1. Introduction
Sensor materials, in the form of coatings on optical fibers or on printed electrodes, opens the possibility for many interesting sensor applications. Optical fiber sensors enable distributed sensing along a fiber at desired points over long distances or in confined spaces. Printed sensors on flexible substrates, such as paper or plastics can, for example, offer production of low-cost sensor devices for point of care applications, construction applications, food packaging and environmental monitoring [
1,
2,
3,
4].
The demand for pH sensors is growing, especially in applications such as chemical instrumentation, industry, process control, medical analyses and environmental monitoring. In the field of environmental monitoring there is a special need for distributed sensors to cover large areas [
5,
6,
7], either as distributed sensors or sensors spread out in numerous locations in for example forests, lakes or landfills. This makes pH responsive coatings that are compatible with the optical or printed sensor technology particularly interesting. Richter et al. [
8] describe two basic principles for transducers based on responsive hydrogels: (a) materials based on mechanical work performed by swelling and shrinking and (b) materials that exhibit a change in properties (e.g., densities, mass, volume, stiffness). For both principles, a pH responsive coating in its cured form must be able to change its mechanical-, optical- or electrical parameters in response to pH variations. Deposition of a material by coating or printing requires a suitable viscosity, wetting on the substrate and the ability to cure the polymer to a solid coating or a printed pattern [
9]. Furthermore, deposition of materials to form coatings on optical fibers is preferably carried out without the use of solvents. Therefore, the precursor forming the responsive coating should be a fluid in its neat form under ambient conditions. Since the material is to form a part of a hydrogel-based sensor, cross-linking during curing must be complete and not result in soluble residues. Otherwise, incomplete curing would result in a gradual leak of soluble material from the gel. For this reason, it is important to establish the right conditions for sufficient curing.
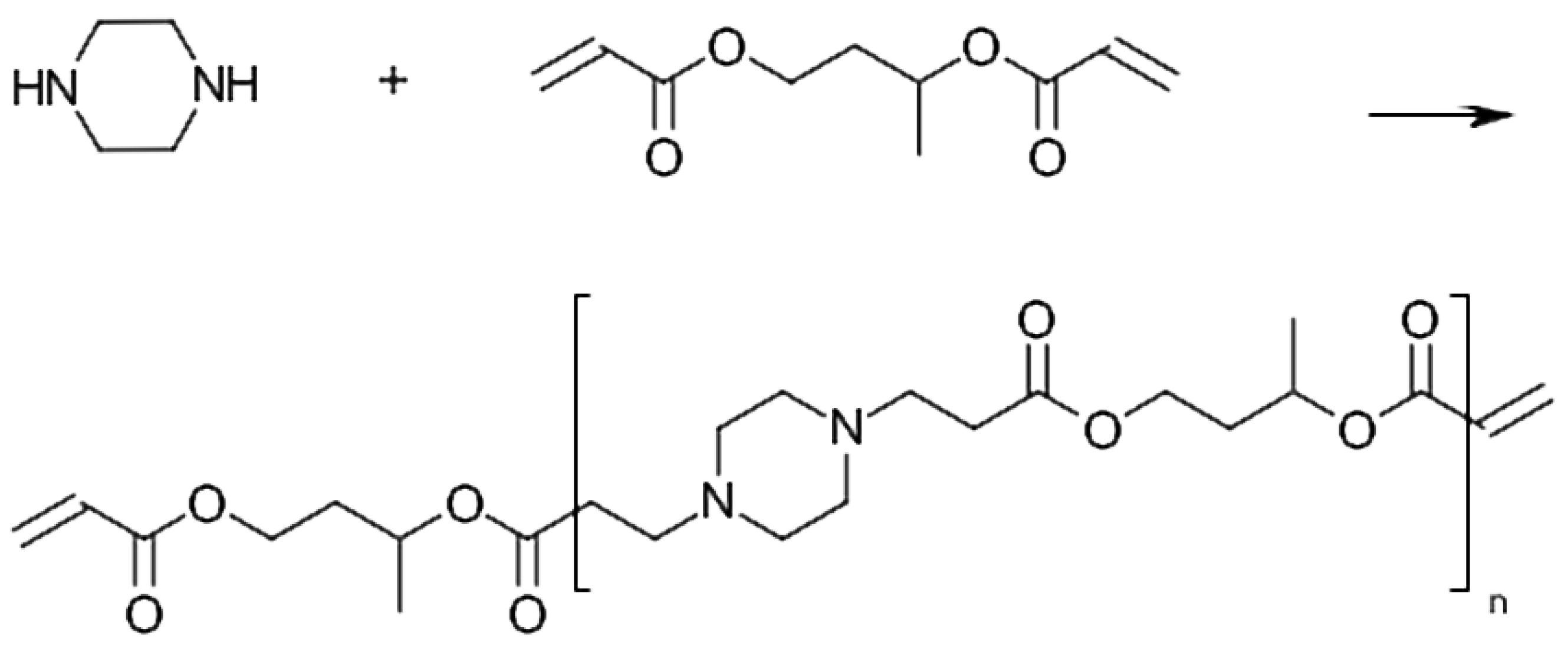
Polymerizable molecules containing amino groups would be suitable starting materials for pH-responsive coatings. We have identified coupling of amines with acryalates to (
-amino esters) as a viable synthetic path to this type of molecules. More specifically, we have explored the stochiometric balance of diamines and diacrylates to produce oligo(
