1. Introduction
The World Health Organization (WHO) reported in 2008 that 17.3 million deaths worldwide were due to cardiovascular disease (CVD) [
1]. A major contributor to CVD is atherosclerosis which is a dynamic process that can begin in childhood and develop or regress, depending on the presence or absence of a range of risk factors, including obesity, inflammation, hyperglycemia, hypertension and hyperlipidemia [
2].
The International Obesity Taskforce estimates that approximately 40–50 million school aged children are obese [
3]. Obese children are at high risk of becoming obese adults, especially if their parents are also obese [
4]. Abdominal obesity in children is associated with low grade inflammation [
5], a significant contributor to the development of atherosclerosis [
2]. Both body mass index (BMI) and waist circumference (WC) correlate with intra-abdominal fat in primary school aged children [
6] and are used as clinical measures to identify CVD risk [
7]. Obese children are also at increased risk of hypertension and dyslipidemia as they age [
8].
A number of risk factors cluster within both adults and children [
9,
10]. Those most commonly identified in overweight and obese children are elevated fasting serum insulin and glucose [
5,
11,
12], high blood pressure [
5,
11,
13,
14], raised triglycerides [
5,
11,
12,
14], total [
5,
14] and LDL-cholesterol [
11,
12,
14]; and low HDL-cholesterol [
11,
12,
13,
14]. There are a number of accepted standard reference ranges used to quantify these risk factors in adults [
15,
16], with age- and sex-specific reference values recently developed for children [
17,
18] using population data from France [
18]. The Framingham method for assessing 30 year risk in adults includes age, sex, systolic blood pressure (SBP), anti-hypertensive medications, smoking, presence of diabetes, total and HDL cholesterol [
19]. Although scoring methods for determining CVD risk in children exist [
20], a straightforward method of assessing and describing a gradation of risk in overweight children by comparing individual clinical data to population-derived percentile bands could assist in estimating future risk of CVD. Therefore the aims of this study were to; (1) develop a multifactor CVD risk score using these reference ranges for pediatric researchers; (2) assess its application in overweight and obese pre-pubertal children; (3) examine the difference in dietary intake between high
