Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Ethanol Extract Preparation of Propolis
2.3. Water Extract Preparation of Propolis
2.4. Gas Liquid Chromatography/Mass Spectrometry (GLC/MS) Analysis of Propolis
2.5. High-Performance Liquid Chromatography (HPLC) Analysis of Propolis
2.6. Antioxidant Activity of Propolis
2.7. Determination of Total Phenolic Content (TPC) of Propolis
2.8. Determination of Total Flavonoid Content (TFC)
2.9. Microorganisms and Culture Media
2.10. Evaluation of Minimum Inhibitory Concentrations (Mics) and Minimal Bactericidal Concentrations (MBC) of Propolis
2.11. Checkerboard Dilution
2.12. Time-Kill Assays
2.13. Data Analysis
3. Results
3.1. Propolis Extracts Analysis
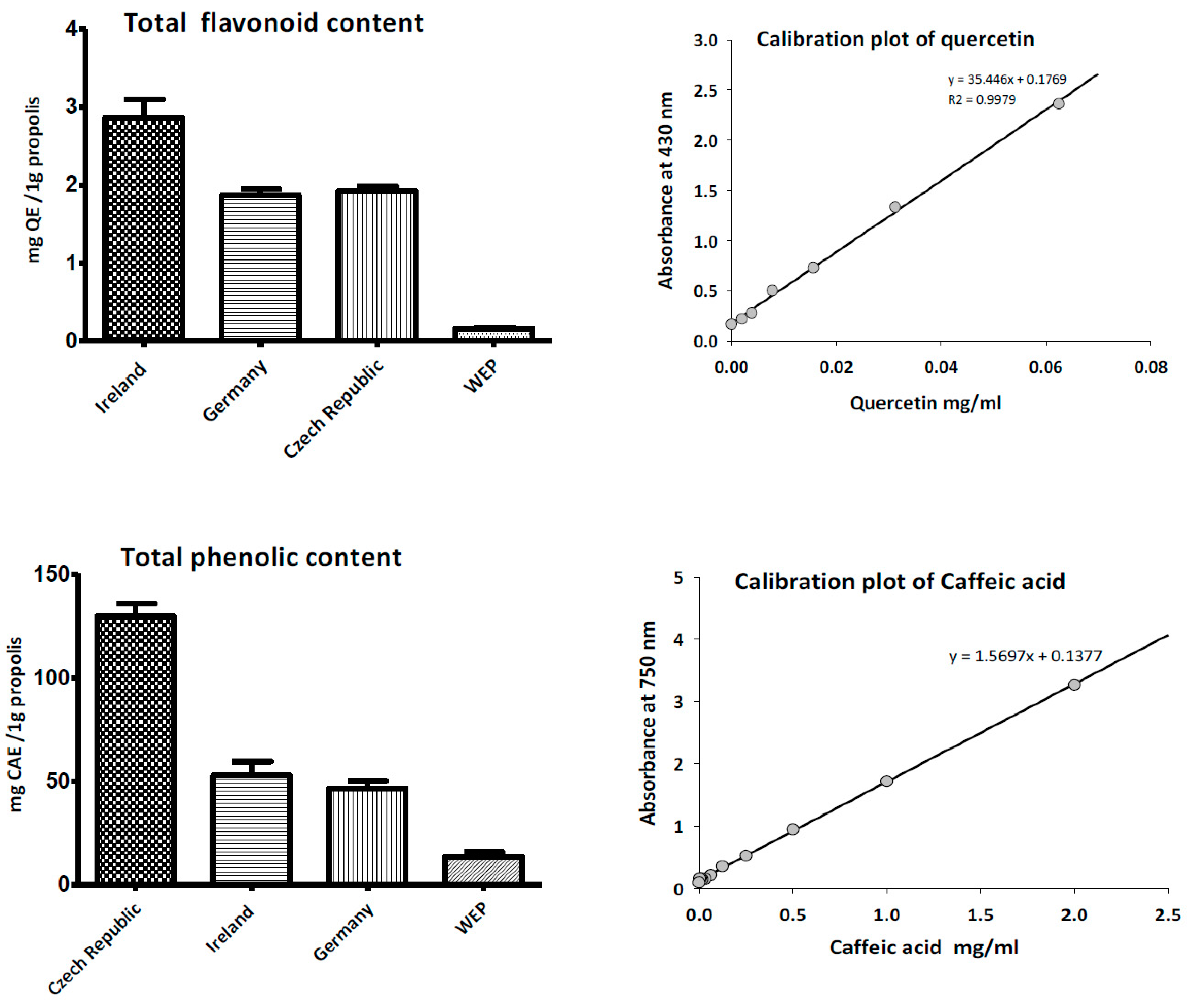
3.2. Total Phenolic and Flavonoid Content of Propolis
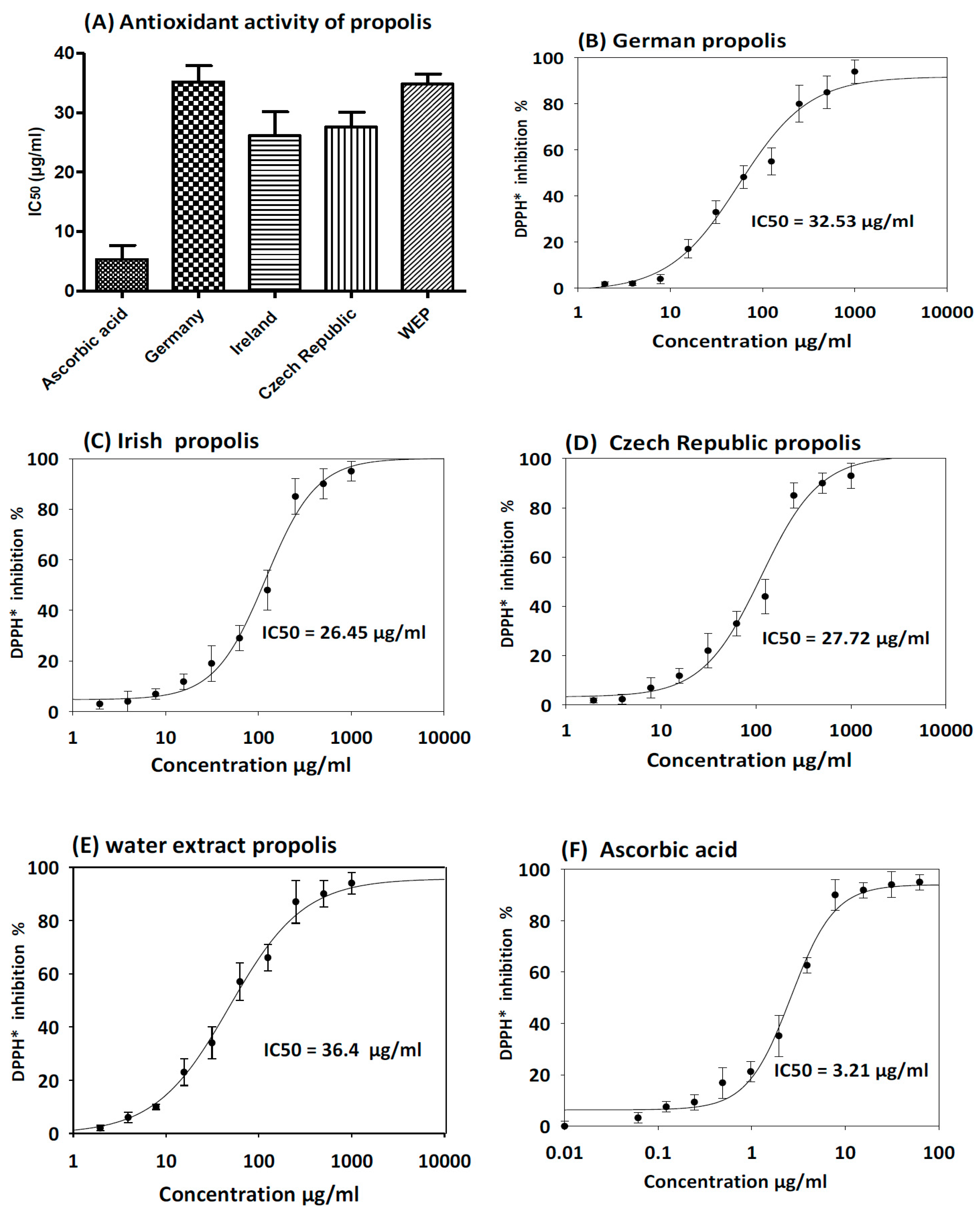
3.3. Antioxidant Activity of Propolis
3.4. Antimicrobial Activity of Propolis
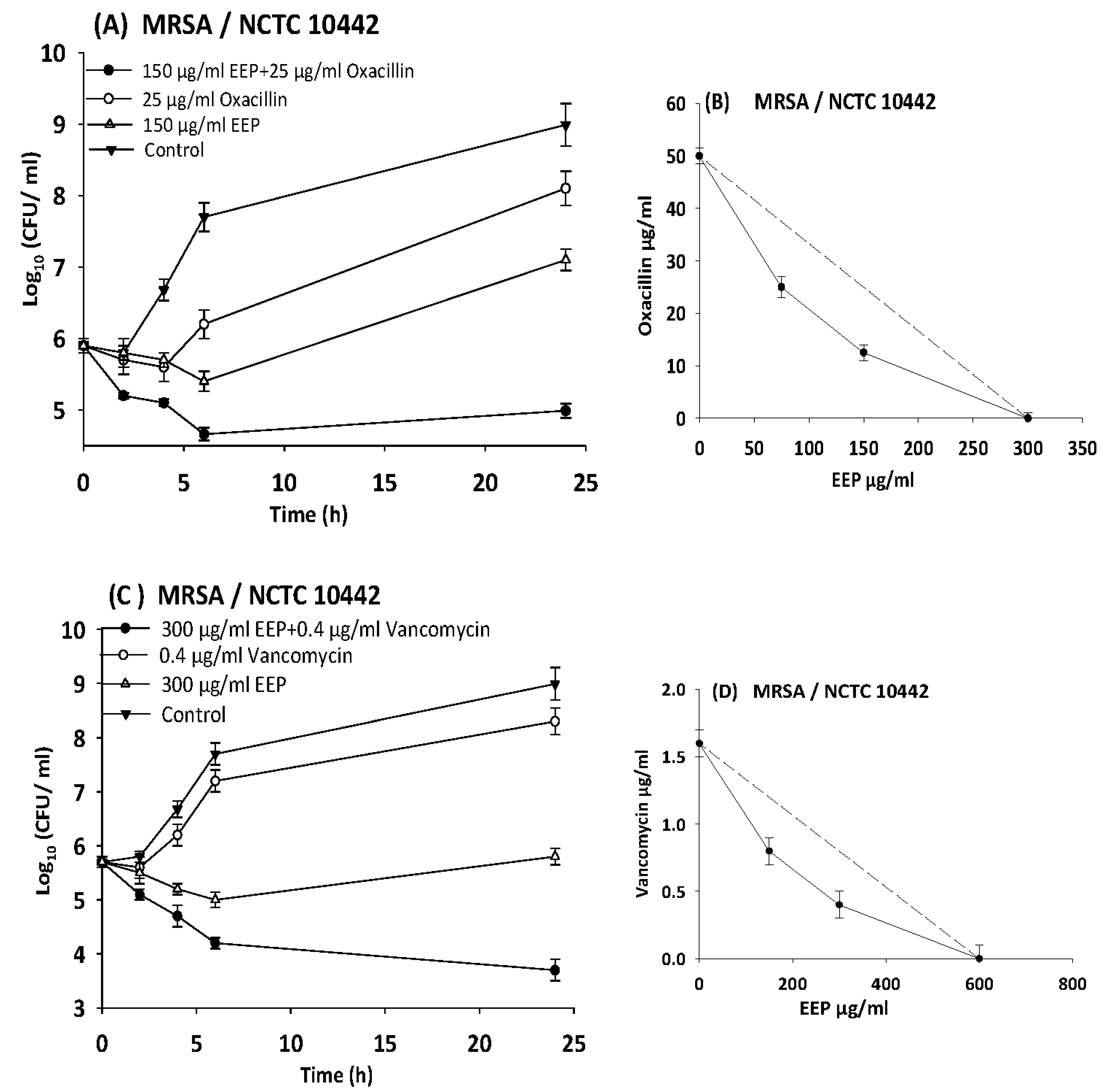
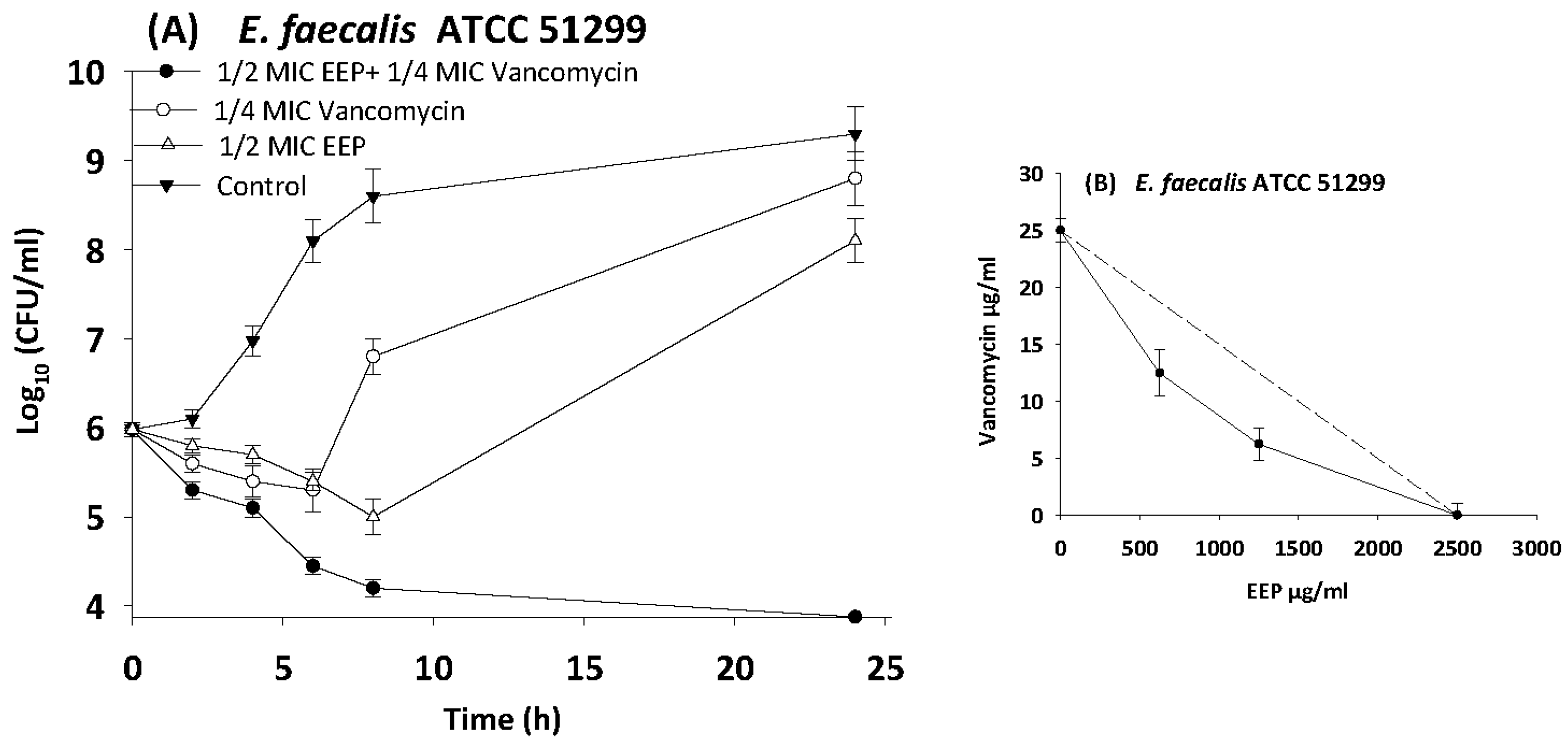
