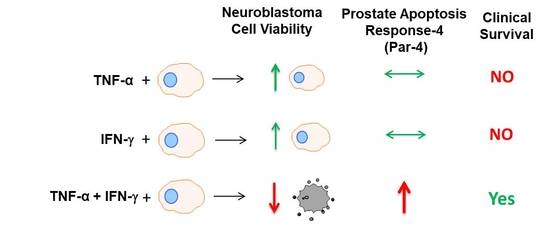
TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Lines
2.3. Cell Viability Assay
2.4. Flow Cytometry Analysis
2.5. DNA Fragmentation Assay
2.6. Western Blot Analysis
2.7. Immunofluorescence Microscopy
2.8. Semi-Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
- Par-4: forward 5′GCAGATCGAGAAGAGGAAGC3′
- reverse 5′GCAGATAGGAACTGCCTGGA3′,
- β-Actin: forward 5′ GTGGGGCGCCCCAGGCACC3′
- reverse 5′CTCCTTAATGTCACGCACGATTTC3′.
2.9. Caspase-8 Activity Assay
2.10. Gene-Expression Omnibus (GEO) Data Acquisition and Analysis
2.11. Statistical Analysis
3. Results
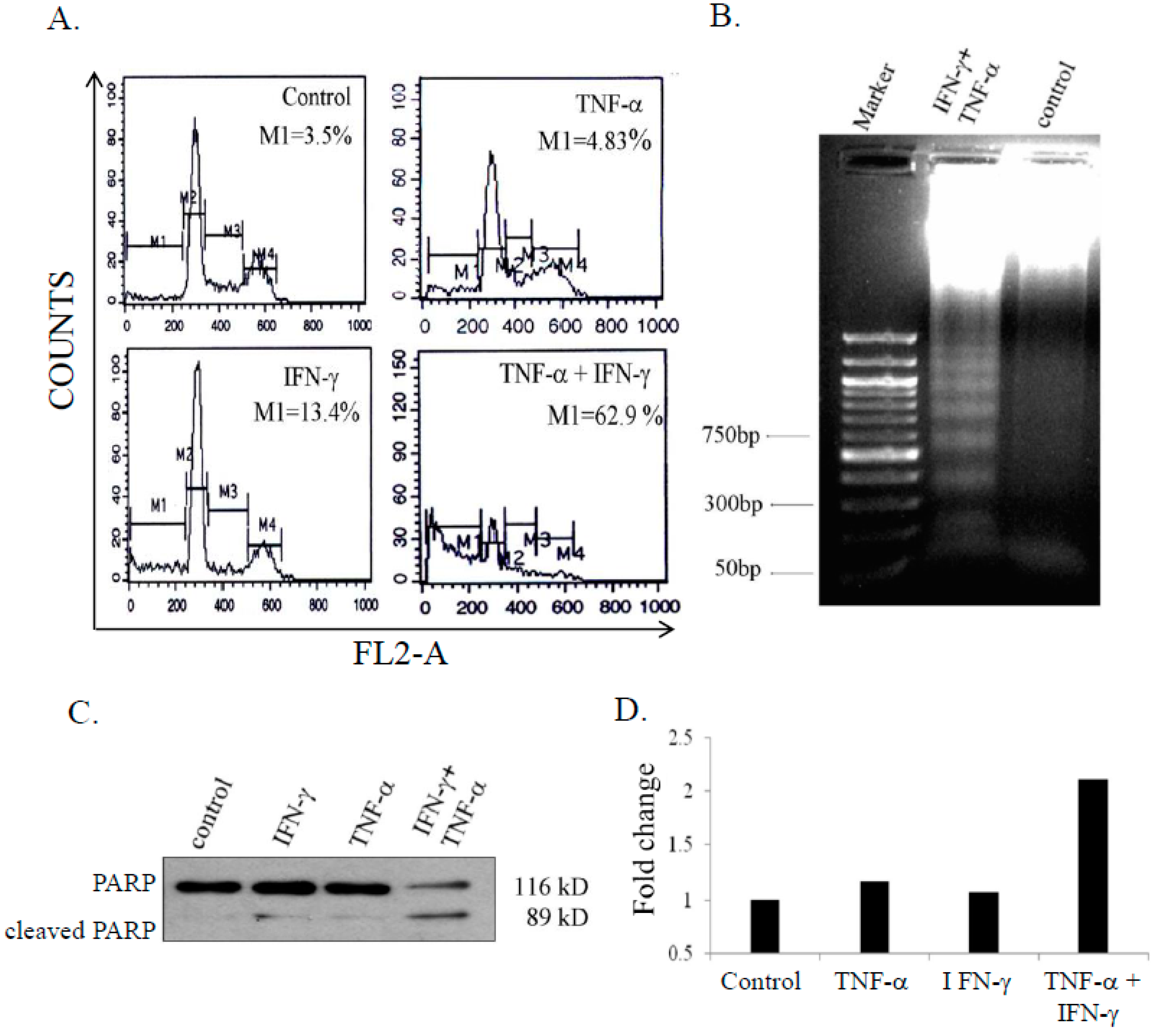
3.1. Neuroblastoma (NB) Cell Lines Sensitive to Combination of IFN-γ and TNF-α Induced Cell Death
3.2. Combination Treatment of IFN-γ (Interferon Gamma) and TNF-α Induced Apoptosis in SK-N-MC Cells
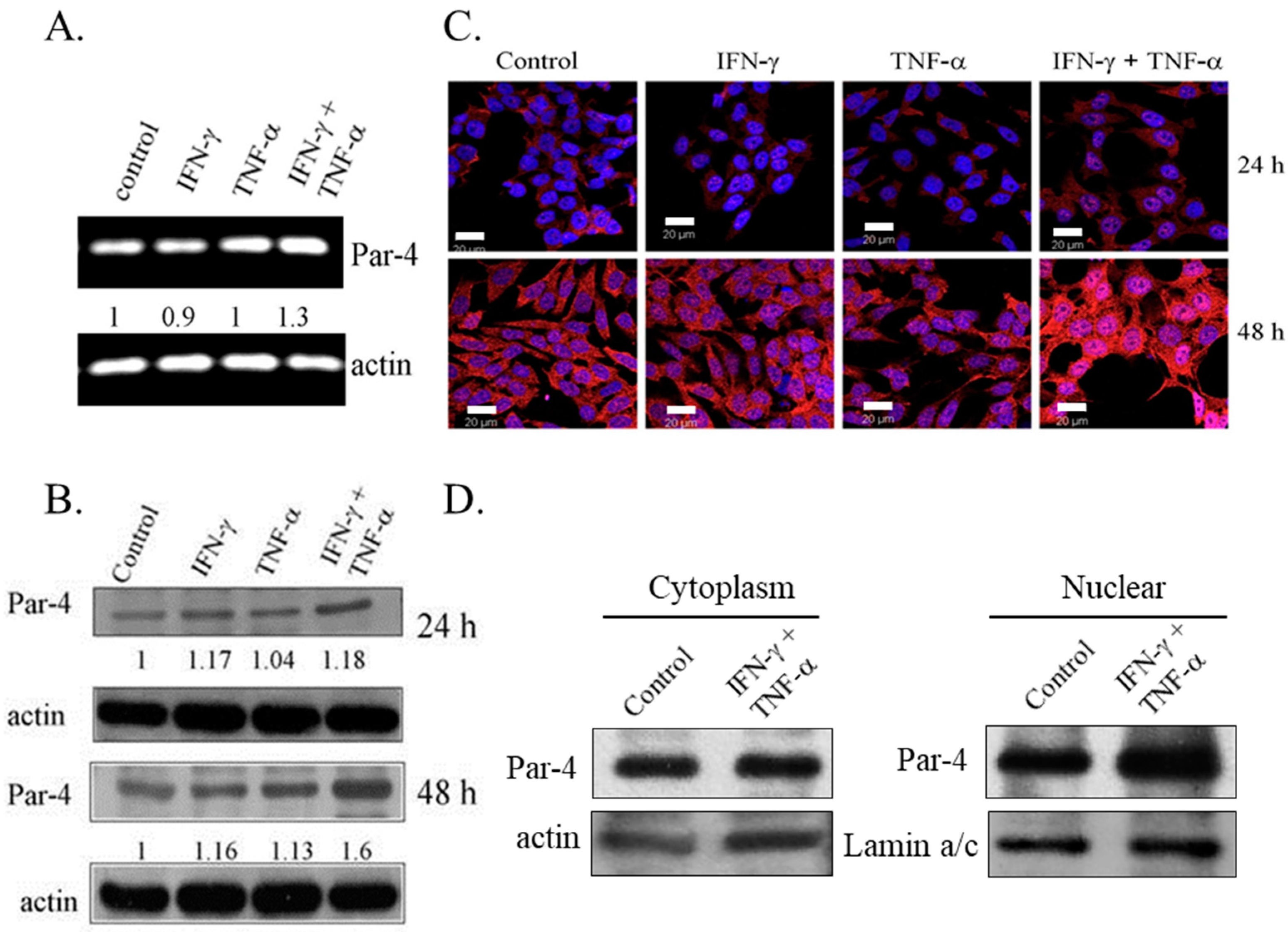
3.3. Combined Treatment of IFN-γ and TNF-α Induced Expression of Pro-Apoptotic Protein-4
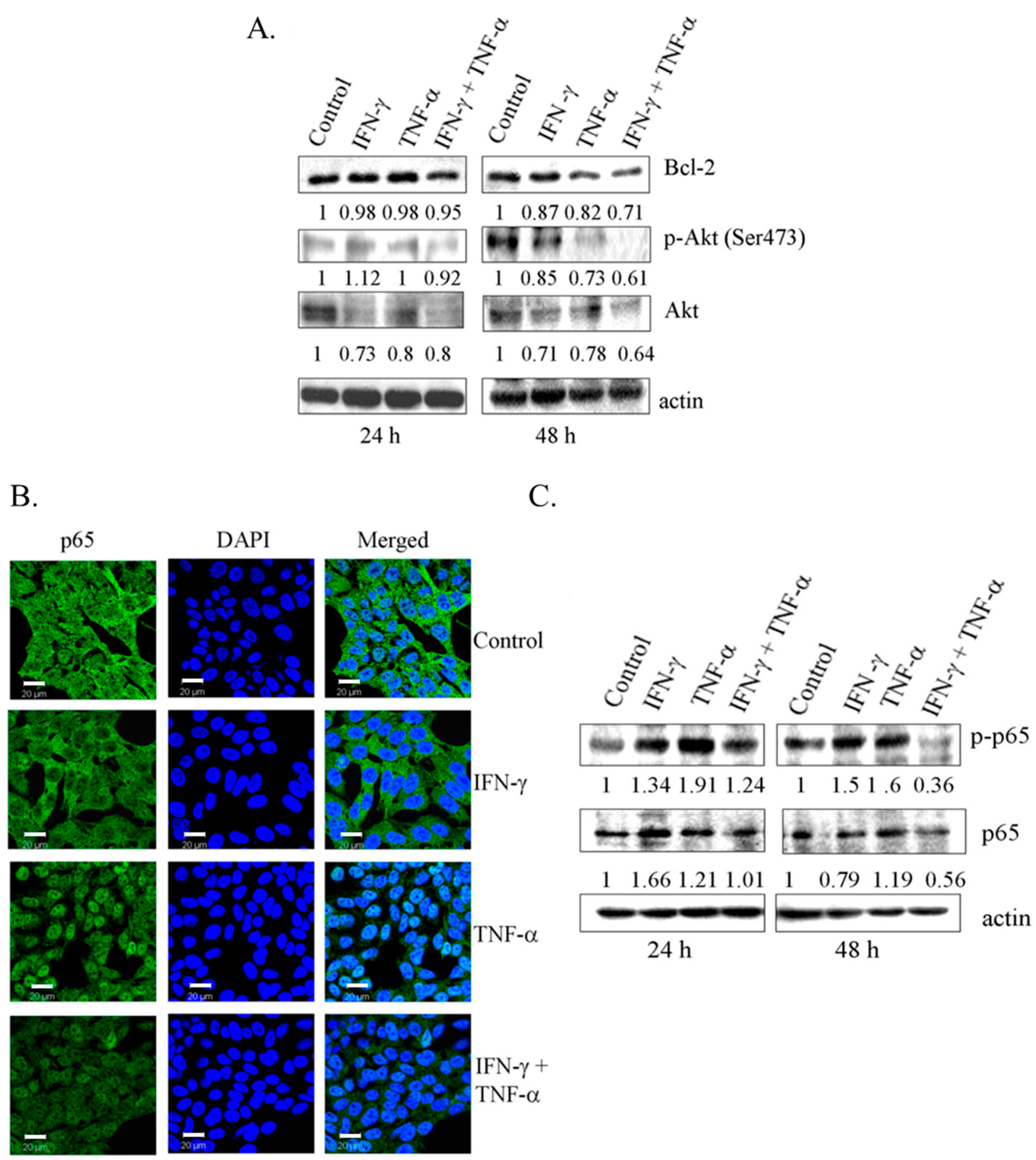
3.4. Co-Treatment with IFN-γ and TNF-α Resulted in Down-Regulation of Bcl-2 and Phosphorylated Akt in SK-N-MC Cells
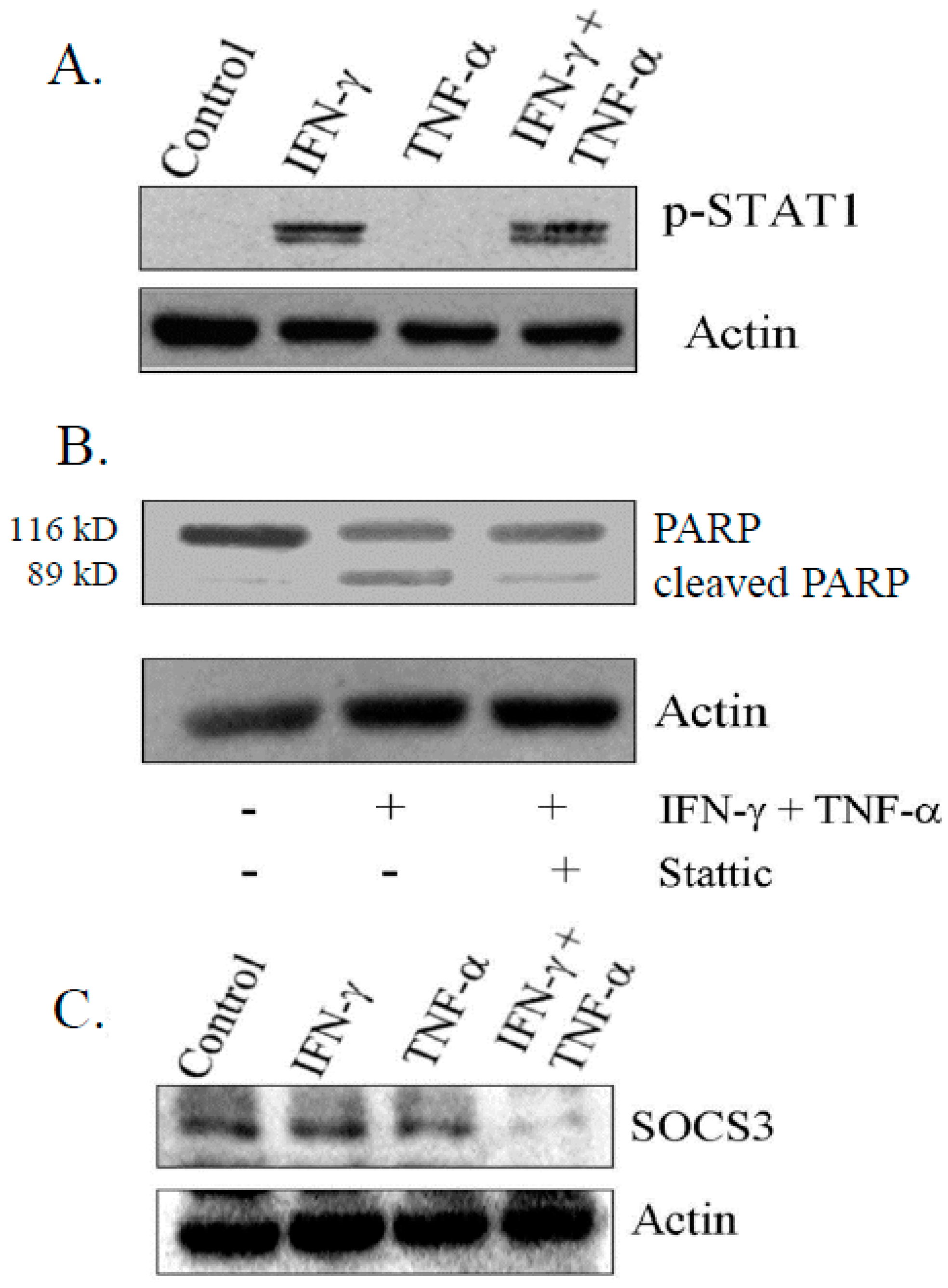
3.5. IFN-γ Signaling Is Critical for Induction of Apoptosis on Combination Treatment
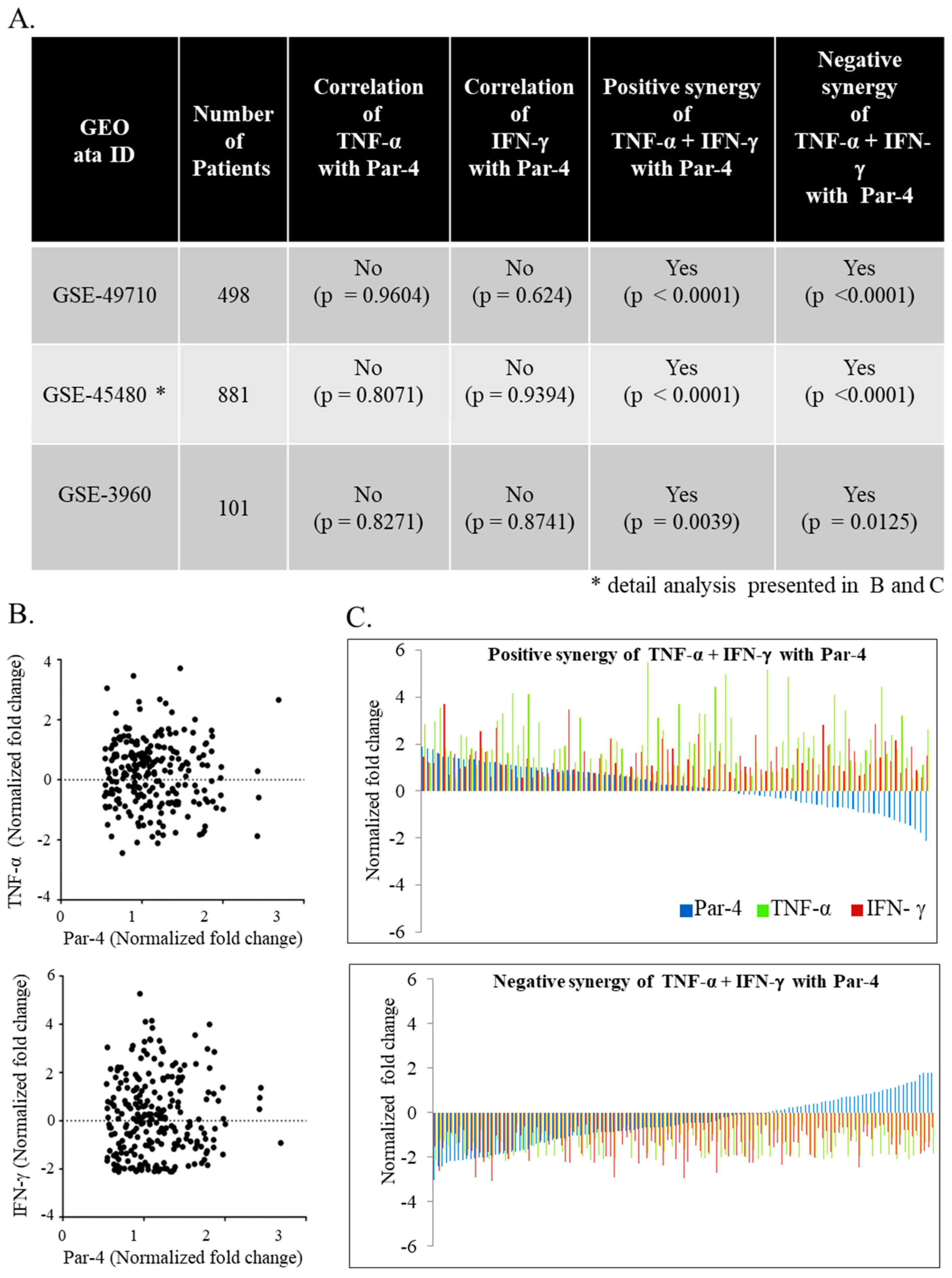
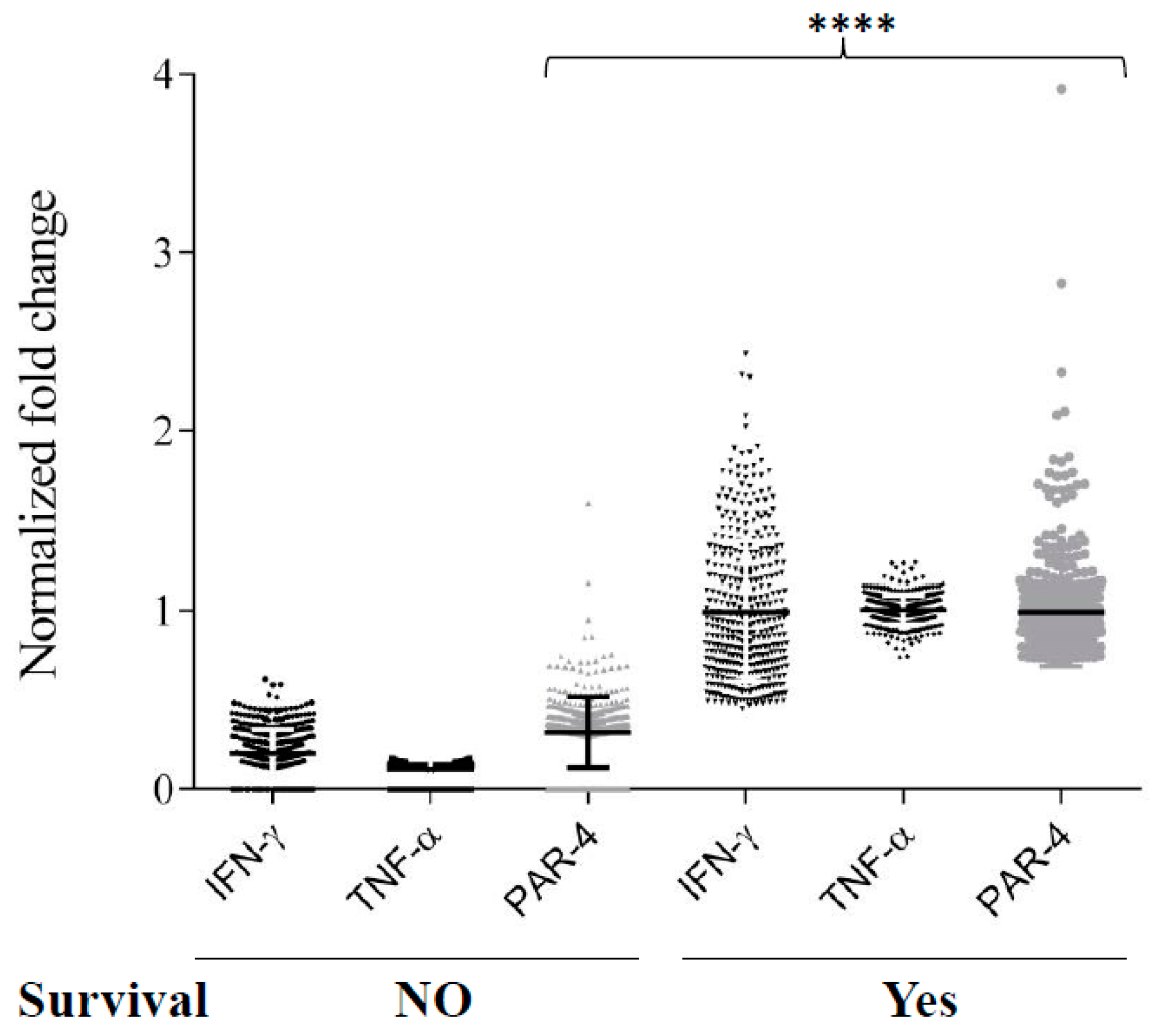
3.6. TNF-α and IFN-γ Co-Expression Synergize with Par-4 (Prostate Apoptosis Response-4) Expression in Neuroblastoma Patients
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Tonini, G.P.; Pistoia, V. Molecularly guided therapy of neuroblastoma: A review of different approaches. Curr. Pharm. Des. 2006, 12, 2303–2317. [Google Scholar] [CrossRef] [PubMed]
