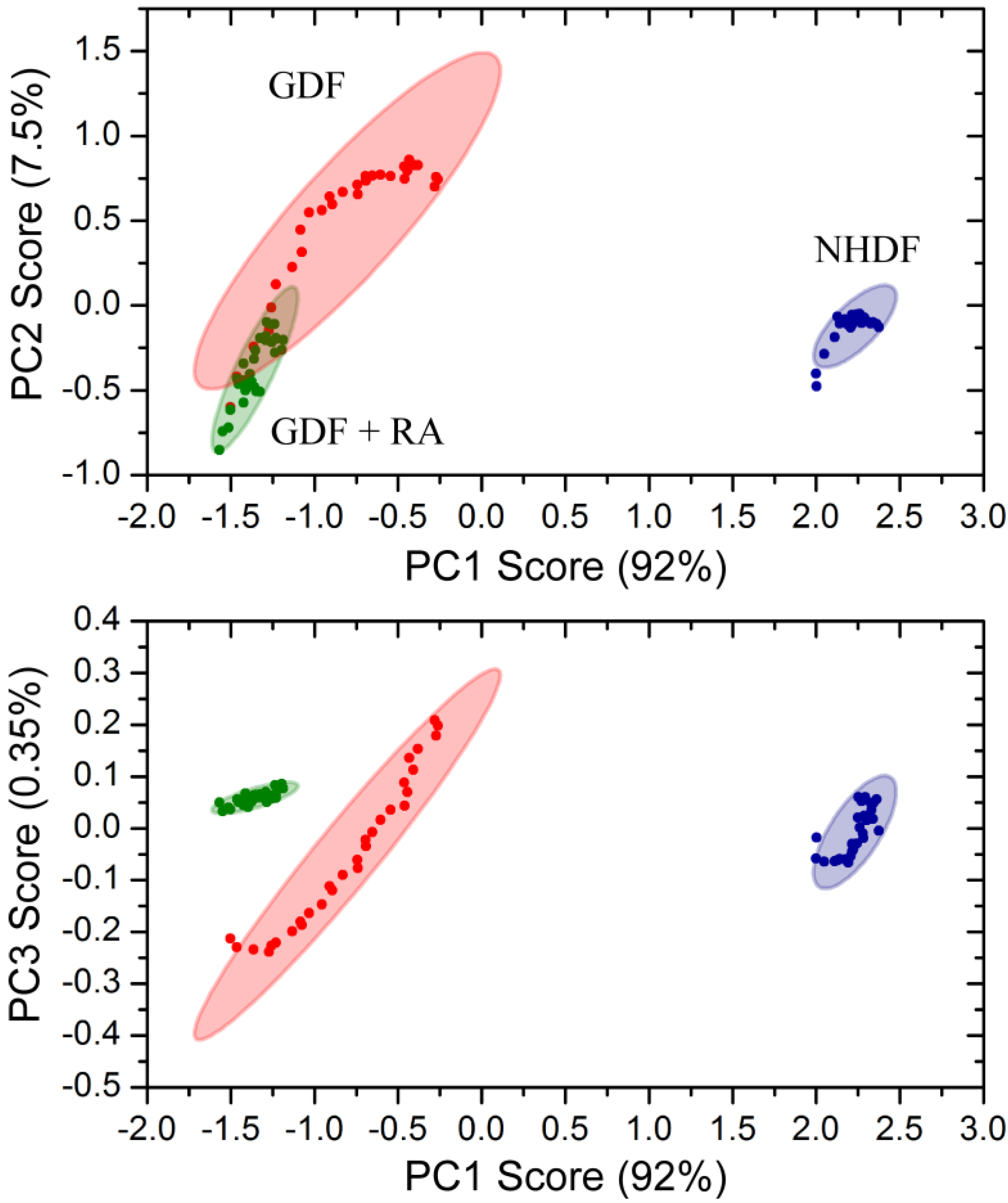
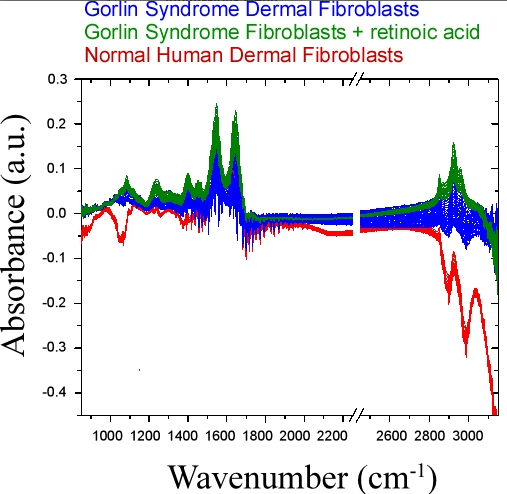
4. Conclusions
4.1. Radiation Carcinogenesis, Retinoic Acid Deficiency and Possible Implications for Secondary Cancers
4.2. DNA Damage Repair and Radiosensitization
4.3. Bulk Chemical Bond Patterns in Live Cells
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kimonis, V.E.; Goldstein, A.M.; Pastakia, B.; Yang, M.L.; Kase, R.; DiGiovanna, J.J.; Bale, A.E.; Bale, S.J. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am. J. Med. Genet. 1997, 69, 299–308. [Google Scholar] [CrossRef]
- Jiang, J.; Hui, C.C. Hedgehog signaling in development and cancer. Dev. Cell 2008, 15, 801–812. [Google Scholar] [CrossRef]
- Wright, A.T.; Magnaldo, T.; Sontag, R.L.; Anderson, L.N.; Sadler, N.C.; Piehowski, P.D.; Gache, Y.; Weber, T.J. Deficient expression of aldehyde dehydrogenase 1A1 is consistent with increased sensitivity of gorlin syndrome patients to radiation carcinogenesis. Mol. Carcinog. 2013. [Google Scholar] [CrossRef]
- Molotkov, A.; Duester, G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J. Biol. Chem. 2003, 278, 36085–36090. [Google Scholar] [CrossRef]
- Schilling, T.F.; Nie, Q.; Lander, A.D. Dynamics and precision in retinoic acid morphogen gradients. Curr. Opin. Genet. Dev. 2012, 22, 562–569. [Google Scholar] [CrossRef]
- Asgari, M.M.; Brasky, T.M.; White, E. Association of vitamin a and carotenoid intake with melanoma risk in a large prospective cohort. J. Investig. Dermatol. 2012, 132, 1573–1582. [Google Scholar] [CrossRef]
- Ostrowski, J.; Jarosz, D.; Butruk, E. Liver vitamin A concentration in patients who died of cancer. Neoplasma 1989, 36, 353–355. [Google Scholar]
- So, P.L.; Fujimoto, M.A.; Epstein, E.H., Jr. Pharmacologic retinoid signaling and physiologic retinoic acid receptor signaling inhibit basal cell carcinoma tumorigenesis. Mol. Cancer Ther. 2008, 7, 1275–1284. [Google Scholar] [CrossRef]
- Burns, F.J.; Tang, M.S.; Frenkel, K.; Nadas, A.; Wu, F.; Uddin, A.; Zhang, R. Induction and prevention of carcinogenesis in rat skin exposed to space radiation. Radiat. Environ. Biophys. 2007, 46, 195–199. [Google Scholar] [CrossRef]
- Fernandes, N.D.; Sun, Y.; Price, B.D. Activation of the kinase activity of ATM by retinoic acid is required for CREB-dependent differentiation of neuroblastoma cells. J. Biol. Chem. 2007, 282, 16577–16584. [Google Scholar] [CrossRef]