versus low category CVD risk scores; and (4) to examine the strength of associations between individual anthropometric and biomedical risk factors in both boys and girls.
3. Results and Discussion
Data were obtained from 56 boys and 80 girls, resulting in 285 sets of observations (121 on boys and 164 on girls), with 51 individuals (37.5%) having a single observation, with 36 (26.5%), 34 (25%) and 15 (11%) having two, three and four observations respectively.
Table 2 reports the baseline data of these children.
Table 2.
Characteristics of the children at baseline.
Table 2.
Characteristics of the children at baseline.
| Variables | Boys (n = 44) * | Girls (n = 44) * | Total (n = 112) * |
|---|
| Age (years) | 8.89 ± 0.8 | 8.53 ± 0.9 | 8.7 ± 0.84 |
| BMI (kg/m2) | 25.48 ± 3.5 | 25.04 ± 4.0 | 25.20 ± 3.8 |
| WC (cm) | 80.50 ± 9.1 | 76.68 ± 9.4 | 78.18 ± 9.4 |
| Systolic BP (mmHg) | 100.65 ± 9.3 | 99.08 ± 8.6 | 99.65 ± 8.9 |
| Diastolic BP (mmHg) | 57.32 ± 6.0 | 55.92 ± 5.5 | 56.47 ± 5.7 |
| Fasting glucose (mmol/L) | 4.22 ± 0.4 | 4.19 ± 0.5 | 4.2 ± 0.5 |
| Insulin (mIU/L) | 11.63 ± 7.0 | 12.05 ± 8.2 | 11.88 ± 7.7 |
| Total Cholesterol (mmol/L) | 4.25 ± 0.6 | 4.37 ± 0.7 | 4.32 ± 0.7 |
| LDL (mmol/L) | 2.44 ± 0.6 | 2.61 ± 0.6 | 2.54 ± 0.6 |
| HDL (mmol/L) | 1.28 ± 0.3 | 1.25 ± 0.3 | 1.27 ± 0.3 |
| TG (mmol/L) | 1.15 ± 0.6 | 1.11 ± 0.6 | 1.12 ± 0.6 |
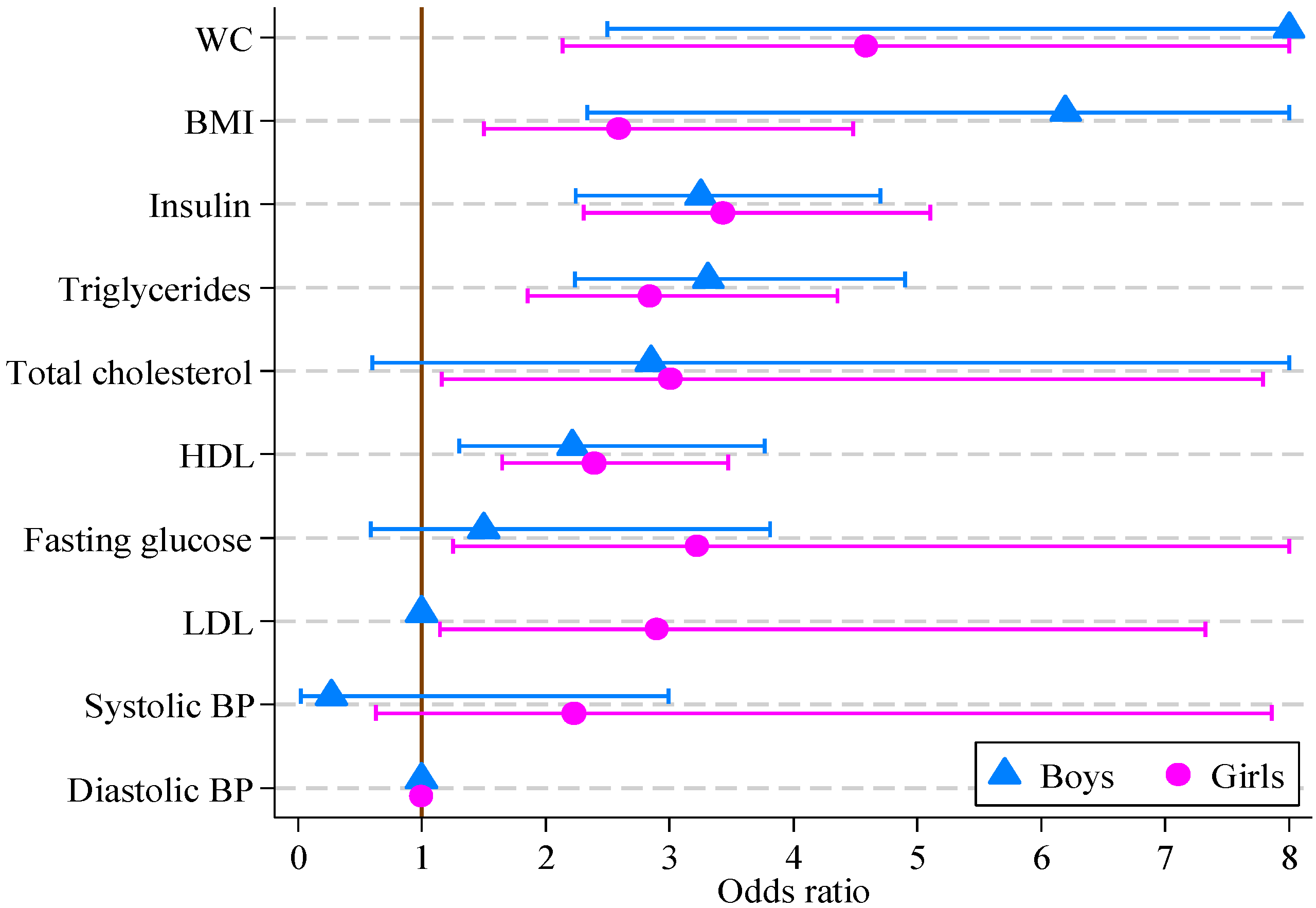
Table 3 reports the count and percentage of observations for boys and girls in each multifactor CVD risk score category for each biomedical measure. Eighty five percent of observations of boys and 75% of observations of girls were at or above the 95th centile (intermediate to high risk) for BMI and WC as expected in this cohort. In addition, 40% of observations of boys and almost 35% of observations of girls were at or above high risk for serum triglycerides and insulin, and over 15% of all observations had low levels of HDL cholesterol. In contrast, five of the other risk factors (systolic and diastolic blood pressure, fasting glucose, total and LDL cholesterol) had 95% or more of observations in the low risk category.
Table 3.
Count and percent of observations by CVD risk score factor level and sex.
