The Current Use of Stem Cells in Bladder Tissue Regeneration and Bioengineering
Abstract
:1. Introduction
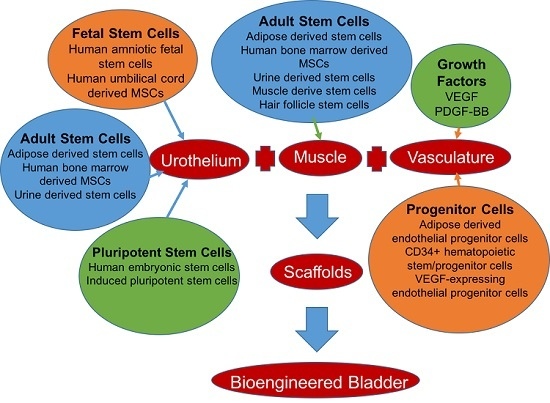
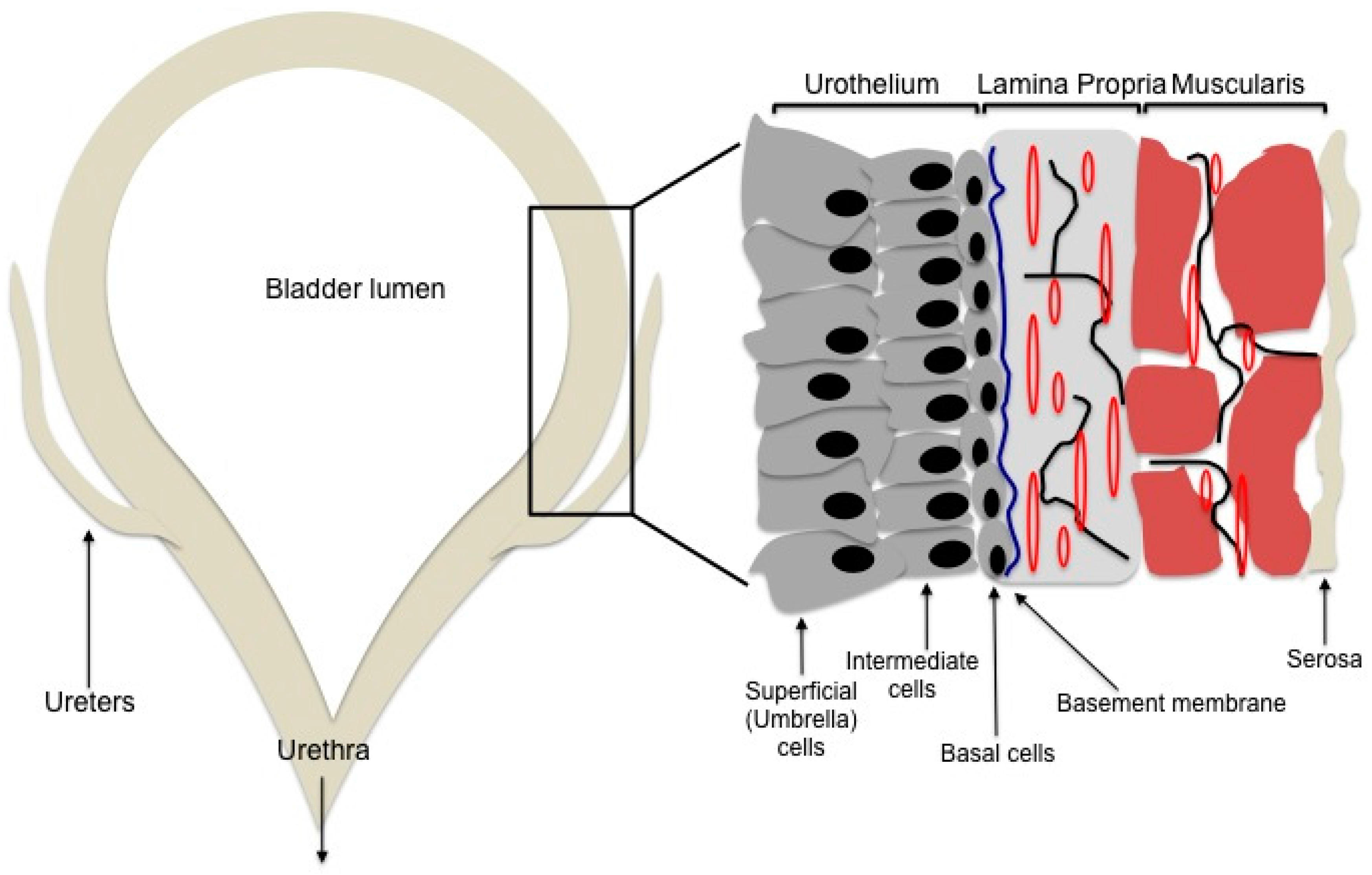
2. Bladder Engineering
2.1. The Scaffold
2.2. Urothelial Generation: Stem Cell Sources
2.2.1. Mesenchymal Stem Cells
2.2.2. Adult Stem Cells
2.2.3. Fetal Stem Cells
2.2.4. Pluripotent Stem Cells
2.3. Muscularis Propia Generation
2.4. Neovascular Generation
3. Future Directions
Author Contributions
Conflicts of Interest
References
- Khandelwal, P.; Abraham, S.N.; Apodaca, G. Cell biology and physiology of the uroepithelium. Am. J. Physiol. Ren. Physiol. 2009, 297, F1477–F1501. [Google Scholar] [CrossRef] [PubMed]
- Colopy, S.A.; Bjorling, D.E.; Mulligan, W.A.; Bushman, W. A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Dev. Dyn. 2014, 243, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, W.; Piszczek, R.; Krajewska, M.; Dembowski, J.; Zdrojowy, R. Urinary diversion metabolic complications—Underestimated problem. Adv. Clin. Exp. Med. 2014, 23, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Merriman, L.S.; Arlen, A.M.; Kirsch, A.J.; Leong, T.; Smith, E.A. Does augmentation cystoplasty with continent reconstruction at a young age increase the risk of complications or secondary surgeries? J. Pediatr. Urol. 2015, 11, 41.e1–41.e5. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Tissue engineering of human bladder. Br. Med. Bull. 2011, 97, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Kwon, I.K.; Kim, Y.H. Tissue engineering of urinary organs. Yonsei Med. J. 2000, 41, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Mousa, N.A.; Abou-Taleb, H.A.; Orabi, H. Stem cell applications for pathologies of the urinary bladder. World J. Stem Cells 2015, 7, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Smolar, J.; Salemi, S.; Horst, M.; Sulser, T.; Eberli, D. Stem cells in functional bladder engineering. Transfus. Med. Hemother. 2016, 43, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Madihally, S.V.; Palmer, B.; Frimberger, D.; Fung, K.M.; Kropp, B.P. Biomatrices for bladder reconstruction. Adv. Drug Deliv. Rev. 2015, 82–83, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Stoppel, W.L.; Ghezzi, C.E.; McNamara, S.L.; Black, L.D., 3rd; Kaplan, D.L. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann. Biomed. Eng. 2015, 43, 657–680. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Xu, Y.M.; Li, Z.B.; Murphy, S.V.; Zhao, W.; Liu, Q.Q.; Zhu, W.D.; Fu, Q.; Zhang, Y.P.; Song, L.J. Tissue performance of bladder following stretched electrospun silk fibroin matrix and bladder acellular matrix implantation in a rabbit model. J. Biomed. Mater. Res. A 2016, 104, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Chung, Y.G.; Gil, E.S.; Tu, D.; Franck, D.; di Vizio, D.; Adam, R.M.; Kaplan, D.L.; Estrada, C.R., Jr.; Mauney, J.R. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials 2013, 34, 4758–4765. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.C.; Davis, M.M.; Smith, E.R., Jr.; Walsh, M.J.; Ellison, P.K.; Rink, R.C.; Kropp, B.P. The ontogeny of canine small intestinal submucosa regenerated bladder. J. Urol. 1997, 158, 1105–1110. [Google Scholar] [CrossRef]