- Wang, X.; Lui, V.C.; Poon, R.T.; Lu, P.; Poon, R.Y. DNA damage mediated s and G(2) checkpoints in human embryonal carcinoma cells. Stem Cells 2009, 27, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Muramoto, G.G.; Russell, J.L.; Safi, R.; Salter, A.B.; Himburg, H.A.; Daher, P.; Meadows, S.K.; Doan, P.; Storms, R.W.; Chao, N.J.; et al. Inhibition of aldehyde dehydrogenase expands hematopoietic stem cells with radioprotective capacity. Stem Cells 2010, 28, 523–534. [Google Scholar]
- Tang, X.H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Petrosino, J.M.; Disilvestro, D.; Ziouzenkova, O. Aldehyde dehydrogenase 1A1: Friend or foe to female metabolism? Nutrients 2014, 6, 950–973. [Google Scholar] [CrossRef]
- Aleksunes, L.M.; Klaassen, C.D. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab. Dispos. 2012, 40, 1366–1379. [Google Scholar] [CrossRef]
- Bakkenist, C.J.; Kastan, M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003, 421, 499–506. [Google Scholar] [CrossRef]
- Valin, A.; Barnay-Verdier, S.; Robert, T.; Ripoche, H.; Brellier, F.; Chevallier-Lagente, O.; Avril, M.F.; Magnaldo, T. Ptch1+/− dermal fibroblasts isolated from healthy skin of gorlin syndrome patients exhibit features of carcinoma associated fibroblasts. PLoS One 2009, 4, e4818. [Google Scholar] [CrossRef]
- Brellier, F.; Valin, A.; Chevallier-Lagente, O.; Gorry, P.; Avril, M.F.; Magnaldo, T. Ultraviolet responses of gorlin syndrome primary skin cells. Br. J. Dermatol. 2008, 159, 445–452. [Google Scholar] [CrossRef]
- Weber, T.J.; Siegel, R.W.; Markillie, L.M.; Chrisler, W.B.; Lei, X.C.; Colburn, N.H. A paracrine signal mediates the cell transformation response to low dose gamma radiation in JB6 cells. Mol. Carcinog. 2005, 43, 31–37. [Google Scholar] [CrossRef]
- Sundaram, S.K.; Sacksteder, C.A.; Weber, T.J.; Riley, B.J.; Addleman, R.S.; Harrer, B.J.; Peterman, J.W. Fourier-transform infrared spectroscopy for rapid screening and live-cell monitoring: Application to nanotoxicology. Nanomedicine (Lond.) 2013, 8, 145–156. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: http://www.r-project.org (access date 2 January 2014).
- COBLENTZ_SOCIETY. National Institute of Standards and Technology—CO2 Infrared Spectrum. Available online: http://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Type=IR-SPEC&Index=1#IR-SPEC (accessed on 3 September 2014).
- Davis, R.; Mauer, L. Fourier transform infrared (FT-IR) spectroscopy: A rapid tool for detection and analysis of foodborne pathogenic bacteria. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; Volume II, pp. 1582–1594. [Google Scholar]