Table 3.
Count and percent of observations by CVD risk score factor level and sex.
| CVD risk factor | Threshold Risk (<90th) | Moderate Risk (90th–95th) | High Risk (95th–97.5th) | Very High Risk (≥97.5th) |
|---|
| Boys |
| BMI | 6 (5.0%) | 13 (10.7%) | 13 (10.7%) | 89 (73.6%) |
| Waist circumference | 5 (4.1%) | 8 (6.6%) | 9 (7.4%) | 99 (81.8%) |
| Systolic BP | 117 (96.7%) | 4 (3.3%) | 0 (0.0%) | 0 (0.0%) |
| Diastolic BP | 120 (99.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) |
| Fasting glucose | 119 (98.3%) | 1 (0.8%) | 0 (0.0%) | 1 (0.8%) |
| Insulin | 54 (44.6%) | 17 (14.0%) | 13 (10.7%) | 37 (30.6%) |
| Total cholesterol | 120 (99.2%) | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) |
| LDL | 117 (96.7%) | 3 (2.5%) | 0 (0.0%) | 1 (0.8%) |
| Triglycerides | 59 (48.8%) | 14 (11.6%) | 9 (7.4%) | 39 (32.2%) |
| | 10th–90th | 5th–10th | 2.5th–5th | ≤2.5th |
| HDL | 70 (57.9%) | 24 (19.8%) | 5 (4.1%) | 19 (15.7%) |
| | | 90–95th | 95th–97.5th | ≥97.5th |
| HDL (protective) | | 1 (0.8%) | 2 (1.7%) | 0 (0.0%) |
| Girls |
| BMI | 9 (5.5%) | 30 (18.3%) | 31 (18.9%) | 94 (57.3%) |
| Waist circumference | 20 (12.2%) | 14 (8.5%) | 22 (13.4%) | 108 (65.9%) |
| Systolic BP | 161 (98.2%) | 2 (1.2%) | 0 (0.0%) | 1 (0.6%) |
| Diastolic BP | 163 (99.4%) | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) |
| Fasting glucose | 158 (96.3%) | 2 (1.2%) | 0 (0.0%) | 4 (2.4%) |
| Insulin | 82 (50.0%) | 24 (14.6%) | 7 (4.3%) | 51 (31.1%) |
| Total cholesterol | 159 (97.0%) | 1 (0.6%) | 1 (0.6%) | 3 (1.8%) |
| LDL | 155 (94.5%) | 4 (2.4%) | 1 (0.6%) | 4 (2.4%) |
| Triglycerides | 86 (52.4%) | 19 (11.6%) | 9 (5.5%) | 50 (30.5%) |
| | 10th–90th | 5th–10th | 2.5th–5th | ≤2.5th |
| HDL | 113 (68.9%) | 17 (10.4%) | 7 (4.3%) | 23 (14.0%) |
| | | 90th–95th | 95th–97.5th | ≥97.5th |
| HDL (protective) | | 4 (2.4%) | 0 (0.0%) | 0 (0.0%) |
Table 4 reports median and IQR of dietary measures by low or high CVD risk score separately for boys and girls. For boys, but not girls, the median of all dietary measures was significantly higher in the high CVD risk score group (with the exception of saturated fat). For girls, there were no significant differences between the medians of high and low CVD risk score groups in any dietary measures.
Table 4.
Median and IQR of dietary measures by CVD risk score category (<9 and ≤9) and sex.
Table 4.
Median and IQR of dietary measures by CVD risk score category (<9 and ≤9) and sex.
