Voltammetric Determination of Anti-Hypertensive Drug Hydrochlorothiazide Using Screen-Printed Electrodes Modified with L-Glutamic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Procedures
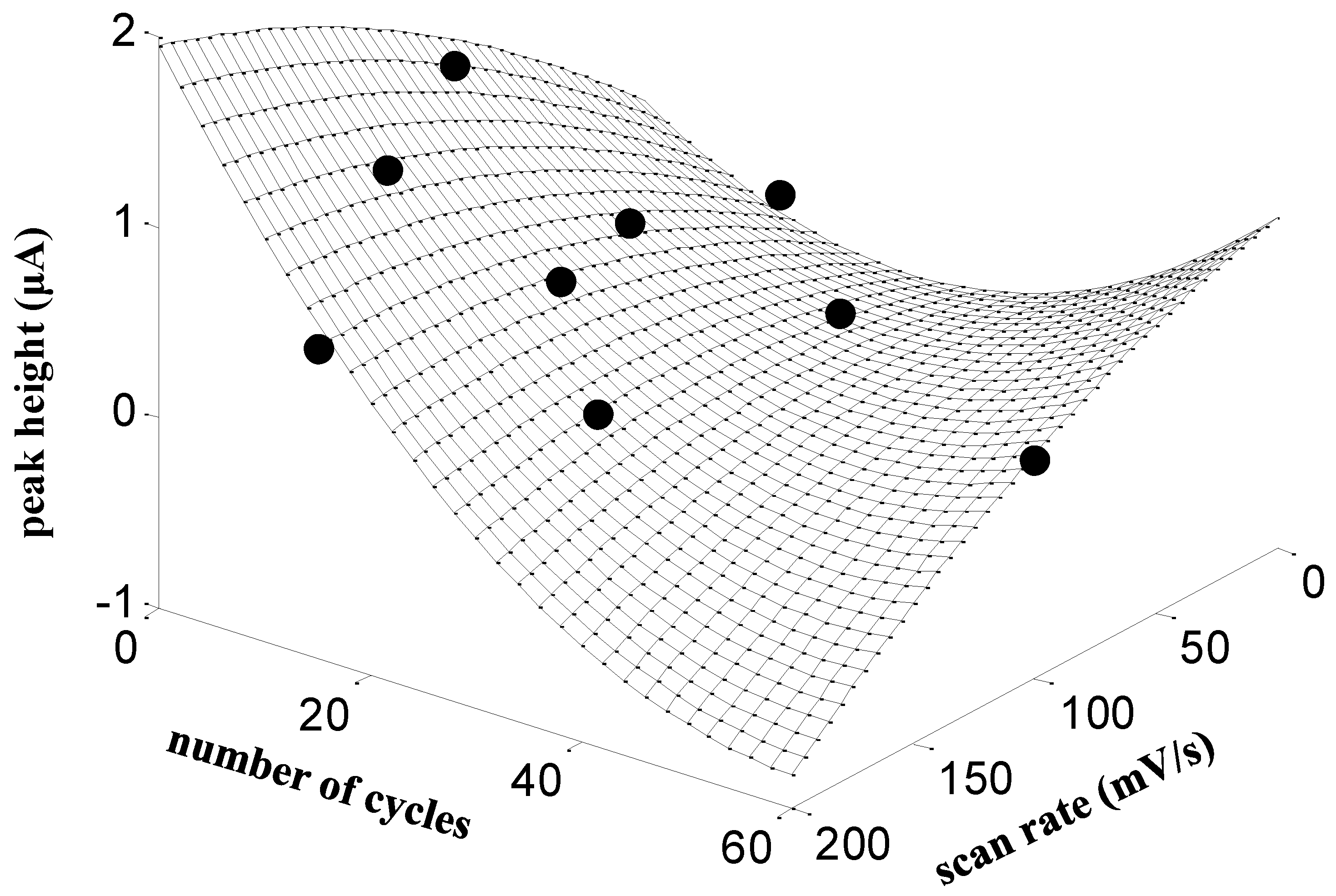
2.3.1. Preparation of Modified SPCEs by Electropolymerization with L-Glutamic Acid (SPCE/PGA)
2.3.2. Preparation of Modified SPCEs by Electrografting with L-Glutamic Acid (SPCE/EGA)
Diazonium Salt Electrografting
Covalent Immobilization of L-Glutamic Acid via Carbodiimide Coupling
2.3.3. Voltammetric Measurements
3. Results and Discussion
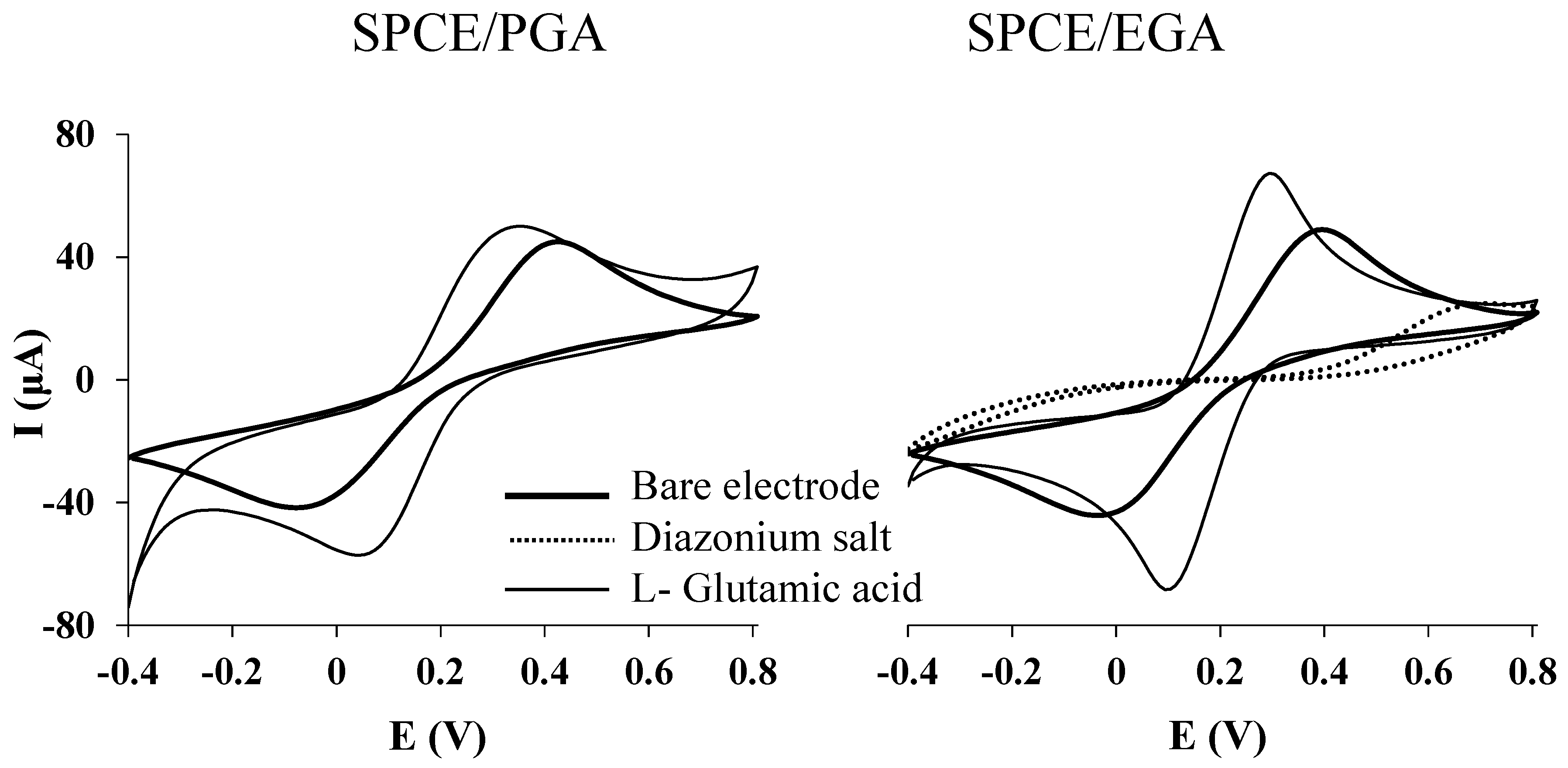
3.1. Electrochemical Characterization
3.2. Repeatability and Reproducibility
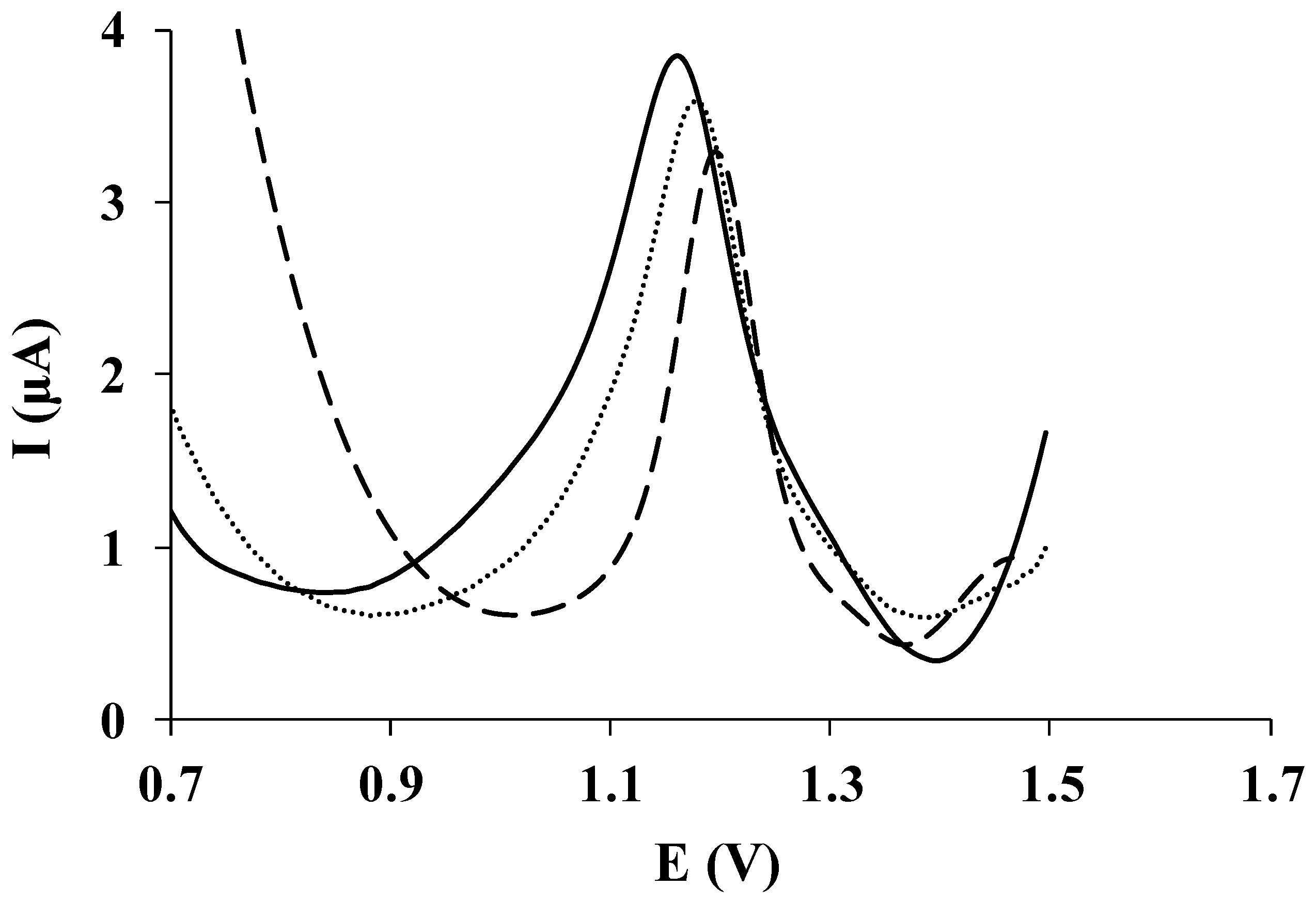
3.3. Calibration Data
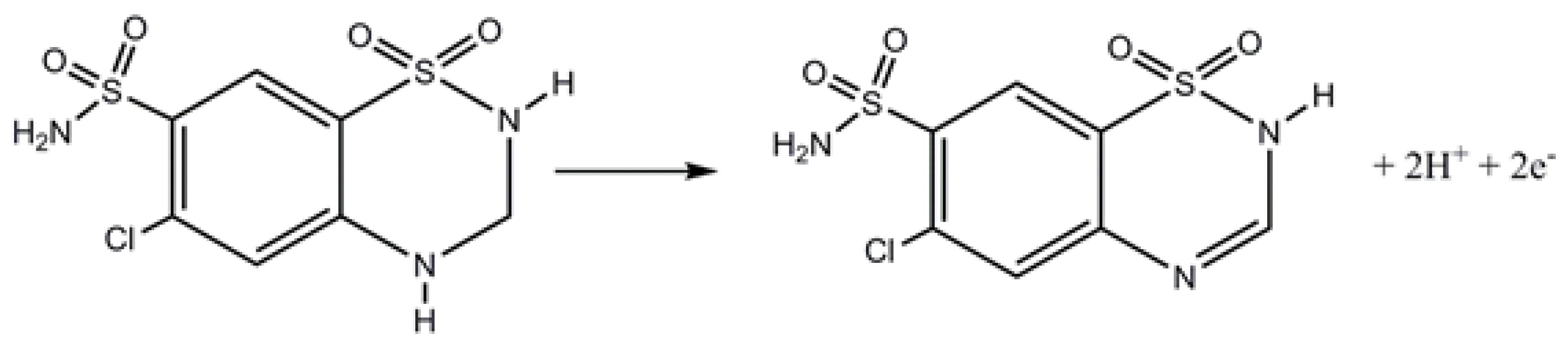
3.4. Application to the Analysis of an Anti-Hypertensive Drug
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schoenberger, J.A. Losartan with hydrochlorothiazide in the treatment of hypertension. J. Hypertens. Suppl. 1995, 13, S43–S47. [Google Scholar] [PubMed]
- Ramsay, L.E.; Yeo, W.W. Double-blind comparison of losartan, lisinopril and hydrochlorothiazide in hypertensive patients with a previous angiotensin converting enzyme inhibitor-associated cough. J. Hypertens. Suppl. 1995, 13, S73–S76. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, G.; Palumbo, G.; Mazzeo, P.; Quaglia, M.G. Simultaneous determination of losartan and hydrochlorothiazide in tablets by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2000, 23, 185–189. [Google Scholar] [CrossRef]
- Bhat, L.R.; Godge, R.K.; Vora, A.T.; Damle, M.C. Validated RP-HPLC method for simultaneous determination of telmisartan and hydrochlorothiazide in pharmaceutical formulation. J. Liq. Chromatogr. Rel. Technol. 2007, 30, 3059–3067. [Google Scholar] [CrossRef]