- Volchenboum, S.L.; Cohn, S.L. Progress in defining and treating high-risk neuroblastoma: Lessons from the bench and bedside. J. Clin. Oncol. 2009, 27, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Rossi, C.R.; Pilati, P.; Nitti, D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005, 16, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Niitsu, Y.; Umeno, H.; Kuriyama, H.; Neda, H.; Yamauchi, N.; Maeda, M.; Urushizaki, I. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 1988, 48, 2179–2183. [Google Scholar] [PubMed]
- Fish, S.M.; Proujansky, R.; Reenstra, W.W. Synergistic effects of interferon γ and tumour necrosis factor α on T84 cell function. Gut 1999, 45, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Hlaing, M.; Akaike, T. Synergistic induction of apoptosis in murine hepatoma Hepa1-6 cells by IFN-γ and TNF-α. Biochem. Biophys. Res. Commun. 2000, 272, 674–680. [Google Scholar] [CrossRef]
- Suk, K.; Kim, S.; Kim, Y.H.; Kim, K.A.; Chang, I.; Yagita, H.; Shong, M.; Lee, M.S. IFN-γ/TNF-α synergism as the final effector in autoimmune diabetes: A key role for STAT1/IFN regulatory factor-1 pathway in pancreatic β cell death. J. Immunol. 2001, 166, 4481–4489. [Google Scholar] [CrossRef]
- Suk, K.; Kim, Y.H.; Chang, I.; Kim, J.Y.; Choi, Y.H.; Lee, K.Y.; Lee, M.S. IFNα sensitizes ME-180 human cervical cancer cells to TNFα-induced apoptosis by inhibiting cytoprotective NF-κB activation. FEBS Lett. 2001, 495, 66–70. [Google Scholar] [CrossRef]
- Gao, Y.; Theng, S.S.; Mah, W.C.; Lee, C.G. Silibinin down-regulates fat10 and modulate TNF-α/IFN-γ-induced chromosomal instability and apoptosis sensitivity. Biol. Open 2015, 4, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, X.; Zimmerman, M.; Waller, J.L.; Wu, P.; Hayes-Jordan, A.; Lev, D.; Liu, K. TNFα cooperates with IFN-γ to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to trail-mediated apoptosis. PLoS ONE 2011, 6, e16241. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.V.; Pan, T.C.; Ruth, J.; Feng, Y.; Zhou, A.; Pant, D.; Grimley, J.S.; Wandless, T.J.; Demichele, A.; Investigators, I.S.T.; et al. Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell 2013, 24, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.A.; Gerhard, R.; Salaorni, S.; Fregnani, J.H.; Nonogaki, S.; Netto, M.M.; Soares, F.A. Down-regulation of the candidate tumor suppressor gene Par-4 is associated with poor prognosis in breast cancer. Int. J. Oncol. 2010, 37, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Rangnekar, V.M. Regulation of cancer cell survival by Par-4. Ann. N. Y. Acad. Sci. 2005, 1059, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, J.C.; Dawood, P.; Shah, R.D.; Chandrika, G.; Natesh, K.; Shiras, A.; Hegde, A.S.; Ranade, D.; Shastry, P. Expression and regulation of prostate apoptosis response-4 (Par-4) in human glioma stem cells in drug-induced apoptosis. PLoS ONE 2014, 9, e88505. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Qiu, S.G.; Vasudevan, K.M.; Rangnekar, V.M. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 2001, 61, 7255–7263. [Google Scholar] [PubMed]
- El-Guendy, N.; Rangnekar, V.M. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp. Cell Res. 2003, 283, 51–66. [Google Scholar] [CrossRef]
