The Protective Effects of p-Coumaric Acid on Acute Liver and Kidney Damages Induced by Cisplatin
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Ethical Decision
2.2. Drugs and Experimental Models
2.3. Biochemical Investigation and Protein Determination
2.4. Histopathological Examinations
2.5. Statistical Analyse
3. Results
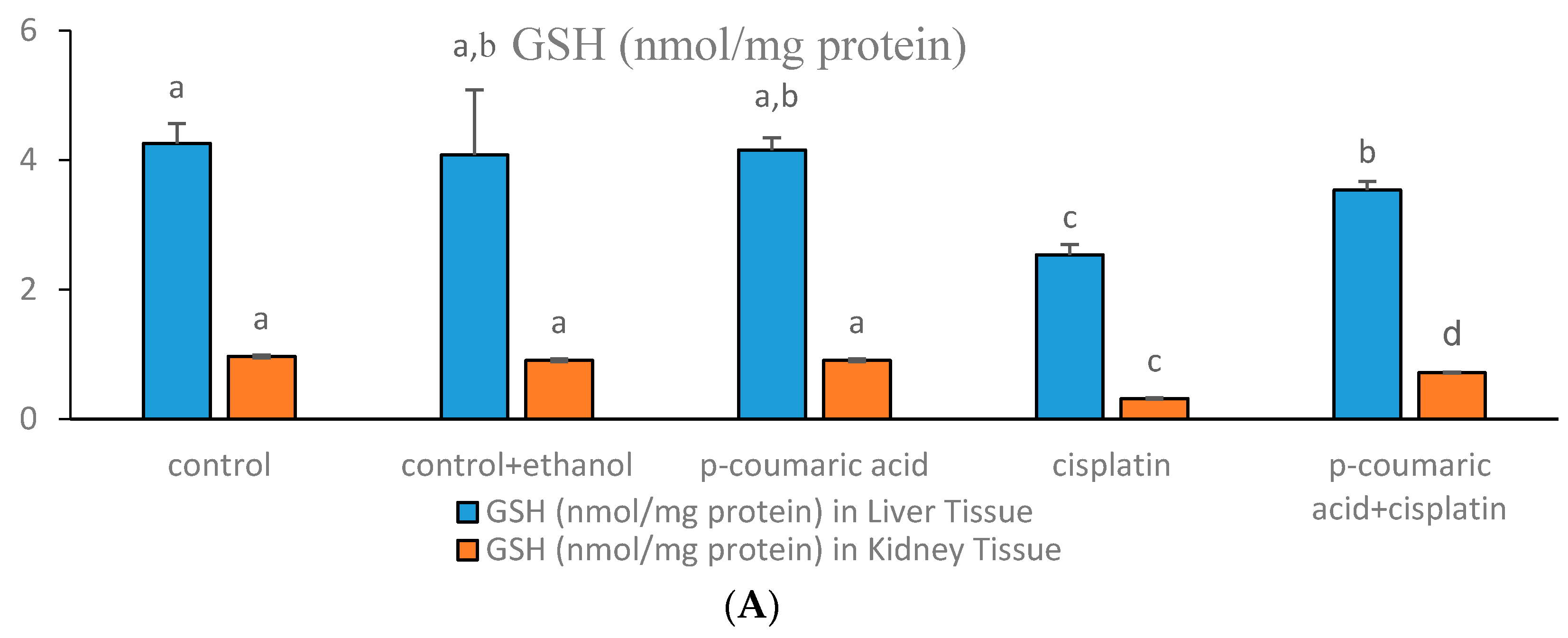
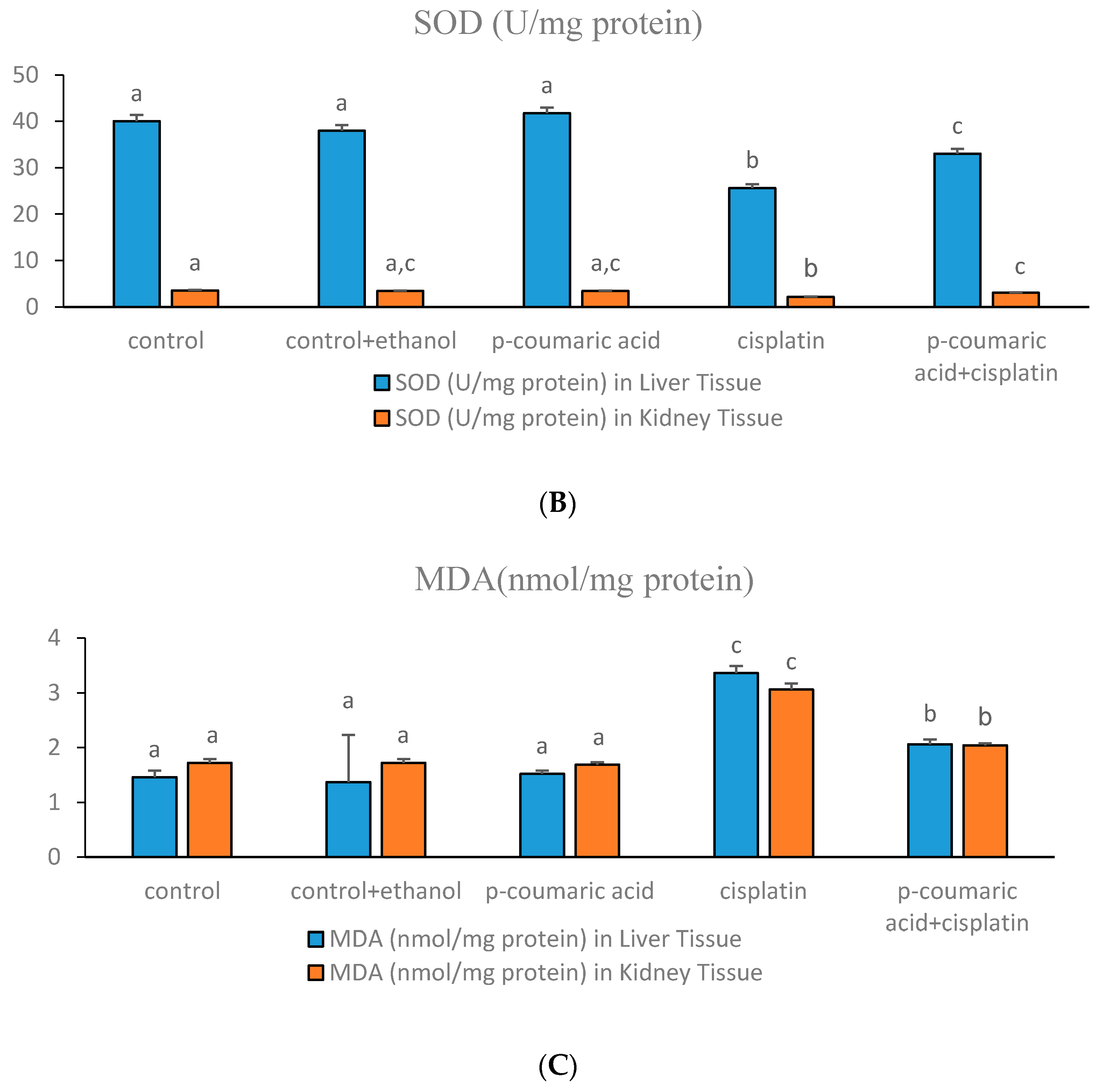
3.1. Markers of Oxidative Stress in Liver and Kidney
3.2. Histological Scores and Changes in Liver and Kidney Samples
3.3. Discussion
4. Conclusions
Author Contributions
Conflicts of interest
References
- Calbreath, D.F. Anathomy and physiology of the kidney. Clin. Chem. A Fundem. Textb. 1992, 11, 240–248. [Google Scholar]
- Alwahsh, S.M.; Dwyer, B.J.; Forbes, S.; van Thiel, D.H.; Starkey Lewis, P.J.; Ramadori, G. Insulin Production and Resistance in Different Models of Diet-Induced Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2017, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Tartaglione, E.V.; Kuver, R.; Haigh, W.G.; Farrell, G.C.; Subramanian, S.; Chait, A.; Yeh, M.M.; Quinn, L.S.; Ioannou, G.N. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology 2013, 57, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Schultze, F.C.; Wilting, J.; Mihm, S.; Raddatz, D.; Ramadori, G. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS ONE 2014, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Martius, G.; Alwahsh, S.M.; Rave-Fränk, M.; Hess, C.F.; Christiansen, H.; Ramadori, G.; Malik, I.A. Hepatic fat accumulation and regulation of FAT/CD36: An effect of hepatic irradiation. Int. J. Clin. Exp. Pathol. 2014, 7, 5379–5392. [Google Scholar] [PubMed]
- Yamamotoya, T.; Nakatsu, Y.; Matsunaga, Y.; Fukushima, T.; Yamazaki, H.; Kaneko, S.; Fujishiro, M.; Kikuchi, T.; Kushiyama, A.; Tokunaga, F.; et al. Reduced SHARPIN and LUBAC formation may contribute to CCl4- or acetaminophen-induced liver cirrhosis in mice. Int. J. Mol. Sci. 2017, 18, 326. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 17, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ruiz, S.; Maksimovic-Ivanic, D.; Mijatovic, S.; Kaluderovic, G.N. On the discovery, biological effects, and use of cisplatin and metallocenes in anticancer chemotherapy. Bioinorg. Chem. Appl. 2012, 2012, 140284. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, E.R.; Lippard, S.J. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.J.; Lu, X.Q.; Lu, C.W.; Li, G.X.; Jin, Y.P.; Tang, H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol. Res. 2008, 57, 125–131. [Google Scholar] [CrossRef]
- Mathe, C.; Szenasi, G.; Sebesteny, A.; Blazovics, A.; Szentmihalyi, K.; Hamar, P.; Albert, M. Protective effect of CV247 against cisplatin nephrotoxicity in rats. Hum. Exp. Toxicol. 2014, 33, 789–799. [Google Scholar] [CrossRef]
- Lynch, E.D.; Gu, R.D.; Pierce, C.; Kil, J. Combined oral delivery of ebselen and allopurinol reduces multiple cisplatin toxicities in rat breast and ovarian cancer models while enhancing anti-tumor activity. Anticancer Drugs 2005, 16, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Shimada, K.; Sakaguchi, Y.; Miyamoto, M. Cisplatin (CDDP)-induced acute toxicity in an experimental model of hepatic fibrosis. J. Toxicol. Sci. 2007, 32, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Chirino, Y.I.; Pedraza-Chaverri, J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009, 61, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Bompart, G. Cisplatin-induced changes in cytochrome P-450, lipid peroxidation and drug-metabolizing enzyme activities in rat kidney cortex. Toxicol. Lett. 1989, 48, 193–199. [Google Scholar] [CrossRef]
- Sezen, O.; Ertekin, M.V.; Demircan, B.; Karslioglu, I.; Erdogan, F.; Kocer, I.; Calik, I.; Gepdiremen, A. Vitamin E and l-carnitine, separately or in combination, in the prevention of radiation-induced brain and retinal damages. Neurosurg. Rev. 2008, 31, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yuce, A.; Atessahin, A.; Ceribasi, A.O.; Aksakal, M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin. Pharmacol. 2007, 101, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [PubMed]