- Campodonico, F.; Benelli, R.; Michelazzi, A.; Ognio, E.; Toncini, C.; Maffezzini, M. Bladder cell culture on small intestinal submucosa as bioscaffold: Experimental study on engineered urothelial grafts. Eur. Urol. 2004, 46, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Lim, G.J.; Kwon, T.G.; Kwak, E.K.; Kim, B.W.; Atala, A.; Yoo, J.J. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials 2007, 28, 4251–4256. [Google Scholar] [CrossRef] [PubMed]
- Shakhssalim, N.; Rasouli, J.; Moghadasali, R.; Aghdas, F.S.; Naji, M.; Soleimani, M. Bladder smooth muscle cells interaction and proliferation on PCL/PLLA electrospun nanofibrous scaffold. Int. J. Artif. Organs 2013, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M.A.; Pourmand, G.; Ai, J.; Ghanbari, H.; Dinarvand, R.; Naji, M.; Faridi-Majidi, R. Electrospun plla nanofiber scaffolds for bladder smooth muscle reconstruction. Int. Urol. Nephrol. 2016, 48, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Milleret, V.; Noetzli, S.; Gobet, R.; Sulser, T.; Eberli, D. Polyesterurethane and acellular matrix based hybrid biomaterial for bladder engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2015. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Hinley, J.; Stahlschmidt, J.; Southgate, J. Tissue engineering potential of urothelial cells from diseased bladders. J. Urol. 2011, 186, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Dozmorov, M.G.; Kropp, B.P.; Hurst, R.E.; Cheng, E.Y.; Lin, H.K. Differentially expressed gene networks in cultured smooth muscle cells from normal and neuropathic bladder. J. Smooth Muscle Res. 2007, 43, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, C.; Li, H.; Chang, J. Bone marrow mesenchymal stem cells differentiate into urothelial cells and the implications for reconstructing urinary bladder mucosa. Cytotechnology 2011, 63, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Bharadwaj, S.; Liu, Y.; Ma, P.X.; Atala, A.; Zhang, Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: Potential for urological tissue engineering. Tissue Eng. A 2010, 16, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Lin, T.; Zhang, C.; Yin, X. Cell-to-cell contact induces human adipose tissue-derived stromal cells to differentiate into urothelium-like cells in vitro. Biochem. Biophys. Res. Commun. 2009, 390, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, M.X.; Zhou, Z.; Zhang, K.; Zhou, J.; Zhao, Y.; Wang, Z.; Lu, M.J. The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation. PLoS ONE 2014, 9, e95583. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.G.; Fu, W.J.; Wang, X.X.; Xu, Y.D.; Li, G.; Hong, B.F.; Hu, K.; Cui, F.Z.; Wang, Y.; Zhang, X. Transdifferentiation of human adipose-derived stem cells into urothelial cells: Potential for urinary tract tissue engineering. Cell Tissue Res. 2012, 347, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Peng, Y.; Zhou, Z.; Zhou, J.; Wang, Z.; Lu, M. Differentiation of human adipose-derived stem cells co-cultured with urothelium cell line toward a urothelium-like phenotype in a nude murine model. Urology 2013, 81, 465.e15–465.e22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.E.; Yoo, J.J.; Atala, A. Urine derived cells are a potential source for urological tissue reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Liu, H.; et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells 2013, 31, 1840–1856. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Kang, J.J.; Kang, H.G.; Chung, S.S. Urothelial differentiation of human amniotic fluid stem cells by urothelium specific conditioned medium. Cell Biol Int. 2014, 38, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Cheng, Z.; Liu, G.; Zhao, X.; Zhong, L.; Zhu, Y.; Zhu, J. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal. Cell. Pathol. 2013, 36, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhuang, Y.; Xiong, J.; Zhi, W.; Liu, L.; Wei, Q.; Han, P. Human umbilical mesenchymal stem cells-seeded bladder acellular matrix grafts for reconstruction of bladder defects in a canine model. PLoS ONE 2013, 8, e80959. [Google Scholar] [CrossRef] [PubMed]
- Osborn, S.L.; Thangappan, R.; Luria, A.; Lee, J.H.; Nolta, J.; Kurzrock, E.A. Induction of human embryonic and induced pluripotent stem cells into urothelium. Stem Cells Transl. Med. 2014, 3, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, H.H.; Han, Y.M. Generation of bladder urothelium from human pluripotent stem cells under chemically defined serum- and feeder-free system. Int. J. Mol. Sci. 2014, 15, 7139–7157. [Google Scholar] [CrossRef] [PubMed]
- Moad, M.; Pal, D.; Hepburn, A.C.; Williamson, S.C.; Wilson, L.; Lako, M.; Armstrong, L.; Hayward, S.W.; Franco, O.E.; Cates, J.M.; et al. A novel model of urinary tract differentiation, tissue regeneration, and disease: Reprogramming human prostate and bladder cells into induced pluripotent stem cells. Eur. Urol. 2013, 64, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, L.V.; Alfonso, Z.; Zhang, R.; Leung, J.; Wu, B.; Ignarro, L.J. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc. Natl. Acad. Sci. USA 2006, 103, 12167–12172. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Bury, M.I.; Fuller, N.J.; Marks, A.J.; Kollhoff, D.M.; Rao, M.V.; Hota, P.V.; Matoka, D.J.; Edassery, S.L.; Thaker, H.; et al. Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Hota, P.V.; Matoka, D.J.; Fuller, N.J.; Jandali, D.; Thaker, H.; Ameer, G.A.; Cheng, E.Y. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials 2010, 31, 6207–6217. [Google Scholar] [CrossRef] [PubMed]
- Drewa, T.; Joachimiak, R.; Kaznica, A.; Sarafian, V.; Pokrywczynska, M. Hair stem cells for bladder regeneration in rats: Preliminary results. Transplant. Proc. 2009, 41, 4345–4351. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Cannon, T.W.; Chermanski, C.; Pruchnic, R.; Somogyi, G.; Sacks, M.; de Groat, W.C.; Huard, J.; Chancellor, M.B. Muscle-derived stem cells seeded into acellular scaffolds develop calcium-dependent contractile activity that is modulated by nicotinic receptors. Urology 2003, 61, 1285–1291. [Google Scholar] [CrossRef]
- Loai, Y.; Yeger, H.; Coz, C.; Antoon, R.; Islam, S.S.; Moore, K.; Farhat, W.A. Bladder tissue engineering: Tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J. Biomed. Mater. Res. A 2010, 94, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xiong, Q.; Xu, G.; Lin, H.; Fang, X.; Cui, D.; Xu, M.; Chen, F.; Geng, H. VEGF-loaded nanoparticle-modified bamas enhance angiogenesis and inhibit graft shrinkage in tissue-engineered bladder. Ann. Biomed. Eng. 2015, 43, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, B.; Sun, C.; Qiu, X.; Sun, Z.; Chen, Y.; Zhang, Y.; Dai, Y. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng. A 2013, 19, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xia, J.; Qiu, X.; Wang, P.; Jia, R.; Chen, Y.; Yang, B.; Dai, Y. In vitro evaluation of endothelial progenitor cells from adipose tissue as potential angiogenic cell sources for bladder angiogenesis. PLoS ONE 2015, 10, e0117644. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Xie, H.; Zhang, S.L.; Geng, H.Q.; Zhou, J.M.; Pan, J.; Chen, F. Tissue engineering of bladder using vascular endothelial growth factor gene-modified endothelial progenitor cells. Int. J. Artif. Organs 2011, 34, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Lai, K.; Li, J.; Zou, Y.; Huang, H.; Liang, J.; Tang, X.; Wei, J.; Zhang, P. A rapid and efficient method for primary culture of human adipose-derived stem cells. Organogenesis 2013, 9, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.J.; Song, Y.S. Treatment of bladder dysfunction using stem cell or tissue engineering technique. Korean J. Urol. 2014, 55, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Anumanthan, G.; Makari, J.H.; Honea, L.; Thomas, J.C.; Wills, M.L.; Bhowmick, N.A.; Adams, M.C.; Hayward, S.W.; Matusik, R.J.; Brock, J.W., 3rd; et al. Directed differentiation of bone marrow derived mesenchymal stem cells into bladder urothelium. J. Urol. 2008, 180, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Cheng, E.Y. Growth factor and small molecule influence on urological tissue regeneration utilizing cell seeded scaffolds. Adv. Drug Deliv. Rev. 2015, 82–83, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Li, N.; Sarojini, H.; An, J.; Lee, M.J.; Wang, E. Secretome from mesenchymal stem cells induces angiogenesis via cyr61. J. Cell. Physiol. 2009, 219, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Neumayer, K.M.; Vaegler, M.; Daum, L.; Amend, B.; Sievert, K.D.; di Giovanni, S.; Kraushaar, U.; Guenther, E.; Stenzl, A.; et al. Cell-based therapy for the deficient urinary sphincter. Curr. Urol. Rep. 2013, 14, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.T.; Freitas-Filho, L.G.; Oliveira, A.S.; Semedo-Kuriki, P.; Laks, M.; Arias, V.E.; Peixoto, P.S. The use of mesenchymal stem cells in bladder augmentation. Pediatr. Surg. Int. 2014, 30, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Long, T.; Deng, J.; Zhang, Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell. Res. Ther. 2014, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Canales, B.K.; Anderson, J.K.; Premoli, J.; Slaton, J.W. Risk factors for upper tract recurrence in patients undergoing long-term surveillance for stage TA bladder cancer. J. Urol. 2006, 175, 74–77. [Google Scholar] [CrossRef]
- Rabbani, F.; Perrotti, M.; Russo, P.; Herr, H.W. Upper-tract tumors after an initial diagnosis of bladder cancer: Argument for long-term surveillance. J. Clin. Oncol. 