- Mashru, R.C.; Sutariya, V.B.; Thakker, A.J. High performance liquid chromatographic method for simultaneous determination of fosinopril sodium and hydrochlorothiazide in tablets formulation. Ars Pharm. 2006, 47, 375–383. [Google Scholar]
- United States Pharmacopoeia. United States Pharmacopoeial Convention; United States Pharmacopoeia: Rockville, MD, USA, 2007. [Google Scholar]
- Li, H.; Wang, Y.; Jiang, Y.; Tang, Y.; Wang, J.; Zhao, L.; Gu, J. A liquid chromatography/tandem mass spectrometry method for the simultaneous quantification of valsartan and hydrochlorothiazide in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Hillaert, S.; Van den Bossche, W. Simultaneous determination of hydrochlorothiazide and several angiotensin-II-receptor antagonists by capillary electrophoresis. J. Pharm. Biomed. Anal. 2003, 31, 329–339. [Google Scholar] [CrossRef]
- Balesteros, M.R.; Faria, A.F.; de Oliveira, M.A.L. Determination of losartan associated with chlorthalidone or hydrochlorothiazide in capsules by capillary zone electrophoresis. J. Braz. Chem. Soc. 2007, 18, 554–558. [Google Scholar] [CrossRef]
- Youssef, A.O. Spectrofluorimetric assessment of hydrochlorothiazide using optical sensor nano-composite terbium ion doped in sol-gel matrix. J. Fluoresc. 2012, 22, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Murillo Pulgarín, J.A.; Alañón Molina, A.; Pérez-Olivares Nieto, G. Determination of hydrochlorothiazide in pharmaceutical preparations by time resolved chemiluminescence. Anal. Chim. Acta 2004, 518, 37–43. [Google Scholar] [CrossRef]