- Diaz-Meco, M.T.; Lallena, M.J.; Monjas, A.; Frutos, S.; Moscat, J. Inactivation of the inhibitory κB protein kinase/nuclear factor κB pathway by Par-4 expression potentiates tumor necrosis factor α-induced apoptosis. J. Biol. Chem. 1999, 274, 19606–19612. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Jang, J.H.; Noh, H.J.; Park, E.J.; Choi, K.S.; Kwon, T.K. Overexpression of Par-4 sensitizes TRAIL-induced apoptosis via inactivation of NF-κB and Akt signaling pathways in renal cancer cells. J. Cell. Biochem. 2010, 109, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.D.; Kline, C.L.; Pastor, D.M.; Olson, T.L.; Frank, B.; Luu, T.; Sharma, A.K.; Robertson, G.; Weirauch, M.T.; Patierno, S.R.; et al. Prostate apoptosis response protein 4 sensitizes human colon cancer cells to chemotherapeutic 5-FU through mediation of an NF κB and microRNA network. Mol. Cancer 2010, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- El-Guendy, N.; Zhao, Y.; Gurumurthy, S.; Burikhanov, R.; Rangnekar, V.M. Identification of a unique core domain of Par-4 sufficient for selective apoptosis induction in cancer cells. Mol. Cell. Biol. 2003, 23, 5516–5525. [Google Scholar] [CrossRef] [PubMed]
- Burikhanov, R.; Shrestha-Bhattarai, T.; Hebbar, N.; Qiu, S.; Zhao, Y.; Zambetti, G.P.; Rangnekar, V.M. Paracrine apoptotic effect of p53 mediated by tumor suppressor Par-4. Cell Rep. 2014, 6, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Burikhanov, R.; Zhao, Y.; Goswami, A.; Qiu, S.; Schwarze, S.R.; Rangnekar, V.M. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell 2009, 138, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Kim, J.H.; Shin, J. P62 forms a ternary complex with PKCζ and Par-4 and antagonizes Par-4-induced PKCζ inhibition. FEBS Lett. 2002, 510, 57–61. [Google Scholar] [CrossRef]