| Diet Measure | Low risk (CVD Score < 9) | High risk (CVD Score ≥ 9) | p-value |
|---|
| Boys | Girls | Boys | Girls | Boys | Girls |
|---|
| n = 60 (49.9%) | n = 89 (54.3%) | n = 61 (50.1%) | n = 75 (45.7%) |
|---|
| Median (IQR) | Median (IQR) |
|---|
| Sugars (×100 g) | 1.65 (1.3–2.0) | 1.64 (1.3–2.2) | 2.10 (1.6–2.7) | 1.67 (1.4–2.1) | <0.001 | 0.629 |
| Energy (×1000 kJ) | 9.87 (8.5–11.0) | 9.82 (7.5–11.6) | 11.45 (9.2–13.8) | 9.72 (8.0–11.5) | 0.004 | 0.936 |
| Protein (×10 g) | 9.30 (7.8–10.6) | 9.06 (8.0–11.3) | 10.68 (8.1–13.3) | 8.98 (7.1–11.5) | 0.018 | 0.493 |
| Carbohydrate (×100 g) | 3.24 (2.6–3.7) | 2.97 (2.5–3.7) | 3.63 (3.0–4.5) | 3.04 (2.7–3.6) | 0.004 | 0.936 |
| Total Fat (×100 g) | 0.74 (0.6–0.9) | 0.74 (0.5–0.9) | 0.84 (0.6–1.1) | 0.71 (0.5–0.9) | 0.047 | 0.941 |
| Saturated fat (×10 g) | 3.24 (2.5–3.9) | 3.18 (2.2–4.1) | 3.49 (2.8–4.8) | 3.00 (2.3–3.8) | 0.099 | 0.803 |
| Monounsaturated fat (×10 g) | 2.59 (2.2–3.1) | 2.61 (1.9–3.2) | 2.84 (2.3–3.7) | 2.60 (1.9–3.4) | 0.048 | 0.986 |
| Polyunsaturated fat (×1 g) | 8.44 (7.3–11.0) | 8.71 (6.6–11.4) | 10.23 (8.6–12.3) | 8.43 (6.9–11.2) | 0.015 | 0.829 |
| Sodium (×1000 mg) | 2.03 (1.6–2.3) | 1.92 (1.6–2.5) | 2.24 (1.8–2.7) | 1.98 (1.5–2.5) | 0.017 | 0.901 |
The results of logistic regression modeling of CVD risk score are reported in
Table 5. For girls, all factors other than systolic and diastolic blood pressure were significantly associated with the multifactor CVD risk score. For boys, the multifactor CVD risk score was associated with all factors other than blood pressure, fasting glucose, total and LDL cholesterol. The greatest odds ratios for both boys and girls were for waist circumference (14.36 and 4.59, respectively), with BMI, insulin, triglycerides and HDL also being significant. The odds ratios and 95% confidence intervals are displayed in
Figure 1.
Table 5.
Odds ratios, 95% confidence intervals (CI) and p-values from multivariate logistic regression models of CVD risk score on individual multifactor CVD risk score factors and total energy (e indicates total energy was significant in the model at the 5% level).
Table 5.
Odds ratios, 95% confidence intervals (CI) and p-values from multivariate logistic regression models of CVD risk score on individual multifactor CVD risk score factors and total energy (e indicates total energy was significant in the model at the 5% level).
| CVD Risk Score Factor | Boys (n = 121) | Girls (n = 164) |
|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value |
|---|
| BMI (kg/m2) | 6.20 e | 2.33, 16.46 | <0.001 | 2.59 | 1.50, 4.48 | 0.001 |
| Waist circ. (cm) | 14.01 e | 2.50, 78.49 | 0.003 | 4.59 | 2.14, 9.83 | <0.001 |
| Systolic BP (mmHg) | 0.27 e | 0.02, 2.99 | 0.286 | 2.23 | 0.63, 7.86 | 0.213 |
| Diastolic BP (mmHg) | 1.00 | 1.00, 1.00 | 1.000 | 1.00 | 1.00, 1.00 | 1.000 |
| Fasting glucose (mmol/L) | 1.50 e | 0.59, 3.81 | 0.395 | 3.23 | 1.25, 8.30 | 0.015 |
| Insulin (mIU/L) | 3.25 e | 2.25, 4.71 | <0.001 | 3.43 | 2.31, 5.10 | <0.001 |
| Total cholesterol (mmol/L) | 2.85 e | 0.61, 13.40 | 0.185 | 3.01 | 1.16, 7.79 | 0.023 |
| LDL (mmol/L) | 1.00 | 1.00, 1.00 | 1.000 | 2.90 | 1.15, 7.32 | 0.024 |
| HDL (mmol/L) | 2.22 e | 1.30, 3.77 | <0.001 | 2.39 | 1.65, 3.47 | <0.001 |
| Triglycerides (mmol/L) | 3.31 | 2.24, 4.90 | <0.001 | 2.84 | 1.85, 4.35 | <0.001 |
Figure 1.
Odds ratios, with 95% confidence intervals, for boys and girls from multivariate logistic regression models of CVD risk score on individual CVD risk score factors (boys, n = 121; girls, n = 164).
Figure 1.
Odds ratios, with 95% confidence intervals, for boys and girls from multivariate logistic regression models of CVD risk score on individual CVD risk score factors (boys, n = 121; girls, n = 164).