- Ouyang, J.; Baeyens, W.R.G.; Delanghe, J.; Van der Weken, G.; Calokerinos, A.C. Cerium (IV)-based chemiluminescence analysis of hydrochlorothiazide. Talanta 1998, 46, 961–968. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. First report for simultaneous determination of methyldopa and hydrochlorothiazide using a nanostructured based electrochemical sensor. J. Electroanal. Chem. 2013, 704, 137–144. [Google Scholar] [CrossRef]
- Beitollahi, H.; Ghorbani, F. Benzoylferrocene-modified carbon nanotubes paste electrode as a voltammetric sensor for determination of hydrochlorothiazide in pharmaceutical and biological samples. Ionics 2013, 19, 1673–1679. [Google Scholar] [CrossRef]
- Gardenal Santos, M.C.; Teixeira Tarley, C.R.; Dall’Antonia, L.H.; Sartori, E.R. Evaluation of boron-doped diamond electrode for simultaneous voltammetric determination of hydrochlorothiazide and losartan in pharmaceutical formulations. Sens. Actuators B Chem. 2013, 188, 263–270. [Google Scholar] [CrossRef]
- Machini, W.B.S.; David-Parra, D.N.; Teixeira, M.F.S. Electrochemical investigation of the voltammetric determination of hydrochlorothiazide using a nickel hydroxide modified nickel electrode. Mater. Sci. Eng. C 2015, 57, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Damiri, S. Multiwalled carbon nanotubes modified electrode as a sensor for adsorptive stripping voltammetric determination of hydrochlorothiazide. IEEE Sens. J. 2008, 9, 1523–1529. [Google Scholar] [CrossRef]
- Pires Eisele, A.P.; Mansano, G.R.; de Oliveira, F.M.; Casarin, J.; Teixeira Tarley, C.R.; Romão Sartori, E. Simultaneous determination of hydrochlorothiazide and valsartan in combined dosage forms: Electroanalytical performance of cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2014, 732, 46–52. [Google Scholar] [CrossRef]
- Yao, C.; Sun, H.; Fu, H.-F.; Tan, Z.-C. Sensitive simultaneous determination of nitrophenol isomers at poly(p-aminobenzene sulfonic acid) film modified graphite electrode. Electrochim. Acta 2015, 156, 163–170. [Google Scholar] [CrossRef]