2001, 19, 94–100. [Google Scholar] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. A 2011, 17, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.D.; Arnott, E.C.; Whitehouse, C.J.; Lawrence, C.M.; Reynolds, A.J.; Hole, N.; Jahoda, C.A. Plasticity of rodent and human hair follicle dermal cells: Implications for cell therapy and tissue engineering. J. Investig. Dermatol. Symp. Proc. 2005, 10, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Drewa, T.; Joachimiak, R.; Bajek, A.; Gagat, M.; Grzanka, A.; Bodnar, M.; Marszalek, A.; Debski, R.; Chlosta, P. Hair follicle stem cells can be driven into a urothelial-like phenotype: An experimental study. Int. J. Urol. 2013, 20, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, I.; Iezzi, I.; Morizio, E.; Mastrangelo, F.; Pantalone, A.; Mattioli-Belmonte, M.; Gigante, A.; Salini, V.; Calabrese, G.; Tete, S.; et al. Isolation of osteogenic progenitors from human amniotic fluid using a single step culture protocol. BMC Biotechnol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Siegel, N.; Rosner, M.; Hanneder, M.; Freilinger, A.; Hengstschlager, M. Human amniotic fluid stem cells: A new perspective. Amino Acids 2008, 35, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Siegel, N.; Valli, A.; Fuchs, C.; Rosner, M.; Hengstschlager, M. Induction of mesenchymal/epithelial marker expression in human amniotic fluid stem cells. Reprod. Biomed. Online 2009, 19, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Perin, L.; Giuliani, S.; Jin, D.; Sedrakyan, S.; Carraro, G.; Habibian, R.; Warburton, D.; Atala, A.; de Filippo, R.E. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007, 40, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Achermann, J.; Odermatt, B.; Genoni, M.; Zund, G.; Hoerstrup, S.P. Cryopreserved amniotic fluid-derived cells: A lifelong autologous fetal stem cell source for heart valve tissue engineering. J. Heart Valve Dis. 2008, 17, 446–455. [Google Scholar] [PubMed]
- Oottamasathien, S.; Wang, Y.; Williams, K.; Franco, O.E.; Wills, M.L.; Thomas, J.C.; Saba, K.; Sharif-Afshar, A.R.; Makari, J.H.; Bhowmick, N.A.; et al. Directed differentiation of embryonic stem cells into bladder tissue. Dev. Biol. 2007, 304, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Ramachandran, A.; Yu, R.N.; Daley, G.Q.; Adam, R.M.; Estrada, C.R. All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PLoS ONE 2010, 5, e11513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Deng, B.; Zhao, Y.; Xie, S.; Nie, R. Differentiated markers in undifferentiated cells: Expression of smooth muscle contractile proteins in multipotent bone marrow mesenchymal stem cells. Dev. Growth Differ. 2013, 55, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Osborn, S.L.; So, M.; Hambro, S.; Nolta, J.A.; Kurzrock, E.A. Inosculation of blood vessels allows early perfusion and vitality of bladder grafts—Implications for bioengineered bladder wall. Tissue Eng. A 2015, 21, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kropp, B.P.; Moore, P.; Cowan, R.; Furness, P.D., 3rd; Kolligian, M.E.; Frey, P.; Cheng, E.Y. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: Potential applications for tissue engineering technology. J. Urol. 2000, 164, 928–934. [Google Scholar] [CrossRef]
- Kajbafzadeh, A.M.; Esfahani, S.A.; Sadeghi, Z.; Elmi, A.; Monajemzadeh, M. Application of different scaffolds for bladder wall regeneration: The bladder as a natural bioreactor. Tissue Eng. A 2012, 18, 882–887. [Google Scholar] [CrossRef] [PubMed]
| Bladder Tissue Layer | Cell Source or Growth Factor | Model System | Major Findings | Reference(s) |
|---|---|---|---|---|