-amino esters) with acrylic terminal groups with controlled molecular weight. However, there have to our knowledge been no reports about any investigation on AOBAEs used as a coating material. The AOBAEs are closely related to the poly(
-amino esters) (PBAEs), which were introduced by Lynn and Langer [
10] as gene transfer vector materials, that have been extensively explored for its pH-responsiveness. PBAEs are readily obtained by reacting equimolar amounts of diamines with diacrylates. Coupling of primary amines with acrylates requires heating and once a secondary (
-amino ester) is formed, the secondary amino group readily reacts with another acrylate to form a PBAE of tertiary amino ester. The obtained PBAEs are bio-compatible, have low toxicity and are suitable for use in vivo and in environmental monitoring [
11]. For example, PBAEs obtained from 1,4-butanediol diacrylate (1,4-BDDA) and piperazine (PIP) have previously been studied as pH responsive polymers [
12]. However, initial tests with acryl-terminated oligomers obtained from reacting 1,4-BDDA and PIP resulted in solid and crystalline materials in room temperature and are hence not suitable for the process of coating of optical fibers. Synthesis of non-crystalline AOBAEs that are fluid at ambient temperatures are therefore needed to produce pH-responsive optical fiber coatings. The crystallinity of polymers depends on the symmetry properties of the polymer structural units. Polymers with symmetric structural units typically have a higher tendency to crystallize than polymers with asymmetric structural units. AOBAEs prepared from 1,4-BDDA and piperazine have symmetric structural units, which may contribute to their observed and in this case undesired, tendency to form crystalline solids. We have therefore explored AOBAEs with asymmetric structural units. By preparing AOBAE from piperazine and 1,3-BDDA we incorporate the asymmetric 1,3-butylene group in the AOBAE. The 1,3-butylene group have a chiral carbon atom, which introduces chirality, as well as a directional “head-to-tail” isomerism due to the un-equivalence of the 1 and 3 positions. It is well known that both tacticity, the arrangement of chiral atoms in a polymer and directional isomerism influence crystallinity in polymers [
13,
14]. The coupling reaction forming (
-amino esters) is likely to produce polymers that are atactic and with an irregular directional arrangement of 1,3-butylene groups. AOBAEs synthesized from 1,3-BDDA are therefore more likely to produce non-crystalline materials, which is the aim of the present investigation.