- Şentürk, M.; Gülçin, İ.; Daştan, A.; Küfrevioğlu, Ö.İ.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg. Med. Chem. 2009, 17, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Öztürk Sarıkaya, S.B.; Gülçin, İ.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem. Biol. Drugs Des. 2010, 75, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Öztürk Sarıkaya, S.B.; Gülçin, İ.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg. Med. Chem. 2010, 18, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Young, G. Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet. Assoc. 1999, 99, 213–218. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur. Food Res. Technol. 2006, 223, 759–767. [Google Scholar] [CrossRef]
- Gülçin, İ. Comparison of in vitro antioxidant and antiradical activities of l-tyrosine and l-Dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.W.; Abu-Amsha Caccetta, R.; Puddey, I.B.; Croft, K.D. Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2000, 27, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Daştan, A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J. Enzym. Inhib. Med. Chem. 2007, 22, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.J.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. 2000, 9, 1163–1170. [Google Scholar]
- Gülçin, İ. Antioxidant activity of l-Adrenaline: An activity-structure insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Ursini, F.; Tubaro, F.; Rong, J.; Sevanian, A. Optimization of nutrition: Polyphenols and vascular protection. Nutr. Rev. 1999, 57, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gürsul, C.; Akdemir, F.N.E.; Akkoyun, T.; Can, İ.; Gul, M.; Gulcin, İ. Protective effect of naringin on experimental hindlimb ischemia-reperfusion injury in rats. J. Enzym. Inhib. Med. Chem. 2016, 31, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ekinci Akdemir, F.N.; Gulcin, İ.; Karagöz, B.; Soslu, R. Quercetin protects rat skeletal muscle from ischemia reperfusion injury. J. Enzym. Inhib. Med. Chem. 2016, 31, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Ekinci Akdemir, F.N.; Gulcin, İ.; Alwasel, S. A Comparative study on the antioxidant effects of hesperidin and ellagic acid against skeletal muscle ischemia/reperfusion injury. J. Enzym. Inhib. Med. Chem. 2016, 31, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 24, 192–205. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [PubMed]