- Goswami, A.; Burikhanov, R.; de Thonel, A.; Fujita, N.; Goswami, M.; Zhao, Y.; Eriksson, J.E.; Tsuruo, T.; Rangnekar, V.M. Binding and phosphorylation of Par-4 by Akt is essential for cancer cell survival. Mol. Cell 2005, 20, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; See, R.H.; Sells, S.F.; Wang, J.; Muthukkumar, S.; Englert, C.; Haber, D.A.; Licht, J.D.; Sugrue, S.P.; Roberts, T.; et al. A novel repressor, Par-4, modulates transcription and growth suppression functions of the Wilms’ tumor suppressor WT1. Mol. Cell. Biol. 1996, 16, 6945–6956. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, J.Y.; Ge, Z.; Zhang, L.; Zhou, G.P. Par-4/THAP1 complex and Notch3 competitively regulated pre-mRNA splicing of CCAR1 and affected inversely the survival of T-cell acute lymphoblastic leukemia cells. Oncogene 2013, 32, 5602–5613. [Google Scholar] [CrossRef] [PubMed]
- Treude, F.; Kappes, F.; Fahrenkamp, D.; Muller-Newen, G.; Dajas-Bailador, F.; Kramer, O.H.; Luscher, B.; Hartkamp, J. Caspase-8-mediated Par-4 cleavage is required for TNFα-induced apoptosis. Oncotarget 2014, 5, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Vetterkind, S.; Boosen, M.; Scheidtmann, K.H.; Preuss, U. Ectopic expression of Par-4 leads to induction of apoptosis in CNS tumor cell lines. Int. J. Oncol. 2005, 26, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Ahmed, M.; Sells, S.F.; Mohiuddin, M.; Weinstein, M.H.; Rangnekar, V.M. Mutually exclusive expression patterns of Bcl-2 and Par-4 in human prostate tumors consistent with down-regulation of Bcl-2 by Par-4. Oncogene 1999, 18, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z. STAT1 in cancer: Friend or foe? Discov. Med. 2017, 24, 19–29. [Google Scholar] [PubMed]