A class of pH sensitive AOBAEs not previously studied have been examined. The aim is to use the AOBAEs as a coating material, compatible with optical- and printed electronics sensor technology for various applications. Cross-linked AOBAE coatings were obtained by polymerization of AOBAE in the presence of a photo-initiator on substrates pretreated with primers to enhance the adhesion. Curing parameters, such as the irradiation dose and atmospheric conditions (air or inert gas) were investigated, as well as the effect of adhesion promoting primers. NMR was used for molecular structural characterization of AOBAE. FTIR spectroscopy was used to monitor the effect of curing parameters. The pH responsivity of cured AOBAE coatings were investigated by monitoring the film thickness change in response to pH variation by using an interferometric measurement technique. The swell responsivity indicates also that the permittivity and the RI will change. Even a small change of swelling can have a large impact on a materials permittivity and RI. For example Bolthauser et al. [
15] built a humidity sensor in which they measured the effective dielectric constant change in polyimide (polyimide absorbs 1.2 wt % water [
16]). And Dubendorfer et al. [
17] reached a pH resolution of
when measuring RI changes for a hydrogel that swelled 35%/pH unit. Therefore we believe that the AOBAE can be an interesting candidate as a pH sensitive coating material for the development pH sensors using optical- or printed electronics technology.
2. Materials and Methods
2.1. Materials
1,3-butanediol diacrylate (1,3-BDDA), piperazine (PIP), diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide (TPO) and 3-(trimethoxysilyl) propyl acrylate were purchased from Merck and used without further purification.
2.2. Instruments and Measurement Setups
The viscosity measurements were made with Mettler RM180 rheomat at 21 C (Mettler Toledo, Columbus, OH, USA) and the testing speeds used were between 50 and 1000 rpm. FTIR measurements were obtained using a NicoletTM 6700 FTIR (Nicolet Instrument Technologies Inc., Madison, WI, USA) with a DTGS KBr detector (Nicolet Instrument Technologies Inc., Madison, WI, USA) and a Smart Orbit accessory. For the FTIR measurements, curing was made directly on the Smart Orbit diamond by using a 365 nm fiber coupled UV-LED (M365F1, Thorlabs, Newton, MA, USA) and a 600 m Ocean Optic patchcord (QP600-2-SR-BX, Ocean Optics inc., Largo, FL, USA). Three different intensities were used for the curing: 2.9, 5.8 and 11.9 mW/cm, which were measured using a photodiode power sensor (S120VC) and readout unit (PM100D) from Thorlabs. Film thicknesses in the FTIR experiments were measured after curing with a caliper, Mahr Millitast 1083 (Mahr GmbH, Göttingen, Germany). H NMR spectra were recorded with a Bruker Ultrashield 500 MHz NMR spectrometer. For the NMR measurements, the AOBAE was dissolved in deuterated chloroform CDCl. The swell experiments were performed with an interferometric thin film measurement setup, consisting of an Avantes Avaspec-2048 optical spectrum analyzer (Avantes, Apeldoorn, The Netherlands), Ocean Optics HL-2000-FHSA light source, Ocean Optics QR450-7-XSR reflection probe (Ocean Optics inc., Largo, FL, USA) and Avantes AvaSoft-ThinFilm software (Avantes, Apeldoorn, The Netherlands) to calculate the thickness of the film. The used pH buffer solutions were controlled by using a Metrohm 632 pH-meter prior to measurements.
2.3. AOBAE
Batches of 1,3-BDDA:PIP with a mole ratio of 2:1 were made by dissolving PIP into 1,3-BDDA during stirring at 60
C, without the use of any solvents. Stirring continued until there were no visible PIP crystals in the solution and then for another 30 min, making it a total of 120 min. The solution was then left to cool to room temperature before further processing.
Figure 1 shows the principle synthesis of 1,3-BDDA:PIP with a mole ratio of 2:1. The viscosity of 1,3-BDDA:PIP with a mole ratio of 2:1 was measured to 770 cP.