- Bayir, Y.; Karagoz, Y.; Karakus, E.; Albayrak, A.; Sengul, O.; Can, I.; Yayla, N.; Kuskun, U.; Keles, M.S. Nigella sativa reduces tissue damage in rat ovaries subjected to torsion and detorsion: Oxidative stress, proinflammatory response and histopathological evaluation. Gynecol. Obstet. Investig. 2012, 74, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Berashvili, D.; Gepdiremen, A. Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J. Ethnopharmacol. 2005, 101, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Polidoro, G.; Dillio, C.; La Rovere, G.; Fedrici, G.S. Superoxide dismutase, reduced glutathione and TBA-reactive products in erythrocytes of patients with multiple sclerosis. Int. J. Biochem. 1984, 16, 505–509. [Google Scholar] [CrossRef]
- Polat Köse, L.; Gülçin, İ.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Gulcin, I.; Beydemir, S. Phenolic compounds as antioxidants: Carbonic anhydrase isoenzymes inhibitors. Mini Rev. Med. Chem. 2013, 13, 408–430. [Google Scholar] [CrossRef] [PubMed]
- Sehitoglu, M.H.; Han, H.; Kalin, P.; Gulcin, I.; Ozkan, A.; Aboul-Enein, H.Y. Pistachio (Pistacia vera L.) Gum: A potent inhibitor of reactive oxygen species. J. Enzym. Inhib. Med. Chem. 2015, 30, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Park, Y.J.; Namiesnik, J.; Gülçin, İ.; Kim, T.C.; Kim, H.C.; Heo, B.G.; Gorinstein, S.; Ku, Y.G. Effects of artificial lighting on bioactivity of sweet red pepper (Capsicum annuum L.). Int. J. Food Sci. Technol. 2016, 51, 1378–1385. [Google Scholar] [CrossRef]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, X.; Zhang, A.; Li, C.; Chen, G.; Ge, X.; Pan, K.; Dong, J.H. Selective bowel decontamination improves the survival of 90% hepatectomy in rats. J. Surg. Res. 2015, 195, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Seyhan, H.A.; Ahmad, S.; Mihm, S.; Ramadori, G.; Schultze, F.C. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J. Gastroenterol. 2014, 20, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant and antiradical activities of l-Carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Buyukokuroglu, M.E.; Gulcin, I.; Oktay, M.; Kufrevioglu, O. In vitro antioxidant properties of dantrolene sodium. Pharmacol. Res. 2001, 44, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufrevioglu, O.I. In vitro antioxidant properties of dantrolene sodium. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. Subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef]