- Bluyssen, H.A.; Rastmanesh, M.M.; Tilburgs, C.; Jie, K.; Wesseling, S.; Goumans, M.J.; Boer, P.; Joles, J.A.; Braam, B. IFN γ-dependent SOCS3 expression inhibits IL-6-induced STAT3 phosphorylation and differentially affects IL-6 mediated transcriptional responses in endothelial cells. Am. J. Physiol. Cell Physiol. 2010, 299, C354–C362. [Google Scholar] [CrossRef]
- Chin, Y.E.; Kitagawa, M.; Kuida, K.; Flavell, R.A.; Fu, X.Y. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cell. Biol. 1997, 17, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Puhr, M.; Santer, F.R.; Neuwirt, H.; Susani, M.; Nemeth, J.A.; Hobisch, A.; Kenner, L.; Culig, Z. Down-regulation of suppressor of cytokine signaling-3 causes prostate cancer cell death through activation of the extrinsic and intrinsic apoptosis pathways. Cancer Res. 2009, 69, 7375–7384. [Google Scholar] [CrossRef]
- Teitz, T.; Wei, T.; Valentine, M.B.; Vanin, E.F.; Grenet, J.; Valentine, V.A.; Behm, F.G.; Look, A.T.; Lahti, J.M.; Kidd, V.J. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med. 2000, 6, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Teitz, T.; Lahti, J.M.; Kidd, V.J. Aggressive childhood neuroblastomas do not express caspase-8: An important component of programmed cell death. J. Mol. Med. (Berl.) 2001, 79, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Kufer, M.U.; Meyer, E.; van Valen, F.; Dockhorn-Dworniczak, B.; Debatin, K.M. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene 2001, 20, 5865–5877. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. 5-Aza-2′-deoxycytidine and IFN-γ cooperate to sensitize for trail-induced apoptosis by upregulating caspase-8. Oncogene 2006, 25, 5125–5133. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, K.F.; Hsu, H.Y.; Hsu, L.P.; Shih, W.L.; Chu, Y.C.; Hsiao, W.T.; Liu, P.F. Protein expression and intracellular localization of prostate apoptosis response-4 (Par-4) are associated with apoptosis induction in nasopharyngeal carcinoma cell lines. Cancer Lett. 2007, 257, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, M.; Hashimura, M.; Kuwata, T.; Okayasu, I. Transcriptional regulation of pro-apoptotic Par-4 by NF-κB/p65 and its function in controlling cell kinetics during early events in endometrial tumourigenesis. J. Pathol. 2010, 221, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Krishnan, S.; Ananth, S.; Sells, S.F.; Shi, Y.; Walther, M.M.; Linehan, W.M.; Sukhatme, V.P.; Weinstein, M.H.; Rangnekar, V.M. Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene 1999, 18, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.L.; Shanmugavelandy, S.S.; Kester, M.; Irby, R.B. Delivery of Par-4 plasmid in vivo via nanoliposomes sensitizes colon tumor cells subcutaneously implanted into nude mice to 5-FU. Cancer Biol. Ther. 2009, 8, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Meco, M.T.; Municio, M.M.; Frutos, S.; Sanchez, P.; Lozano, J.; Sanz, L.; Moscat, J. The product of Par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell 1996, 86, 777–786. [Google Scholar] [CrossRef]
- Alvarez, S.; Blanco, A.; Fresno, M.; Munoz-Fernandez, M.A. TNF-α contributes to caspase-3 independent apoptosis in neuroblastoma cells: Role of NFAT. PLoS ONE 2011, 6, e16100. [Google Scholar] [CrossRef] [PubMed]
- Abadie, A.; Besancon, F.; Wietzerbin, J. Type I interferon and TNFα cooperate with type II interferon for TRAIL induction and triggering of apoptosis in SK-N-MC EWING tumor cells. Oncogene 2004, 23, 4911–4920. [Google Scholar] [CrossRef] [PubMed]