2.4. FTIR and NMR
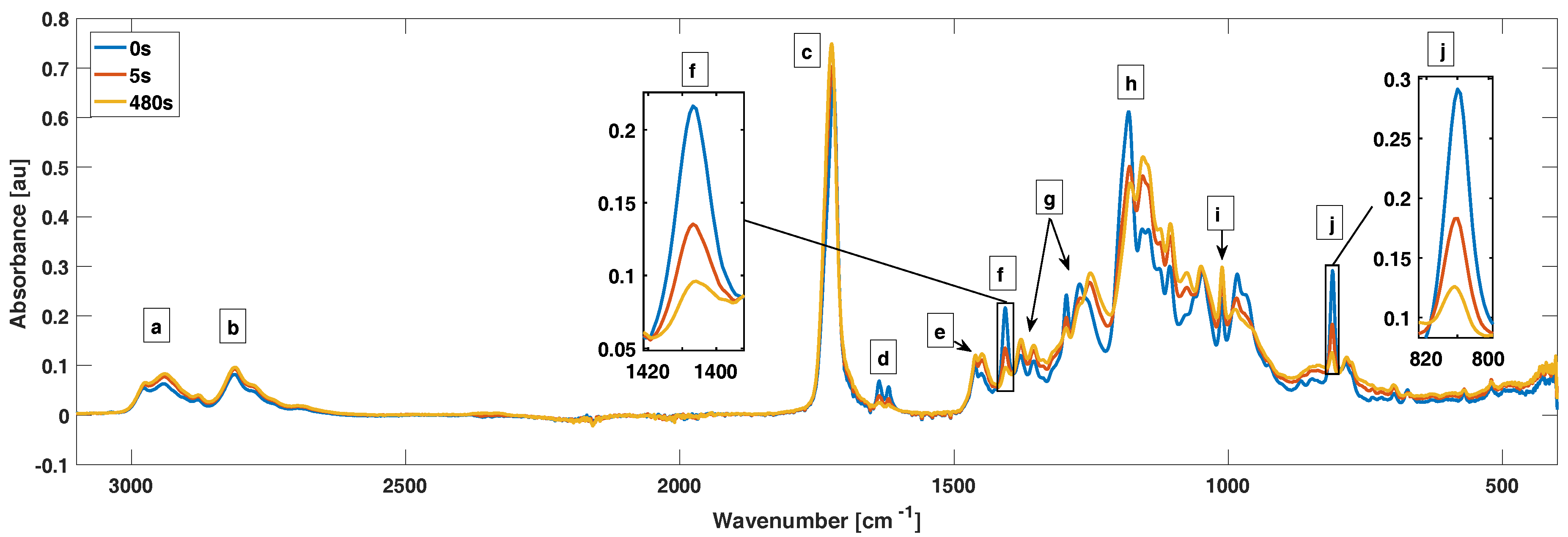
For the FTIR measurements, we put a drop of AOBAE directly on top of the Smart Orbit crystal and the film thicknesses were measured after curing with a m caliper. The AOBAE drops provided film thicknesses between 200 and 400 m. The Smart Orbit accessory uses a reflective measurement method and can therefore only measure a few m into the sample. Because of the relative thick films used, we ensured that we only measured on AOBAE. The resolution was set to 2 cm and an average of 32 sample spectrums were used. For measurements in inert atmosphere, a dark box was built around the Smart Orbit diamond that was flooded with N gas and purged for a minimum of 15 min before the measurements. The box was also used during tests in ambient air atmosphere, but without N flowing. Prior to the FTIR measurements, 2.5 or 1 wt % of TPO was mixed into the AOBAE solution. The estimated conversion error is ±3%, calculated from three batches of 1,3-AOBAE with 2.5 wt % of TPO.
To establish the relation of power output from the UV-led and curing of the 1,3-AOBAE, intensities of 2.9, 5.8 and 11.9 mW/cm
were evaluated in both air and N
. The curing experiments were conducted on a 200–400
m thick film with 2.5 or 1 wt % of (TPO). The degree of conversion (x) is directly related to the decrease of the IR absorbance and was calculated from Equation (
1)
where
and
represent the absorbance peak of the IR band [
18,
19].