- Waters, K.M.; Stenoien, D.L.; Sowa, M.B.; von Neubeck, C.H.; Chrisler, W.B.; Tan, R.; Sontag, R.L.; Weber, T.J. Annexin A2 modulates radiation-sensitive transcriptional programming and cell fate. Radiat. Res. 2013, 179, 53–61. [Google Scholar] [CrossRef]
- Wilson, K.A.; Stern, D.F. NFBD1/MDC1, 53BP1 and BRCA1 have both redundant and unique roles in the ATM pathway. Cell Cycle 2008, 7, 3584–3594. [Google Scholar] [CrossRef]
- Olofsson, B.A.; Kelly, C.M.; Kim, J.; Hornsby, S.M.; Azizkhan-Clifford, J. Phosphorylation of SP1 in response to DNA damage by ataxia telangiectasia-mutated kinase. Mol. Cancer Res. 2007, 5, 1319–1330. [Google Scholar] [CrossRef]
- Shin, M.H.; Yuan, M.; Zhang, H.; Margolick, J.B.; Kai, M. ATM-dependent phosphorylation of the checkpoint clamp regulates repair pathways and maintains genomic stability. Cell Cycle 2012, 11, 1796–1803. [Google Scholar] [CrossRef]
- Salsman, S.; Lu, S.; Benbrook, D.M. The mechanism of retinoic acid radiosensitization is independent of AP-1 repression in a cervical carcinoma cell line. Gynecol. Oncol. 1999, 73, 253–256. [Google Scholar] [CrossRef]
- Moulas, A.N.; Gerogianni, I.C.; Papadopoulos, D.; Gourgoulianis, K.I. Serum retinoic acid, retinol and retinyl palmitate levels in patients with lung cancer. Respirology 2006, 11, 169–174. [Google Scholar] [CrossRef]
- Busch, A.M.; Galimberti, F.; Nehls, K.E.; Roengvoraphoj, M.; Sekula, D.; Li, B.; Guo, Y.; Direnzo, J.; Fiering, S.N.; Spinella, M.J.; et al. All-trans-retinoic acid antagonizes the hedgehog pathway by inducing patched. Cancer Biol. Ther. 2014, 15, 463–472. [Google Scholar] [CrossRef]
- Gongal, P.A.; March, L.D.; Holly, V.L.; Pillay, L.M.; Berry-Wynne, K.M.; Kagechika, H.; Waskiewicz, A.J. Hmx4 regulates sonic hedgehog signaling through control of retinoic acid synthesis during forebrain patterning. Dev. Biol. 2011, 355, 55–64. [Google Scholar] [CrossRef]
- Kitagawa, R.; Kastan, M.B. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 99–109. [Google Scholar] [CrossRef]
- Da Silva, R.; dos Santos-Valente, E.C.; Burim Scomparini, F.; Saccardo Sarni, R.O.; Costa-Carvalho, B.T. The relationship between nutritional status, vitamin A and zinc levels and oxidative stress in patients with ataxia-telangiectasia. Allergol. Immunopathol. (Madr.) 2013, 22, 427–431. [Google Scholar]
- Reichenbach, J.; Schubert, R.; Schwan, C.; Muller, K.; Bohles, H.J.; Zielen, S. Anti-oxidative capacity in patients with ataxia telangiectasia. Clin. Exp. Immunol. 1999, 117, 535–539. [Google Scholar] [CrossRef]