- Lezi, N.; Kokkinos, C.; Economou, A.; Prodromidis, M.I. Voltammetric determination of trace Tl(I) at disposable screen-printed electrodes modified with bismuth precursor compounds. Sensors Actuators B Chem. 2013, 182, 718–724. [Google Scholar] [CrossRef]
- Shi, F.; Xi, J.; Hou, F.; Han, L.; Li, G.; Gong, S.; Chen, C.; Sun, W. Application of three-dimensional reduced graphene oxide-gold composite modified electrode for direct electrochemistry and electrocatalysis of myoglobin. Mater. Sci. Eng. C 2016, 58, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Pereira Santos, D.; Boldrin Zanoni, M.V.; Bergamini, M.F.; Chiorcea-Paquim, A.-M.; Diculescu, V.C.; Oliveira Brett, A.-M. Poly(glutamic acid) nanofibre modified glassy carbon electrode: Characterization by atomic force microscopy, voltammetry and electrochemical impedance. Electrochim. Acta 2008, 53, 3991–4000. [Google Scholar] [CrossRef]
- Gooding, J.J. Advances in interfacial design for electrochemical biosensors and sensors: Aryl diazonium salts for modifying carbon and metal electrodes. Electroanalysis 2008, 20, 573–582. [Google Scholar] [CrossRef]
- Delamar, M.; Hitmi, R.; Pinson, J.; Saveant, J.M. Covalent modification of carbon surfaces by grafting of functionalized aryl radicals produced from electrochemical reduction of diazonium salts. J. Am. Chem. Soc. 1992, 114, 5883–5884. [Google Scholar] [CrossRef]
- Baranton, S.; Bélanger, D. Electrochemical derivatization of carbon surface by reduction of in situ generated diazonium cations. J. Phys. Chem. B. 2005, 109, 24401–24410. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Nguyen, Q.T.; Chow, E.; Böcking, T.; Hibbert, D.B.; Gooding, J.J. Study of factors affecting the performance of voltammetric copper sensors based on Gly-Gly-His modified glassy carbon and gold electrodes. Electroanalysis 2006, 18, 1141–1151. [Google Scholar] [CrossRef]