2.5. Surface Priming of Si-Wafers
A standard Silicon (Si) wafer was used as a substrate for the swell experiments. The adhesive strength between the coated layer and the substrate was improved by tethering acryl groups to the surface. 3-(trimethoxysilyl) propyl acrylate was spin coated on the substrates at 3000 rpm and left to dry at room temperature before the AOBAE coating was applied.
2.6. Spin Coating and Curing on Si-Wafers
Before AOBAE coating and curing on Si-wafers was made, 2.5 wt % of TPO was dissolved into the AOBAE. 1,3-AOBAE was then spin-coated onto the Si-wafers at 9000 rpm in air atmosphere (for 45, 90 and 135 s), forming thin films between 1.4 and 12 m. The Si-wafer was then placed in a box that was flooded with N for 15 min before curing.
2.7. Swell Experiments
The reflection probe was held at approximately 2 mm from the Si-wafer surface and a buffer solution (Britton–Robinson buffer [
20]) with different pH was placed between the probe and the coated Si-wafer. The drop filled the whole space between the probe and the Si-wafer, thus there were no disturbing light reflections from the surface of the pH solution. The 1,3-AOBAE film thickness was first measured in its dry state and used as reference.
4. Discussion
The experiments show that AOBAE can form pH-responsive coatings. One objective was to manufacture AOBAEs that are fluid in the form of neat compounds at ambient temperature. A molecular design preventing crystallization by using asymmetric monomer units of the polymer was successfully explored.
In pH-responsive coatings from cross-linked AOBAEs, swelling is driven by osmotic pressure induced by protonation of amino groups and is restricted by elastic force of the bonds forming cross-linked network [
24]. Consequently, it can be expected that the swelling, which is a response to lowering the pH, is dependent on the molecular weight of the AOBAE. Increasing the molecular weight of the AOBAE will increase the number density of amino groups and decrease the number density of cross-linking bonds in the cured coating, consequently increasing the swelling responsivity to pH changes. However, in devices where a hydrogel coating is immobilized on a device surface, swelling induces both adhesive and cohesive stress that may destroy the sensor structure [