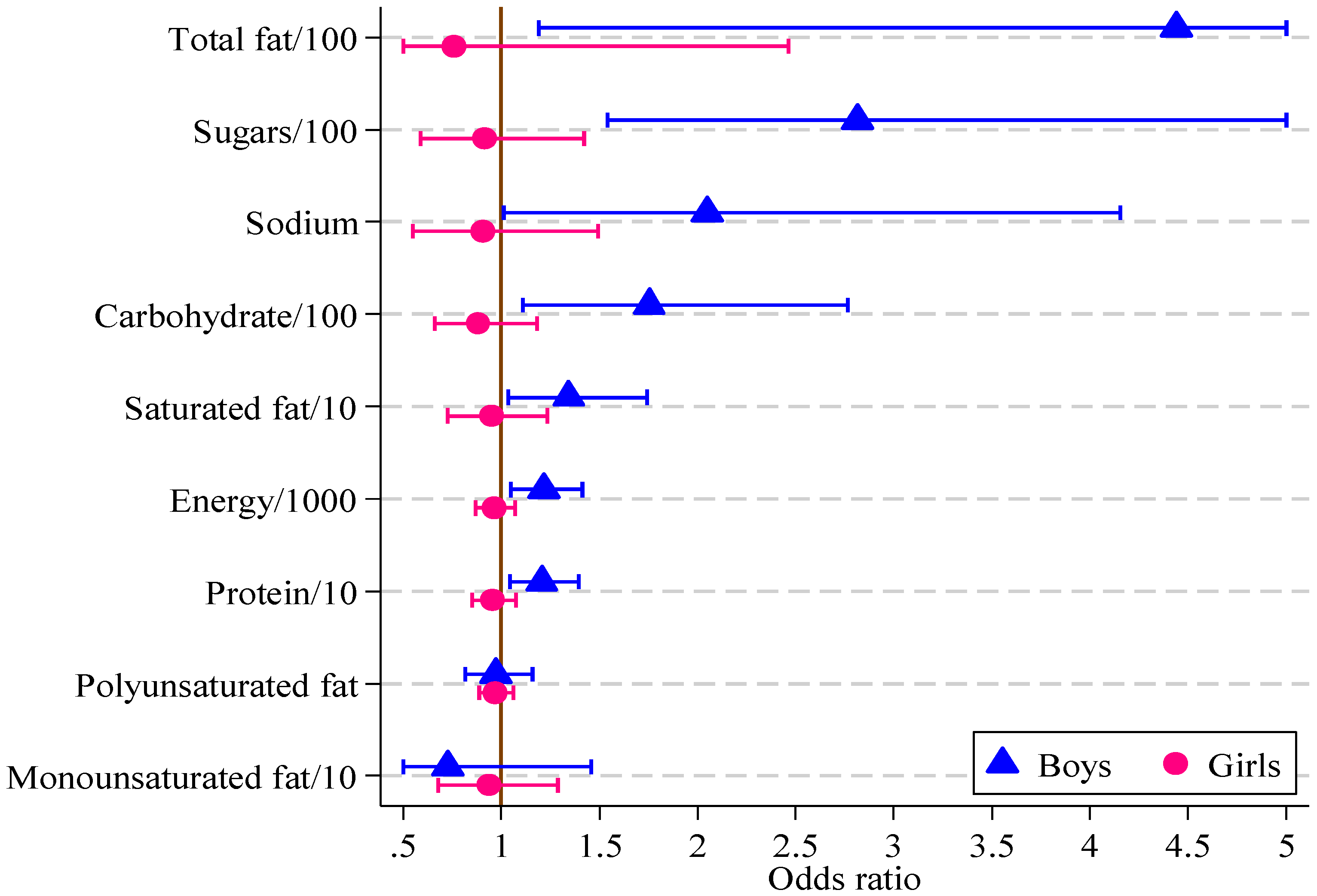
Significant associations were found in boys between the HIKCUPS CVD risk score and all dietary measures except polyunsaturated fat (
Table 6). No significant associations were demonstrated using similar models for girls, or in models using observations from both sexes, with or without the interaction between sex and dietary measures. These odds ratios and 95% confidence intervals are displayed in
Figure 2.
Table 6.
Odds ratios, 95% confidence intervals (CI) and p-values from multivariate logistic regression models of CVD risk score on individual diet measures and total energy (e indicates total energy was significant in the model at the 5% level).