| Urothelium | Human bone marrow-derived mesenchymal stem cells (MSCs) | In vitro co-culture with human urothelial cells or urothelial cell conditioned medium | Induced urothelial-like cells that express cytokeratins typical of urothelium | [21] |
| Exhibited epithelial characteristics via TEM | ||||
| In vitro co-culture with human urothelium or culture in urothelial cell conditioned medium | Induced urothelium that expressed urothelial markers Uroplakin Ia (UPIa) and cytokeratins 7 and 13 | [22] | ||
| Adipose-derived stem cells (ASCs) | In vitro co-culture with human urothelial cells or urothelial cell conditioned medium | Induction of uroplakin-expressing urothelial cells in vitro | [23,24,25] | |
| ASCs mixed with human urothelial cell line and implanted subcutaneously into athymic mice | High expression of UPIa and Uroplakin II(UPII) at 4 weeks post-implant | [26] | ||
| Urine-derived stem cells (USCs) | In vitro culture in urothelial specific medium and in vivo implantation of induced urothelial cells | High expression of Uroplakins in induced urothelium in vitro and in vivo | [27,28,29] | |
| Barrier function in vitro | ||||
| Stratified layers of induced urothelium in vivo | ||||
| Human amniotic fetal stem cells | In vitro co-culture with bladder cancer cell conditioned medium | Morphologically resemble urothelial cells and express UPII, cytokeratin 8 and Fibroblast growth factor 10 (FGF10) | [30] | |
| Human umbilical cord-derived mesenchymal stromal cells (HUMSCs) | In vitro co-culture with urothelial cell conditioned medium | Morphologically resemble urothelial cells and express UPII and cytokeratins | [31] | |
| HUMSCs seeded on BAMGs were used to repair bladder defects in vivo using a canine transplant model | Bladder acellular matrix grafts (BAMGs) seeded with HUMSCs had better urothelial and muscle regeneration than did non-seeded grafts | [32] | ||
| Human embryonic stem cells (ESCs) | In vitro culture through definitive endoderm (DE) intermediary step, then induction to urothelial cells with urothelial cell-specific medium | Expression of proteins involved in urothelial fate specification during induction | [33] | |
| High production of urothelium determined by uroplakin expression | ||||
| Induced pluripotent stem cells (iPSCs) | In vitro culture through DE intermediary step, then induction to urothelial cells with urothelial cell-specific medium | High production of urothelium determined by uroplakin expression | [33,34] | |
| Urinary tract-derived iPSCs cultured in vitro culture with urothelial cell conditioned medium | Differentiation of urothelial cells expressing UPs, cytokeratins and claudins | [35] | ||
| Muscle | Adipose-derived stem cells (ASCs) | In vitro culture in smooth muscle differentiation medium | Induced SMCs exhibited upregulation of smooth muscle proteins and contraction/relaxation properties in vitro | [36] |
| Human bone marrow-derived MSCs | In vitro differentiated smooth muscle cells (via co-culture with human bladder SMCs or conditioned medium from the SMCs) were seeded onto scaffolds and transplanted in vivo | Induced smooth muscle cells increased expression of desmin in vivo and improved contractility in seeded grafts versus non-seeded grafts in vitro | [22] | |