- Shilkrut, M.; Belkacemi, Y.; Kuten, A. Secondary malignancies in survivors of breast cancer: How to overcome the risk. Crit. Rev. Oncol. Hematol. 2012, 84, e86–e89. [Google Scholar] [CrossRef]
- Weinstein, J.L.; Ayyanar, K.; Watral, M.A. Secondary neoplasms following treatment for brain tumors. Cancer Treat. Res. 2009, 150, 239–273. [Google Scholar]
- Tran, C.; Sorg, O.; Carraux, P.; Didierjean, L.; Saurat, J.H. Topical delivery of retinoids counteracts the UVB-induced epidermal vitamin A depletion in hairless mouse. Photochem. Photobiol. 2001, 73, 425–431. [Google Scholar] [CrossRef]
- Sorg, O.; Tran, C.; Carraux, P.; Didierjean, L.; Falson, F.; Saurat, J.H. Oxidative stress-independent depletion of epidermal vitamin A by UVA. J. Investig. Dermatol. 2002, 118, 513–518. [Google Scholar] [CrossRef]
- Li, T.; Molteni, A.; Latkovich, P.; Castellani, W.; Baybutt, R.C. Vitamin A depletion induced by cigarette smoke is associated with the development of emphysema in rats. J. Nutr. 2003, 133, 2629–2634. [Google Scholar]
- Fletcher, N.; Hanberg, A.; Hakansson, H. Hepatic vitamin A depletion is a sensitive marker of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in four rodent species. Toxicol. Sci. 2001, 62, 166–175. [Google Scholar] [CrossRef]
- Moolgavkar, S.H.; Luebeck, E.G.; Krewski, D.; Zielinski, J.M. Radon, cigarette smoke, and lung cancer: A re-analysis of the colorado plateau uranium miners’ data. Epidemiology 1993, 4, 204–217. [Google Scholar] [CrossRef]
- Hellmann-Regen, J.; Herzog, I.; Fischer, N.; Heuser, I.; Regen, F. Do tetracyclines and erythromycin exert anti-acne effects by inhibition of P450-mediated degradation of retinoic acid? Exp. Dermatol. 2014, 23, 290–293. [Google Scholar]
- Mancuso, M.; Pasquali, E.; Leonardi, S.; Tanori, M.; Rebessi, S.; di Majo, V.; Pazzaglia, S.; Toni, M.P.; Pimpinella, M.; Covelli, V.; et al. Oncogenic bystander radiation effects in patched heterozygous mouse cerebellum. Proc. Natl. Acad. Sci. USA 2008, 105, 12445–12450. [Google Scholar] [CrossRef]
- Aszterbaum, M.; Epstein, J.; Oro, A.; Douglas, V.; LeBoit, P.E.; Scott, M.P.; Epstein, E.H., Jr. Ultraviolet and ionizing radiation enhance the growth of bccs and trichoblastomas in patched heterozygous knockout mice. Nat. Med. 1999, 5, 1285–1291. [Google Scholar] [CrossRef]
- Tonge, P.D.; Andrews, P.W. Retinoic acid directs neuronal differentiation of human pluripotent stem cell lines in a non-cell-autonomous manner. Differentiation 2010, 80, 20–30. [Google Scholar] [CrossRef]