3.5. Evaluating Synergistic Interactions of Propolis Extracts with Antibiotics in Checkerboard Assays
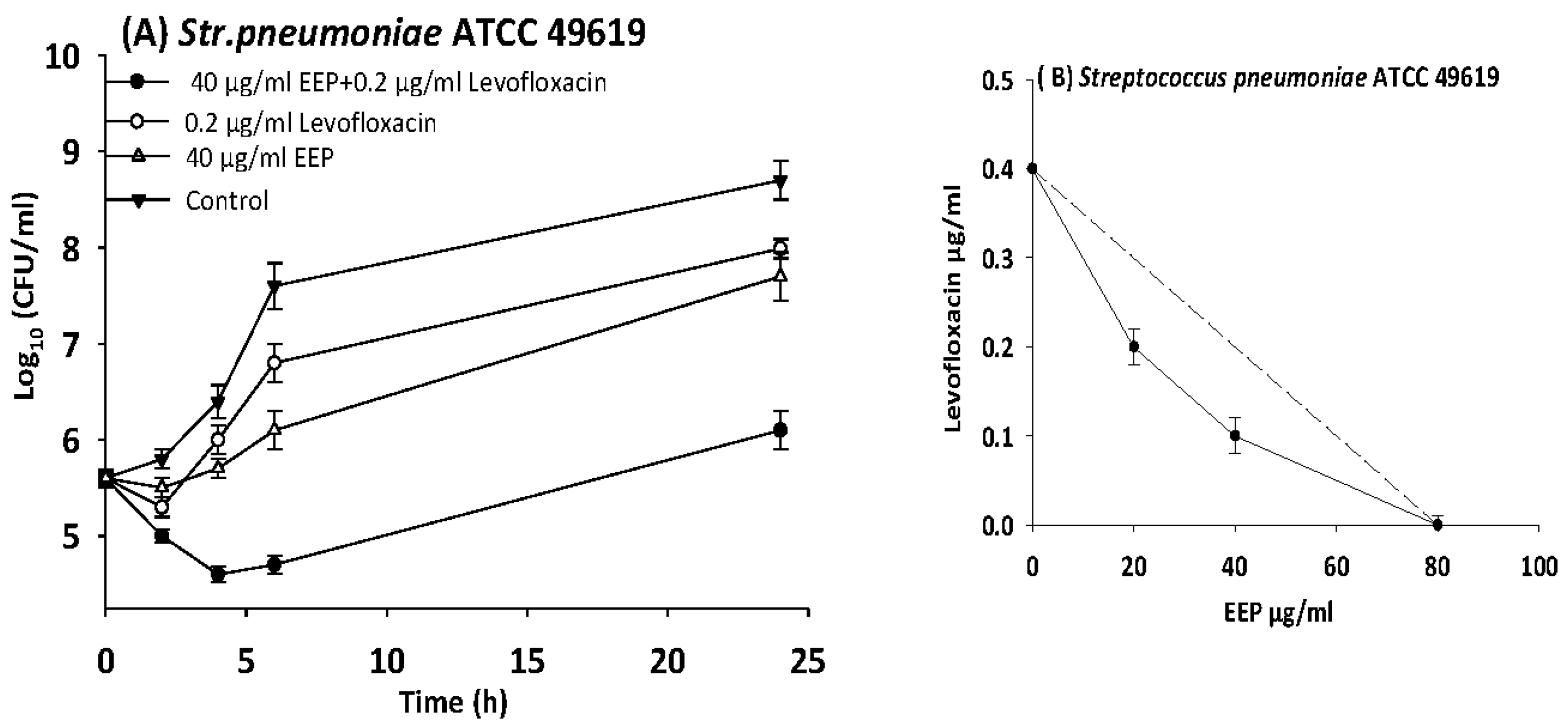
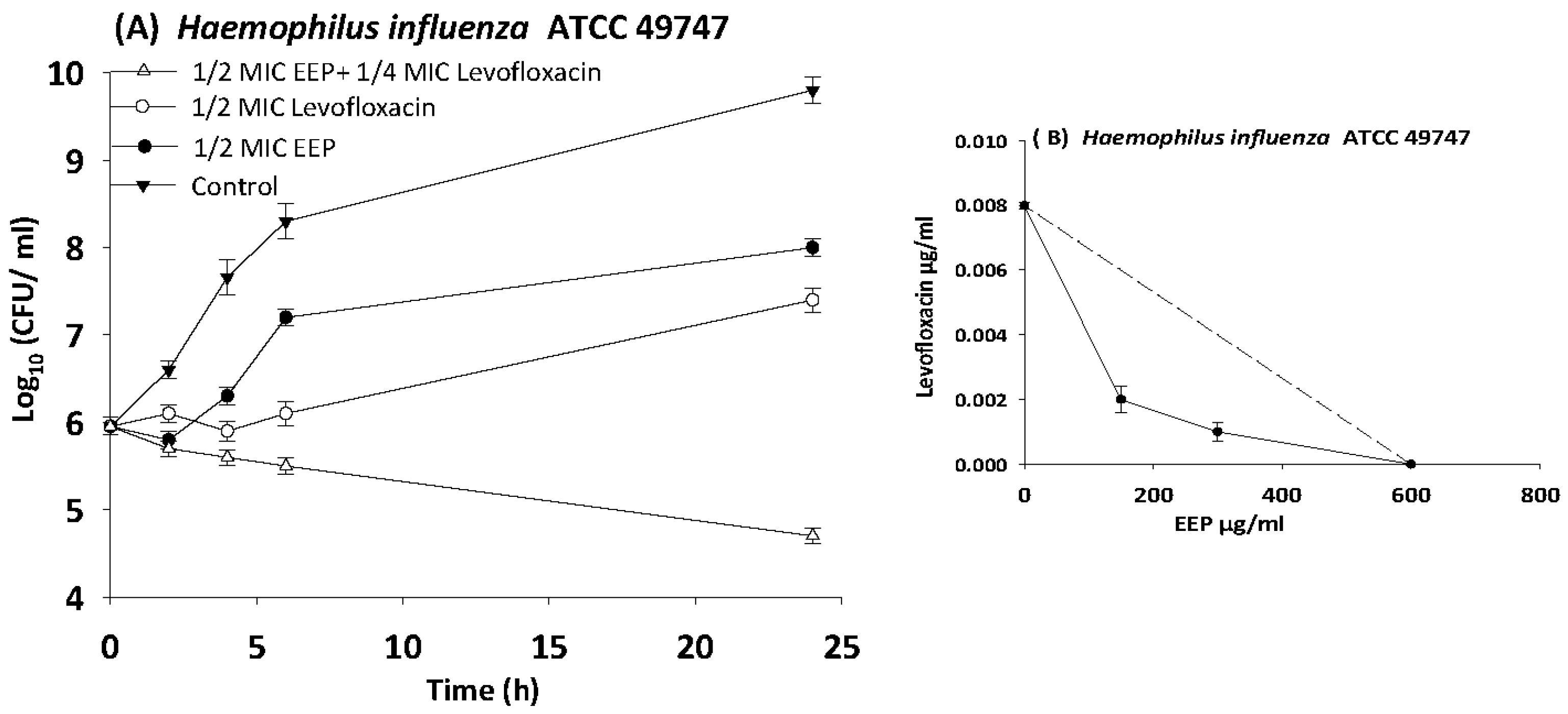
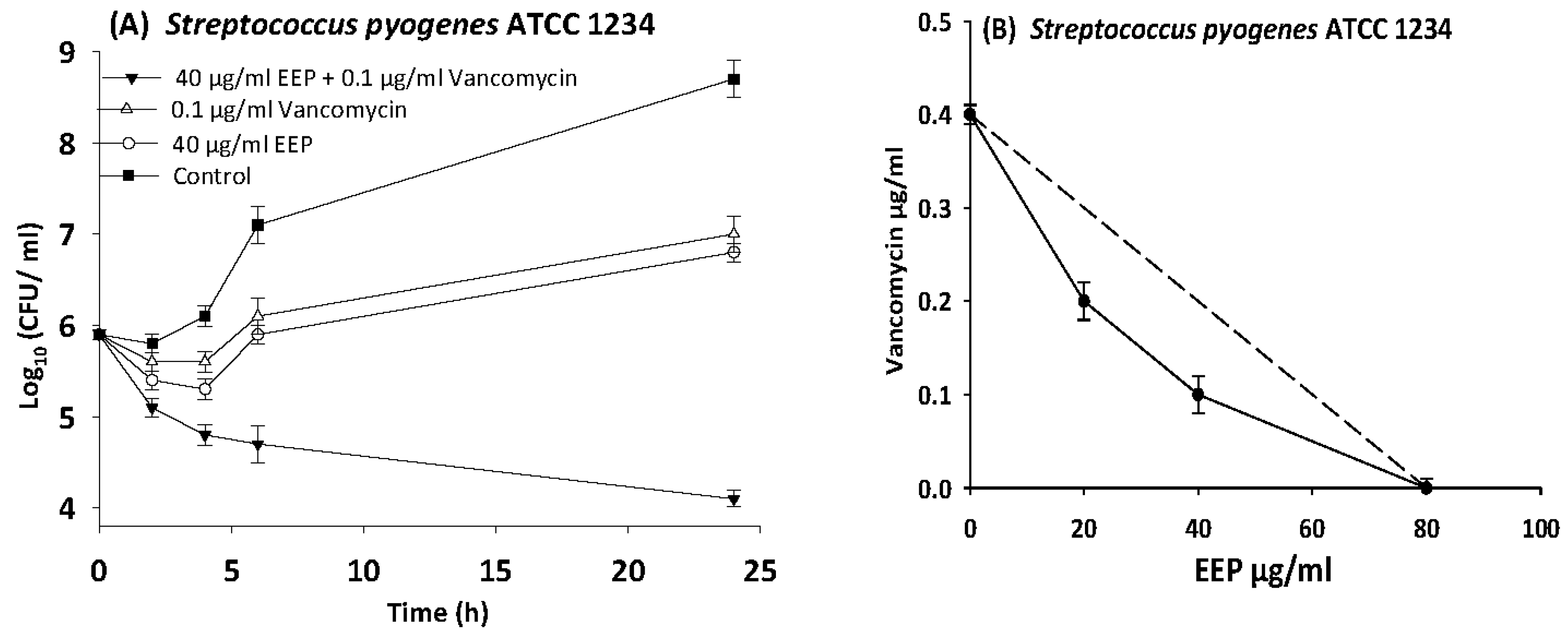
3.6. Time Kill Assays
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simone-Finstrom, M.; Borba, R.S.; Wilson, M.; Spivak, M. Propolis counteracts some threats to honey bee health. Insects 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Tosic, S.; Stojanovic, G.; Mitic, S.; Pavlovic, A.; Alagic, S. Mineral composition