| Poly (1,8-octaneodiol-co-citrate) elastomeric scaffolds were seeded with MSCs and transplanted onto cystectomized rat bladders | MSCs differentiated into SMCs within the graft and formed more organized muscular networks than did non-MSC seeded grafts | [37,38] | ||
| Urine-derived stem cells (USCs) | USCs induced into SMCs via conditioned medium in vitro then seeded onto cellulose scaffolds and implanted subcutaneously in athymic mice | Increased SMC marker expression and functional contraction in vitro | [27,29] | |
| 3D formation of bladder tissue in vivo | ||||
| Hair follicle stem cells | BAMGs seeded with hair follicle stem cells in vitro then transplanted to the rat bladder | Seeded grafts showed better muscle regeneration than did non-seeded grafts | [39] | |
| Muscle-derived stem cells | Small intestinal submucosa (SIS) scaffolds seeded with muscle-derived stem cells were cultured in vitro | Seeded grafts exhibited spontaneous contractile activities in vitro | [40] | |
| Blood Vessels | Vascular endothelial growth factor (VEGF) | BAMGs were hydrated with various concentrations of VEGF and utilized in a porcine model of bladder augmentation | Significant increase in vascularization, epithelialization and muscle regeneration in vivo in VEGF-hydrated BAMGs | [41] |
| BAMGs seeded with VEGF-loaded nanoparticles were transplanted onto bladders of rabbits after partial cystectomy | VEGF-loaded BAMGs showed significant increase in microvessel density with decreased rate of graft contracture | [42] | ||
| Platelet-derived growth factor-BB (PDGF-BB) + VEGF | Porcine BAMGs were loaded with Platelet derived growth factor-BB (PDGF-BB) and VEGF and transplanted into rabbits after partial cystectomy | Porcine BAMGs loaded with PDGF-BB and VEGF improved smooth muscle regeneration, vascularization and contractility | [43] | |
| Adipose-derived endothelial progenitor cells (ADEPCs) | ADEPCs were isolated from rat adipose tissue and cultured in vitro | ADEPCs expressed endothelial cell markers and formed capillary-like structures in BAMGs | [44] | |
| CD34+ hematopoietic stem/progenitor cells (HPSCs) + Bone marrow-derived MSCs | CD34+ HPSCs and MSCs were seeded onto poly (1,8-octaneodiol-co-citrate) elastomeric scaffolds and transplanted onto rat bladders after partial cystectomy | CD34+ HSPCs and MSCs increased vascularization of grafts and induced de novo vascularization and peripheral nerve growth | [37] | |
| VEGF-expressing endothelial progenitor cells (EPCs) | BAMGs were seeded with EPCs modified to express VEGF and used in a porcine model of partial cystectomy and transplantation | Seeded BAMGs showed enhanced vascularization versus non-EPC/VEGF seeded grafts | [45] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.Y.; Sandlin, S.K.; Kurzrock, E.A.; Osborn, S.L. The Current Use of Stem Cells in Bladder Tissue Regeneration and Bioengineering. Biomedicines 2017, 5, 4. https://doi.org/10.3390/biomedicines5010004
Chan YY, Sandlin SK, Kurzrock EA, Osborn SL. The Current Use of Stem Cells in Bladder Tissue Regeneration and Bioengineering. Biomedicines. 2017; 5(1):4. https://doi.org/10.3390/biomedicines5010004
Chicago/Turabian StyleChan, Yvonne Y., Samantha K. Sandlin, Eric A. Kurzrock, and Stephanie L. Osborn. 2017. "The Current Use of Stem Cells in Bladder Tissue Regeneration and Bioengineering" Biomedicines 5, no. 1: 4. https://doi.org/10.3390/biomedicines5010004