- Cheema, S.K.; Mishra, S.K.; Rangnekar, V.M.; Tari, A.M.; Kumar, R.; Lopez-Berestein, G. Par-4 transcriptionally regulates Bcl-2 through a WT1-binding site on the Bcl-2 promoter. J. Biol. Chem. 2003, 278, 19995–20005. [Google Scholar] [CrossRef] [PubMed]
- Glienke, W.; Chow, K.U.; Bauer, N.; Bergmann, L. Down-regulation of WT1 expression in leukemia cell lines as part of apoptotic effect in arsenic treatment using two compounds. Leuk. Lymphoma 2006, 47, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Opel, D.; Poremba, C.; Simon, T.; Debatin, K.M.; Fulda, S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007, 67, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Finnegan, C.E.; Rogers, K.M.; Purcell, J.W.; Trimble, A.; Johnston, P.G.; Boland, M.P. STAT1: A modulator of chemotherapy-induced apoptosis. Cancer Res. 2004, 64, 8357–8364. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.C.; Metaxas, S.; Kobayashi, K.; Harris, M.; Werther, G.A. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology 2004, 145, 4103–4112. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelke, G.V.; Jagtap, J.C.; Kim, D.-K.; Shah, R.D.; Das, G.; Shivayogi, M.; Pujari, R.; Shastry, P. TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas. Biomedicines 2018, 6, 4. https://doi.org/10.3390/biomedicines6010004
Shelke GV, Jagtap JC, Kim D-K, Shah RD, Das G, Shivayogi M, Pujari R, Shastry P. TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas. Biomedicines. 2018; 6(1):4. https://doi.org/10.3390/biomedicines6010004
Chicago/Turabian StyleShelke, Ganesh V., Jayashree C. Jagtap, Dae-Kyum Kim, Reecha D. Shah, Gowry Das, Mruthyunjaya Shivayogi, Radha Pujari, and Padma Shastry. 2018. "TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas" Biomedicines 6, no. 1: 4. https://doi.org/10.3390/biomedicines6010004