25]. By altering the mole ratio or the density of cross-linked acrylic groups, the sensitivity of the material is also altered. A higher concentration of amino groups increases the swelling and a higher density of cross-linked acrylic groups decreases the swelling [
26,
27]. Therefore, the responsive material needs to be optimized for the specific application where it is to be used.
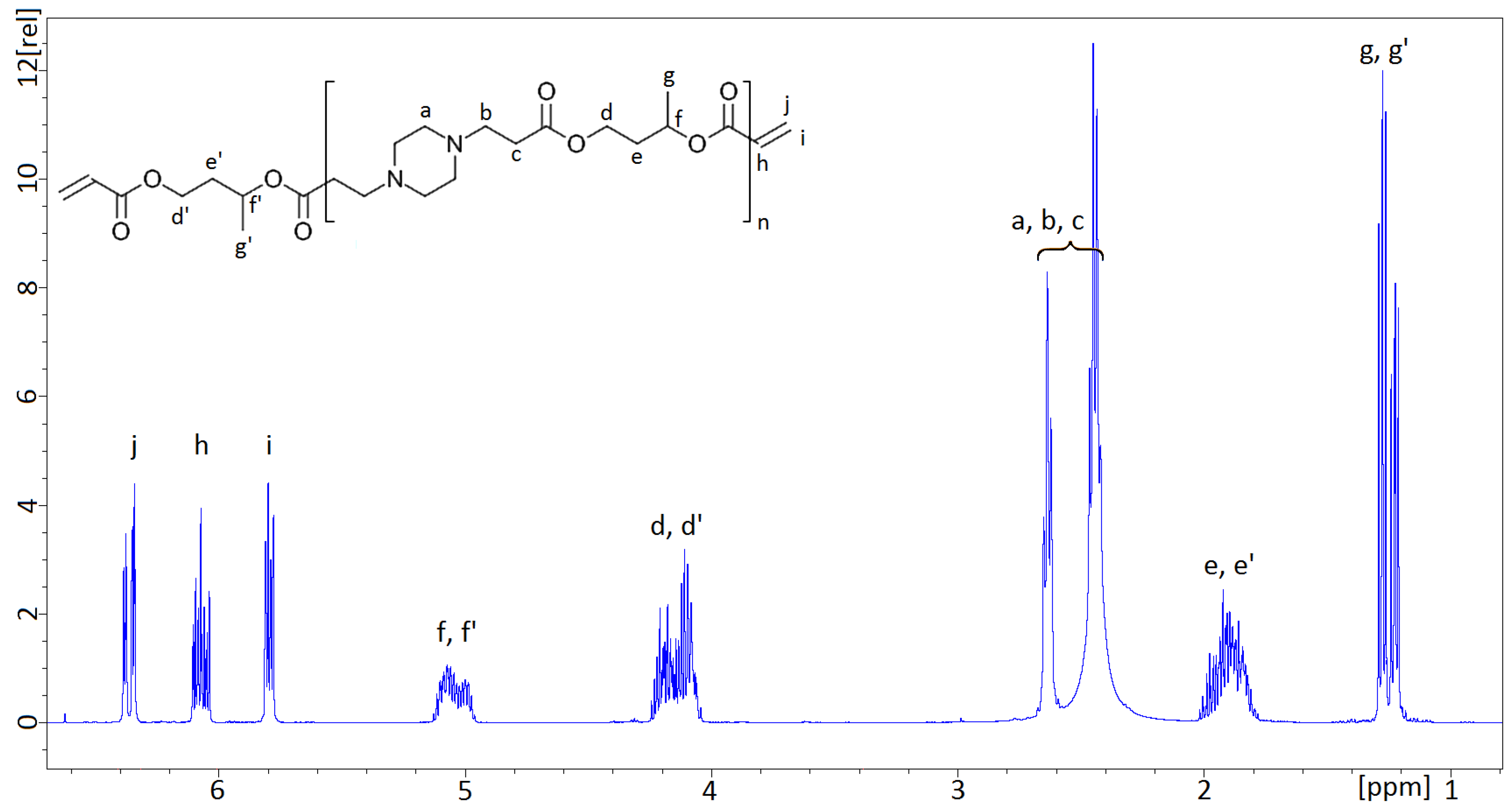
The NMR spectra obtained from the AOBAEs, synthesized from 1,3-BDDA and PIP, were consistent with the proposed structure. The NMR spectrum of 1,3-AOBAE was compared with NMR-spectra previously recorded about PBAEs, based on 1,4-BDDA and PIP, cf. reference [
12]. It can be noted that the additional peaks from the methyl group (g and g’), as well as the NMR signals originating from the 1,3-butylene group, are broader. The 1,3-butylene group introduces asymmetry in two ways: by the chirality of the methine carbon and by the possibility of coupling to the either the side of the 1 or 3, “head and tail”, positions of the molecule. The stereo and directional isomers are not assigned to specific spectral peaks in this study, but their presence can explain why the spectral peaks of the 1,3-butylene group exhibits a broad and more complicated pattern than the NMR-peaks of the symmetric 1,4-butylene group shown in reference [
12]. More importantly, the isomerism present in 1,3-AOBEA prevents crystallization of the resulting monomers, which is in line with the intention of this work.
Surface priming with a molecule having an acrylic group covalently tethered to the substrate resulted in the samples with the best adhesion for swelling/de-swelling experiments. Depending on the surface to be coated (material and structure), a different surface priming might be needed. Adhesion of the coating requires priming the substrate with polymerize-able groups forming covalent bonds between the substrate and the pH responsive coating. This will reduce the risk for the film to detach from the substrate due to the physical forces from swelling. Apparently, the acrylic groups of the priming agent partake in the polymerization under curing. Further work in this class of sensor material coating should be aimed at providing coatings with improved stability for adhesion and swelling.