- Feng, J.; Zou, J.; Li, L.; Zhao, Y.; Liu, S. Antisense oligodeoxynucleotides targeting ATM strengthen apoptosis of laryngeal squamous cell carcinoma grown in nude mice. J. Exp. Clin. Cancer Res. 2011, 30, e43. [Google Scholar] [CrossRef]
- Roossink, F.; Wieringa, H.W.; Noordhuis, M.G.; ten Hoor, K.A.; Kok, M.; Slagter-Menkema, L.; Hollema, H.; de Bock, G.H.; Pras, E.; de Vries, E.G.; et al. The role of ATM and 53BP1 as predictive markers in cervical cancer. Int. J. Cancer 2012, 131, 2056–2066. [Google Scholar] [CrossRef]
- Keijer, J.; Bunschoten, A.; Palou, A.; Franssen-van Hal, N.L. Beta-carotene and the application of transcriptomics in risk-benefit evaluation of natural dietary components. Biochim. Biophys. Acta 2005, 1740, 139–146. [Google Scholar] [CrossRef]
- Knobf, M.T.; Sun, Y. A longitudinal study of symptoms and self-care activities in women treated with primary radiotherapy for breast cancer. Cancer Nurs. 2005, 28, 210–218. [Google Scholar] [CrossRef]
- Dorr, W. Skin and other reactions to radiotherapy—Clinical presentation and radiobiology of skin reactions. Front. Radiat. Ther. Oncol. 2006, 39, 96–101. [Google Scholar]
- Lopez, E.; Guerrero, R.; Nunez, M.I.; del Moral, R.; Villalobos, M.; Martinez-Galan, J.; Valenzuela, M.T.; Munoz-Gamez, J.A.; Oliver, F.J.; Martin-Oliva, D.; et al. Early and late skin reactions to radiotherapy for breast cancer and their correlation with radiation-induced DNA damage in lymphocytes. Breast Cancer Res. 2005, 7, R690–R698. [Google Scholar] [CrossRef]
- McQuestion, M. Evidence-based skin care management in radiation therapy: Clinical update. Semin. Oncol. Nurs. 2011, 27, e1–e17. [Google Scholar] [CrossRef]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Int. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Weber, T.J.; Magnaldo, T.; Xiong, Y. ALDH1A1 Deficiency in Gorlin Syndrome Suggests a Central Role for Retinoic Acid and ATM Deficits in Radiation Carcinogenesis. Proteomes 2014, 2, 451-467. https://doi.org/10.3390/proteomes2030451
Weber TJ, Magnaldo T, Xiong Y. ALDH1A1 Deficiency in Gorlin Syndrome Suggests a Central Role for Retinoic Acid and ATM Deficits in Radiation Carcinogenesis. Proteomes. 2014; 2(3):451-467. https://doi.org/10.3390/proteomes2030451
Chicago/Turabian StyleWeber, Thomas J., Thierry Magnaldo, and Yijia Xiong. 2014. "ALDH1A1 Deficiency in Gorlin Syndrome Suggests a Central Role for Retinoic Acid and ATM Deficits in Radiation Carcinogenesis" Proteomes 2, no. 3: 451-467. https://doi.org/10.3390/proteomes2030451