- Naqshbandi, A.; Rizwan, S.; Khan, F. Dietary supplementation of flaxseed oil ameliorates the effect of cisplatin on rat kidney. J. Funct. Foods 2013, 5, 316–326. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.P.; Habeebu, S.S.M.; Klaassen, C.D. Metallothionein (MT)-null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 1998, 149, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.A.; Ball, J.G.; Brown, J.M.; Terneus, M.V.; McQuade, E.; van Meter, S.; Hedrick, H.M.; Roy, AA.; Williams, T. Resveratrol attenuates cisplatin renal cortical cytotoxicity by modifying oxidative stress. Toxicol. In Vitro 2014, 28, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Amirshahrokhi, K.; Khalili, A.R. Thalidomide ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in an experimental model. Inflammation 2015, 38, 476–484. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xin, H.; Yan, W.; Zhou, X.X. Amelioration of cisplatin-induced nephrotoxicity by pravastatin in mice. Exp. Toxicol. Pathol. 2011, 63, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, M.K.; Burkhardt, G.; Kohler, H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol. Dial. Transplant. 1997, 12, 2478–2480. [Google Scholar] [CrossRef]
- Naziroglu, M.; Karaoglu, A.; Aksoy, A.O. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 2004, 195, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.H.; Hafez, H.F.; Fahmy, N.M. Silymarin modulates Cisplatin-induced oxidative stress and hepatotoxicity in rats. J. Biochem. Mol. Biol. 2006, 39, 656–661. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, J.E.; Ma, J.Y.; Sablad, M.; Sonee, M.; Varacallo, L.; Louden, C.; Guy, A.; Vegas, J.; Liu, X.; La, D.; et al. Time course of renal proximal tubule injury, reversal, and related biomarker changes in rats following cisplatin administration. Int. J. Toxicol. 2013, 32, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y.; Wang, T. Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients 2017, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miriam, B.; Lavine, J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013, 57, 2525–2531. [Google Scholar]