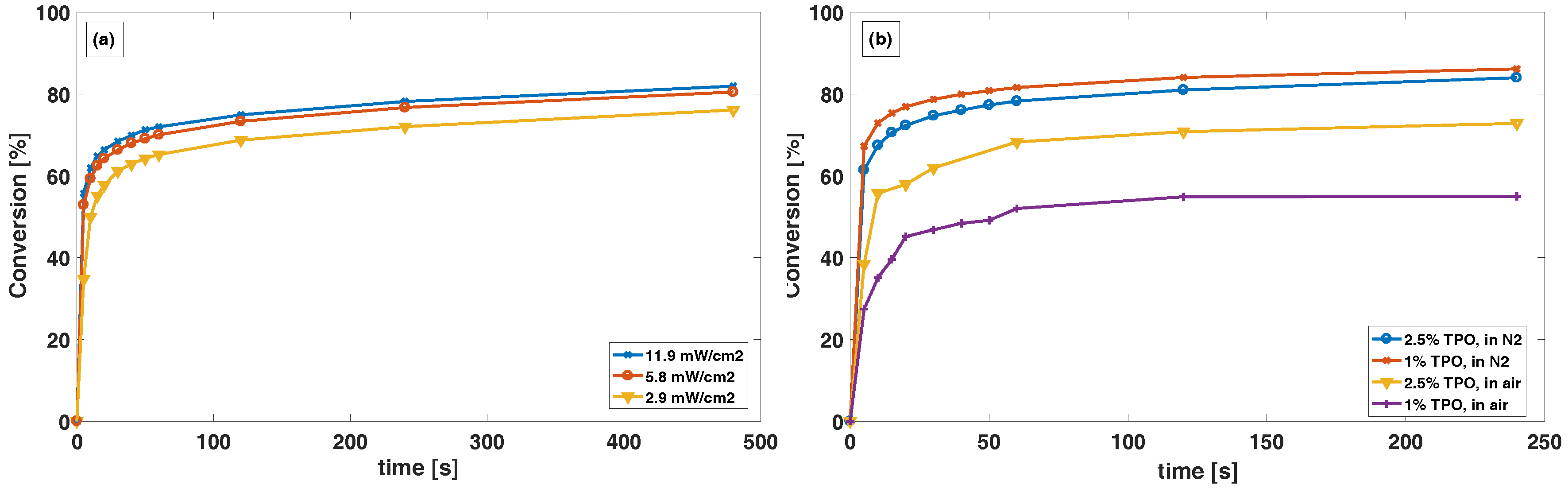
The curing conditions are crucial for a successful creation of a pH-responsive coating. Curing by photo-initiated radical polymerization of the terminal groups proceeds best under inert atmosphere and agrees with previous reports from Studer et al. [
18,
19]. In
Figure 4a, the monomer conversion in N
for 5.8 and 11.9 mW/cm
are very comparable, while slightly lower for 2.9 mW/cm
. This can be explained by a lower rate of photolytic generation of radicals from the photo initiator and possibly termination of radicals by oxygen that may be present even after flushing with nitrogen gas. Our experiments also showed that a sufficient amount of photo initiator is necessary, especially for curing in air, to overcome the inhibitory effect of oxygen. This is shown in
Figure 4b, where only a small difference in conversion is observed between 1 and 2.5% TPO in N
atmosphere. A larger difference is observed for conversion in air, indicating that free radicals released from the TPO is reacting with oxygen at a fast enough rate to limit the conversion. Thus, we believe that in air atmosphere the rate of photolytic generation of radicals from the photo initiator is not fast enough to overcome the rate of new oxygen that is diffusing into the polymer from the atmosphere. There is a number of factors that influence the polymerization, including curing power, the thickness of the polymer film, the temperature and the oxygen levels in the atmosphere [
18,
19], which should be taken into account and furthered studied.
From the swell tests on a flat surface, we have seen that the film detaches at lower pH due to the physical forces induced by the swelling. Though we believe that when coated on a round surface, such as an optical fiber, this problem will be much smaller. This because the polymer swells in all directions (the x, y and z direction) and therefore also presses toward the fiber.