- Serrano, N.; Prieto-Simón, B.; Cetó, X.; del Valle, M. Array of peptide-modified electrodes for the simultaneous determination of Pb(II), Cd(II) and Zn(II). Talanta 2014, 125, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.; González-Calabuig, A.; del Valle, M. Crown ether-modified electrodes for the simultaneous stripping voltammetric determination of Cd(II), Pb(II) and Cu(II). Talanta 2015, 138, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Penicillamine-modified sensor for the voltammetric determination of Cd(II) and Pb(II) ions in natural samples. Talanta 2015, 144, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Glutathione modified screen-printed carbon nanofiber electrode for the voltammetric determination of metal ions in natural samples. Talanta 2016, 155, 8–13. [Google Scholar] [CrossRef] [PubMed]
- González-Calabuig, A.; Guerrero, D.; Serrano, N.; del Valle, M. Simultaneous voltammetric determination of heavy metals by use of crown ether-modified electrodes and chemometrics. Electroanalysis 2016, 28, 663–670. [Google Scholar] [CrossRef]
- Liu, X.; Luo, L.; Ding, Y.; Ye, D. Poly-glutamic acid modified carbon nanotube-doped carbon paste electrode for sensitive detection of L-tryptophan. Bioelectrochemistry 2011, 82, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Holtzer, A. The helix-coil transition in solutions of polyglutamic acid. J. Am. Chem. Soc. 1964, 86, 538–543. [Google Scholar] [CrossRef]
- Santos, D.P.; Bergamini, M.F.; Fogg, A.G.; Zanoni, M.V.B. Application of a glassy carbon electrode modified with poly(glutamic acid) in caffeic acid determination. Microchim. Acta 2005, 151, 127–134. [Google Scholar] [CrossRef]