- Alwahsh, M.; Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 2017, 91, 1545–1563. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.A.; Mohamed, W.R.; Arafa, E.S.A.; Shehata, B.A.; El Sherbiny, G.A.; Arab, H.H.; Elgendy, A.N. Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacol. Rep. 2016, 68, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kuncha, M.; Putcha, U.K.; Sistla, R. Effect of metformin against cisplatin induced acute renal injury in rats: A biochemical and histoarchitectural evaluation. Exp. Toxicol. Pathol. 2013, 65, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Cagin, Y.F.; Erdogan, M.A.; Sahin, N.; Parlakpinar, H.; Atayan, Y.; Polat, A.; Vardi, N.; Yildiz, A.; Tanbek, K. Protective effects of apocynin on cisplatin-induced hepatotoxicity in rats. Arch. Med. Res. 2015, 46, 517–526. [Google Scholar] [CrossRef] [PubMed]

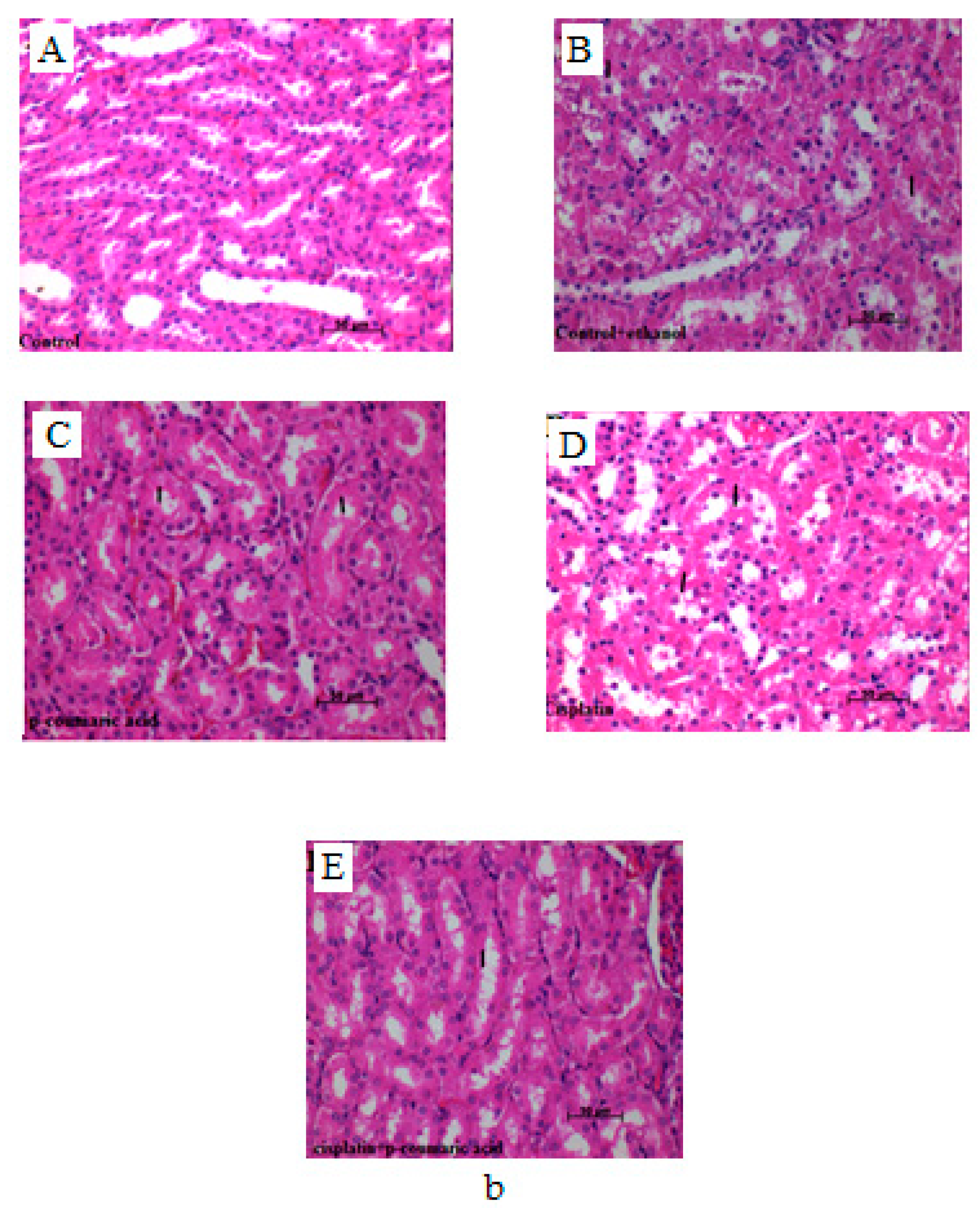
| Groups | Sinusoidal Dilatation in Liver | Vascular Congestion in Liver | Hydropic Degeneration in Liver | Prevalence of Necrosis of Tubular Epithelial Cells in Kidney | Severity of Necrosis of Tubular Epithelial Cells in Kidney |
|---|---|---|---|---|---|
| Control | − a | − a | − a | − a | − a |
| Control + Ethanol | + b | + b | + | + b | + b |
| p-Coumaric acid | − a | + b | − a | + b | + b |
| Cisplatin | +++ d | +++ d | ++ c | +++ d | +++ d |
| p-Coumaric acid + Cisplatin | + b | ++ | + b | ++ c | ++ c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekinci Akdemir, F.N.; Albayrak, M.; Çalik, M.; Bayir, Y.; Gülçin, İ. The Protective Effects of p-Coumaric Acid on Acute Liver and Kidney Damages Induced by Cisplatin. Biomedicines 2017, 5, 18. https://doi.org/10.3390/biomedicines5020018
Ekinci Akdemir FN, Albayrak M, Çalik M, Bayir Y, Gülçin İ. The Protective Effects of p-Coumaric Acid on Acute Liver and Kidney Damages Induced by Cisplatin. Biomedicines. 2017; 5(2):18. https://doi.org/10.3390/biomedicines5020018
Chicago/Turabian StyleEkinci Akdemir, Fazile Nur, Mevlüt Albayrak, Muhammet Çalik, Yasin Bayir, and İlhami Gülçin. 2017. "The Protective Effects of p-Coumaric Acid on Acute Liver and Kidney Damages Induced by Cisplatin" Biomedicines 5, no. 2: 18. https://doi.org/10.3390/biomedicines5020018