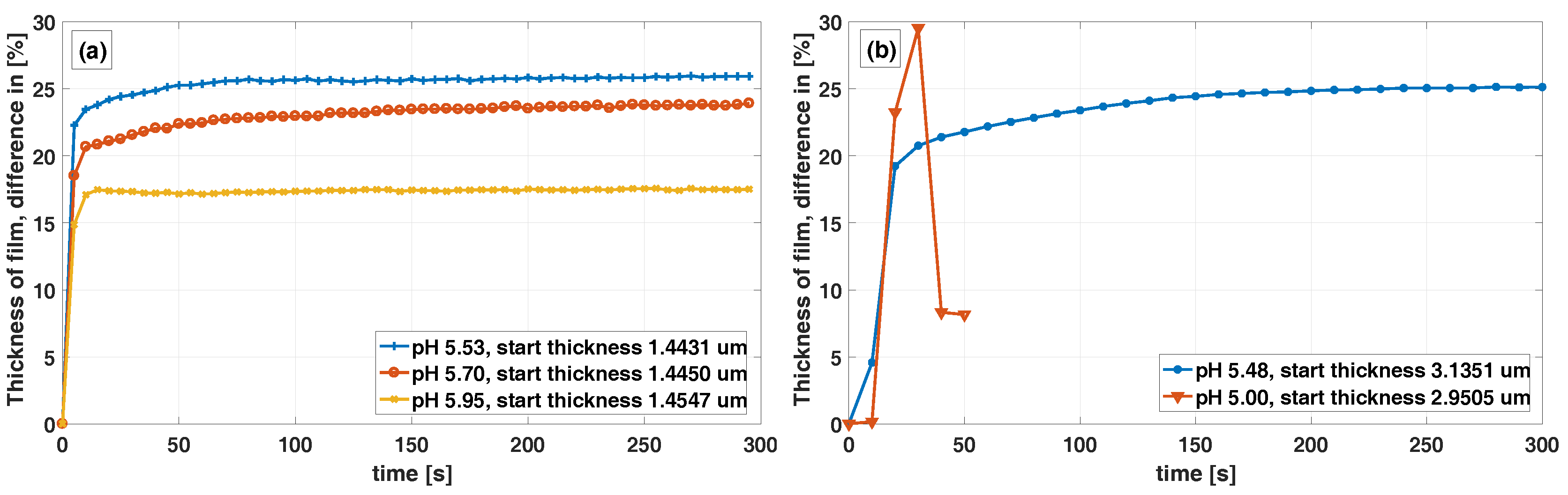
Figure 5a shows that the response curve of the three measurements differ. Possible explanation for this could be that for small degrees of swelling, swelling is fast and the gel network purely elastic, while at larger degrees of swelling, rearrangement of the gel network may be required and rate limiting, although this needs to be studied further for clarification. In
Figure 5a the yellow (
-x-) curve for pH 5.95, we can also see that the thickness is constant over time. This indicates that the polymerization is complete and that no AOBAE material is dissolving out into the pH-buffer. For thicker films, the response time is longer, which is shown in
Figure 5b blue (
-∗-) curve, this is in agreement with Tanaka and Fillmore [
23], who stated that the time constant
for a spherical hydrogel has an
relation
. Where r is the radius of the sphere and D
is the cooperative diffusion coefficient and also in agreement with De et al. [
28], who concluded that the swelling kinetics of hydrogels is a diffusion limited process. The 1,3-AOBAE synthesized from a molar ratio of 2:1 has a swell difference of 9%-units between pH 5.95 and 5.53. Due to film detachment from the Si-wafer, measurements with lower pH than approximately 5.5 could not be made. This swell response to pH might seem small, although this can still induce a large difference in the effective permittivity and RI of the material. Permittivity and RI are physical attributes commonly used in electronic and optical sensing and thus this needs to be studied further.