- Yu, A.-M.; Chen, H.-Y. Electrocatalytic oxidation of hydrazine at the poly(glutamic acid) chemically modified electrode and its amperometric determination. Anal. Lett. 1997, 30, 599–607. [Google Scholar] [CrossRef]
- Yu, A.-M.; Chen, H.-Y. Electrocatalytic oxidation and determination of ascorbic acid at poly(glutamic acid) chemically modified electrode. Anal. Chim. Acta 1997, 344, 181–185. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, X. Covalent modification of glassy carbon electrode with glutamic acid for simultaneous determination of uric acid and ascorbic acid. Analyst 2001, 126, 367–370. [Google Scholar] [CrossRef] [PubMed]
- González-Vargas, C.; Garcia, C.; Celis, F.; Salazar, R. Differential pulse voltammetry determination of anti-hypertensive drug hydrochlorothiazide in pharmaceuticals using glassy-carbon electrode modified by electropolymerization with l- and d-glutamic acids. Int. J. Electrochem. Sci. 2017. submitted. [Google Scholar]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Barton, J.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Diamond, D.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2016, 183, 503–517. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Salamanca-Neto, C.A.R.; Pires Eisele, A.P.; Gouveia Resta, V.; Scremin, J.; Romão Sartori, E. Differential pulse voltammetric method for the individual and simultaneous determination of antihypertensive drug metoprolol and its association with hydrochlorothiazide in pharmaceutical dosage forms. Sens. Actuators B Chem. 2016, 230, 630–638. [Google Scholar] [CrossRef]
- Razak, O.A. Electrochemical study of hydrochlorothiazide and its determination in urine and tablets. J. Pharm. Biomed. Anal. 2004, 34, 433–440. [Google Scholar] [CrossRef]