Table 6.
Odds ratios, 95% confidence intervals (CI) and p-values from multivariate logistic regression models of CVD risk score on individual diet measures and total energy (e indicates total energy was significant in the model at the 5% level).
| Diet Measure | Boys (n = 121) | Girls (n = 164) |
|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value |
|---|
| Sugars/100 (g) | 2.82 | 1.54, 5.15 | 0.001 | 0.92 | 0.59, 1.42 | 0.696 |
| Energy/1000 (kJ) | 1.22 | 1.05, 1.41 | 0.010 | 0.96 | 0.87, 1.07 | 0.479 |
| Protein/10 (g) | 1.21 | 1.04, 1.39 | 0.011 | 0.95 | 0.85, 1.07 | 0.435 |
| Carbohydrate/100 (g) | 1.75 | 1.11, 2.77 | 0.016 | 0.88 | 0.66, 1.18 | 0.400 |
| Total Fat/100 (g) | 4.44 | 1.19, 16.60 | 0.027 | 0.76 | 0.23, 2.46 | 0.646 |
| Saturated fat/10 (g) | 1.34 | 1.03, 1.74 | 0.027 | 0.95 | 0.73, 1.23 | 0.690 |
| Monounsaturated fat/10 (g) | 0.73 e | 0.36, 1.46 | 0.366 | 0.93 | 0.68, 1.29 | 0.678 |
| Polyunsaturated fat (g) | 0.76 e | 0.13, 4.34 | 0.761 | 0.73 | 0.30, 1.80 | 0.493 |
| Sodium/1000 (mg) | 2.05 | 1.01, 4.15 | 0.047 | 0.90 | 0.55, 1.49 | 0.694 |
Figure 2.
Odds ratios, with 95% confidence intervals, for boys and girls from multivariate logistic regression models of CVD risk score on diet measure (boys, n = 121; girls, n = 164).
Figure 2.
Odds ratios, with 95% confidence intervals, for boys and girls from multivariate logistic regression models of CVD risk score on diet measure (boys, n = 121; girls, n = 164).
The current study describes the development of a multifactor CVD risk score that reflects the clustering of CVD risk score factors in pre-pubertal overweight and obese children. The main factors contributing to higher CVD risk score in this pediatric group were high WC and BMI, elevated serum insulin and triglycerides, and low HDL concentrations. Elevated blood pressure was rare. Dietary measures, including high intakes of total and saturated fat, carbohydrate and sugars were significantly related to CVD risk score in boys only with no associations between a range of dietary measures and multifactor CVD risk score in girls.
The risk factors identified in the current study are not entirely consistent with other studies in overweight and obese children. Although 48% of girls and 50% of boys had triglyceride concentrations above the 90th centile in the current study, with similar results for fasting insulin levels, most had values for blood pressure, fasting glucose, total and LDL cholesterol below the 90th percentile. An Australian study of 1430 eight year olds [
40] (boys and girls 15% and 20% overweight or obese, respectively) found significant differences between normal and overweight/obese participants for triglycerides, HDL, systolic and diastolic pressure. Data from the Bogalusa study [
41] showed that overweight children (aged 5–10 years) had a higher prevalence of elevated insulin and triglyceride levels, as well as systolic blood pressure, total and LDL cholesterol, compared to those of normal weight (defined as >95th centile for race, age and sex, >130 mg/dL, >95th centile according to National High Blood Pressure Education Program methods, >200 mg/dL and >130 mg/dL respectively). In a sample of European overweight and obese children [
13] aged 12 ± 3 years (31% pre-pubertal), elevated blood pressure was the most prevalent CVD risk score factor at 35% (assessed by height >95th centile of European reference ranges). Potential influences on the reference ranges include disparities in centile or population reference values based on age, sex, race and height; differences in assay techniques; and possibly diurnal differences in serum risk factor concentrations.
The combination of low levels of HDL with elevated serum insulin and triglyceride concentrations increases CVD risk score and was found in approximately one third of children in the current study. Insulin resistance increases triglyceride production, which in turn facilitates development of small dense LDL particles that are more susceptible to oxidation [
42], further increasing CVD risk. Data compiled from four major child cardiovascular studies (Bogalusa Heart, Muscatine, Young Finns and Childhood Determinants of Adult Health) [
8,
43] were able to predict subclinical atherosclerosis in children aged nine years or older by identifying those with elevated total cholesterol, triglycerides, blood pressure and BMI. In addition, Lawlor
et al. [
44] and Nyugen
et al. [
28] argue that positive and rapid changes in BMI over time further increase the risk of CVD and metabolic syndrome and need to be considered when assessing risk.