- Ahlberg, E.; Helgée, B.; Parker, V.D. The reaction of aryl radicals with metallic electrodes. Acta Chem. Scand. B 1980, 34, 181–186. [Google Scholar] [CrossRef]
- Lourencao, B.C.; Silva, T.A.; Fatibello-Filho, O.; Swain, G.M. Voltammetric studies of propranolol and hydrochlorothiazide oxidation in standard and synthetic biological fluids using anitrogen-containing tetrahedral amorphous carbon (ta-C:N) electrode. Electrochim. Acta 2014, 143, 398–406. [Google Scholar] [CrossRef]
- Alghamdi, A.F. Electrochemical oxidation behavior of hydrochlorothiazide on a glassy carbon electrode and its voltammetric determination in pharmaceutical formulations and biological fluids. J. Food Drug Anal. 2014, 22, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Hamzavi, M.; Torkzadeh-Mahani, M. Electrochemical determination of hydrochlorothiazide and folic acid in real samples using a modified graphene oxide sheet paste electrode. Mater. Sci. Eng. C 2015, 52, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Pishahang, J.; Barzegar Amiri, H.; Sattarahmady, N. Synthesis of nickel nanowrinkles and its application for the electrocatalytic oxidation and sensitive detection of hydrochlorothiazide. Microchem. J. 2017, 130, 205–212. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ganjali, M.R.; Norouzi, P.; Bananezhad, A. Amplified nanostructure electrochemical sensor for simultaneous determination of captopril, acetaminophen, tyrosine and hydrochlorothiazide. Mater. Sci. Eng. C 2017, 73, 472–477. [Google Scholar] [CrossRef] [PubMed]
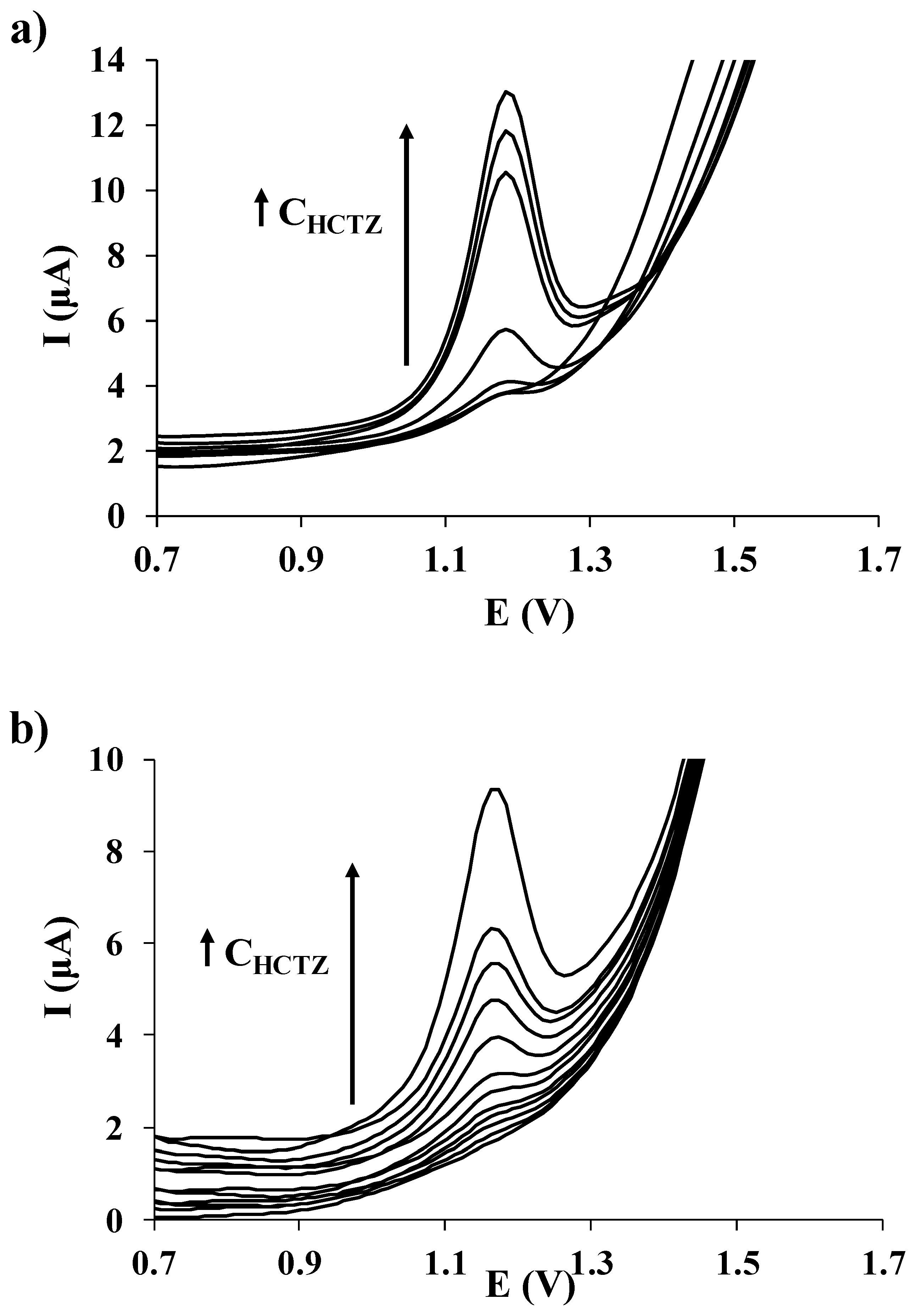
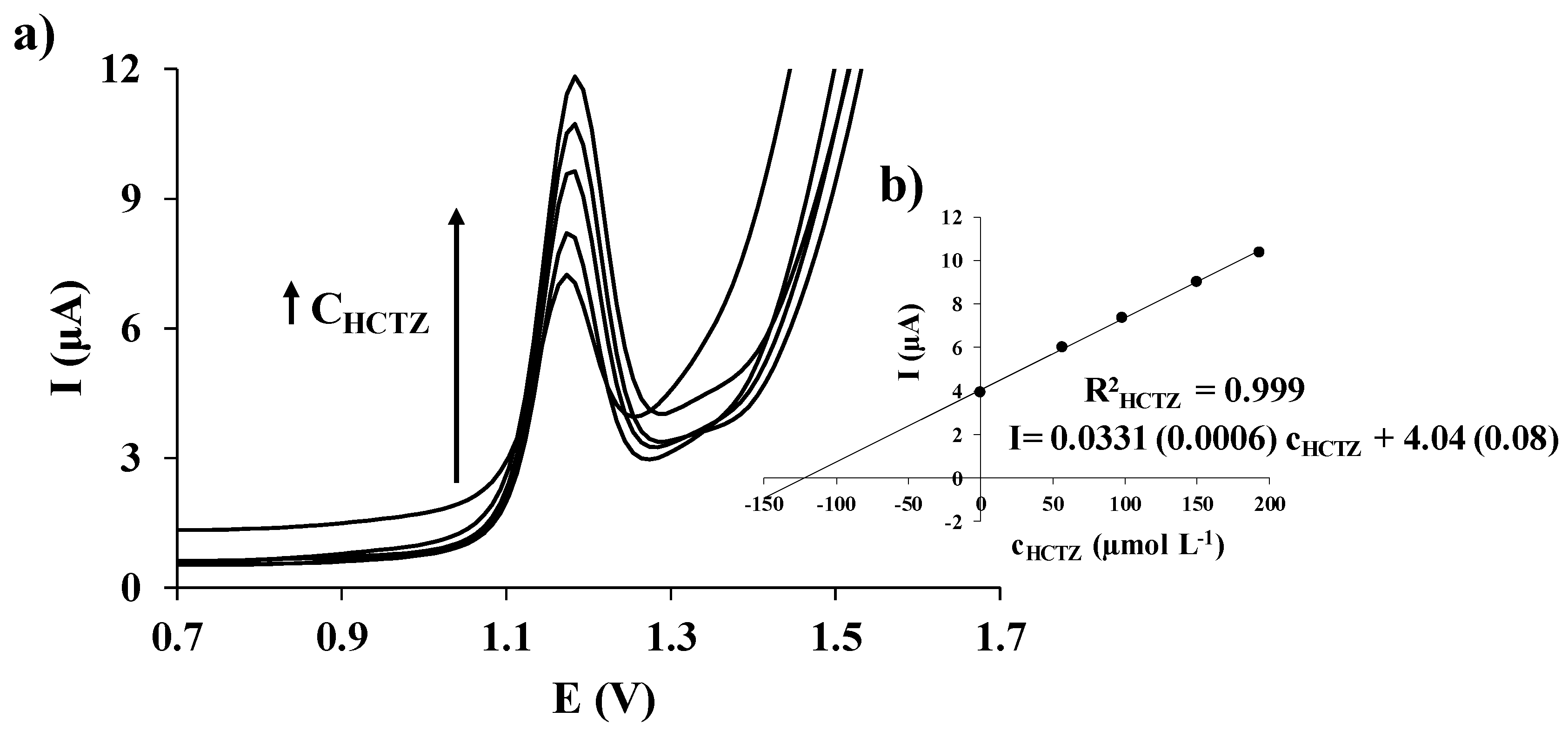
| Electrode | Hydrochlorothiazide | |||
|---|---|---|---|---|
| Sensitivity (μA µmol−1 L) (a) | R2 | Linear range (µmol L−1) (b) | LOD (µmol L−1) | |
| SPCE/PGA | 0.0306 (0.0005) | 0.999 | 28.5–300.0 | 8.55 |