Studies examining sex-based associations between diet and CVD risk in children, particularly amongst those overweight or obese, are limited and findings inconsistent making comparisons across studies difficult. A study of a large European pediatric cohort [
45] found that boys aged 6 to 9 years who consumed nuts and seeds or had high intakes of chocolate and nut-based spreads had a lower CVD risk whereas girls had a higher risk in association with high intakes of manufactured juices and lower risk with chocolate and nut-based spreads. Ambrosini
et al. [
46] found 14 year old girls, but not boys, who had diets high in fat, refined sugars and sodium had greater clustering of risk factors for metabolic syndrome. In a Mexican population of a similar age [
47], positive correlations were found between white bread and fasting insulin concentrations; between fasting glucose and sugar sweetened beverages and between added fats and serum triglycerides. However, no differences were reported by sex or weight status.
Sex-based responses to diet may influence CVD risk secondary to differences in hormone profiles, lipid metabolism or lifestyle behaviors, as suggested previously by Ambrosini
et al. [
46]. Differences in maturation and hormonal status of children may have influenced the results and account for some of the sex differences in this study. Reinehr and Toschke [
48] found that CVD risk factors vary by stage of puberty. In the current study, pubertal status was assessed at baseline only, with follow-up time points up to two years included. Therefore it is not known whether any participants entered puberty during this time. Consequently the results need to be interpreted with some caution [
48] and further investigation is warranted. Reinehr [
49] suggests that future research focus on identifying which high-risk adolescents respond to specific treatment approaches, with the current study supporting a focus on dietary interventions particularly for boys shown to be at elevated CVD risk prior to puberty.
Clustering of CVD risk factors is common [
5,
13,
41,
50]. The strength of the CVD risk score developed in the current study is that it combines graded scores based on ten CVD risk variables [
30,
51]. Deriving a combined CVD risk score from measures of adiposity, blood lipids, carbohydrate metabolism and blood pressure referenced to normal ranges has been used in previous studies [
5,
17,
52]. Kelly
et al. [
53] found that clustered scores were better predictors of metabolic syndrome, and that it overcame issues associated with arbitrary cut-points for some criteria [
54]. The benefit of such a tool is that it is easier than calculating z-scores or quintiles, as is commonly used currently [
53,
55]. Using both BMI and WC allows more accurate identification of adiposity and abdominal obesity, rather than those with greater lean muscle mass.
When attempting to create a risk assessment tool, there are limitations that need to be acknowledged. The current tool would be strengthened by applying it to a larger and more varied cohort in a much longer study that followed children into adulthood to obtain objective CVD outcomes. Whilst this study used the 90th centile as the cut-point across all risk factors to ensure consistency across reference values, it is acknowledged that this may reduce sensitivity in identifying all those at increased risk secondary to high BMI. Some studies [
28,
44] have suggested that change in body weight may be important and this is not included in the current study. Mattsson [
29] suggests that true CVD risk assessment needs to include family history of CVD and metabolic syndrome to account for genetic contributions, which were not assessed as part of the HIKCUPS study. Weight status of parents and family lifestyle behaviors could also be included in future risk assessment [
4,
40]. In practice, BP, BMI, WC and family history are more likely to be measured due to their low cost, whereas blood tests to assess plasma lipids are less regularly performed [
56]. The use of normal reference ranges developed on other pediatric populations also has inherent difficulties. Although reported to be consistent with other Caucasian children for WC, blood pressure, serum lipids and glucose metabolism [
18], the reference ranges used here were developed in French children. Variations exist when obtaining WC measures as an indicator of central adiposity in children (umbilicus, narrowest point, mid-point). This can be difficult to measure in overweight and obese pediatric participants and variations are known to exist within the measurements [
57] compared to measures such as height and weight. In the current study WC measures and reference values were obtained by different methods and may have influenced the results. The use of blood pressure centiles based on height could also yield different risk scores, by allowing higher blood pressure ranges in taller children [
58]. In the current study the CVD risk score for each factor was weighted as equally important but this may an over-simplification and hence results should be interpreted with caution. Future studies should investigate this further, and include additional factors associated with insulin resistance [
54].