| SPCE/EGA | 0.0395 (0.0003) | 0.999 | 3.78–200.0 | 1.13 |
| Method | Electrode | Linear Range (µmol L−1) | LOD (µmol L−1) | LOQ (µmol L−1) | Ref. |
|---|---|---|---|---|---|
| SWV | BDDE | 1.97–88.1 | 0.639 | 2.13 | [18] |
| DPV | GC | 71.5–1000.0 | 21.4 | 71.5 | [38] |
| DPV | GC/L-PAG | 65.0–1000.0 | 19.6 | 65.0 | [38] |
| DPV | GC/D-PAG | 63.3–1000.0 | 19.0 | 63.3 | [38] |
| ASV | GC | 4.0–40.0 | 0.0043 | - | [47] |
| SWV | 2CBFGPE | 0.05–200.0 | 0.02 | - | [48] |
| CV | NCPE | 220.0–5820.0 | 21.2 | 70.6 | [49] |
| SWV | NiO/CNTs/DPID/CPE | 10.0–600.0 | 5.0 | - | [50] |
| DPV | SPCE/PGA | 28.5–300.0 (a) | 8.55 | 28.5 | This work |
| DPV | SPCE/EGA | 3.78–200.0 (a) | 1.13 | 3.78 | This work |
| CHCTZ (mg/tablet) | RSD (%) | Relative error (%) | |
|---|---|---|---|
| SPCE/EGA | 52.1 | 5.6 | 4.3 |
| Reported value | 50 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Vargas, C.; Serrano, N.; Ariño, C.; Salazar, R.; Esteban, M.; Díaz-Cruz, J.M. Voltammetric Determination of Anti-Hypertensive Drug Hydrochlorothiazide Using Screen-Printed Electrodes Modified with L-Glutamic Acid. Chemosensors 2017, 5, 25. https://doi.org/10.3390/chemosensors5030025
González-Vargas C, Serrano N, Ariño C, Salazar R, Esteban M, Díaz-Cruz JM. Voltammetric Determination of Anti-Hypertensive Drug Hydrochlorothiazide Using Screen-Printed Electrodes Modified with L-Glutamic Acid. Chemosensors. 2017; 5(3):25. https://doi.org/10.3390/chemosensors5030025
Chicago/Turabian StyleGonzález-Vargas, Camilo, Núria Serrano, Cristina Ariño, Ricardo Salazar, Miquel Esteban, and José Manuel Díaz-Cruz. 2017. "Voltammetric Determination of Anti-Hypertensive Drug Hydrochlorothiazide Using Screen-Printed Electrodes Modified with L-Glutamic Acid" Chemosensors 5, no. 3: 25. https://doi.org/10.3390/chemosensors5030025