of selected Serbian propolis samples. J. Apic. Sci. 2017, 61, 5–15. [Google Scholar] [CrossRef]
- De Figueiredo, S.M.; Binda, N.S.; Almeida, B.D.M.; Lemos Abreu, S.R.; de Abreu, J.A.S.; Pastore, G.M.; Sato, H.H.; Toreti, V.C.; Tapia, E.V.; Park, Y.K.; et al. Green propolis: Thirteen constituents of polar extract and total flavonoids evaluated during six years through RP-HPLC. Curr. Drug Discov. Technol. 2015, 12, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Paramás, A.M. Phenolic composition of propolis. In Bee Products-Chemical and Biological Properties; Springer: Gewerbestrasse, Switzerland, 2017; pp. 99–111. [Google Scholar]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.Z.; Shi, J.; Zhang, C.; Hu, F. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus×canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.S.; De Mendonça, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Lagareiro Netto, A.A.; Lira, I.S.; López, B.G.-C.; Negrão, V.; Marcucci, M.C. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Hacievliyagil, S.; Kutlu, N.O.; Aydin, N.E.; Kurkcuoglu, M.; Iraze, M.; Durmazf, R. Effect of water extract of Turkish propolis on tuberculosis infection in guinea-pigs. Pharmacol. Res. 2004, 49, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Ahmad, R.S.; Arshad, M.U.; Niaz, B.; Batool, R.; Naz, R.; Ansar Rasul Suleria, H. Propolis to curb lifestyle related disorders: An overview. Int. J. Food Prop. 2016, 19, 420–437. [Google Scholar] [CrossRef]
- Demir, S.; Aliyazicioglu, Y.; Turan, I.; Misir, S.; Mentese, A.; Yaman, S.O.; Deger, O. Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line. Nutr. Cancer 2016, 68, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Soltani, E.-K.; Cerezuela, R.; Charef, N.; Mezaache-Aichour, S.; Esteban, M.A.; Zerroug, M.M. Algerian propolis extracts: Chemical composition, bactericidal activity and in vitro effects on gilthead seabream innate immune responses. Fish Shellfish Immunol. 2017, 62, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Rubio, O.; Fernández, M.C.; Hernández, I.M.; Jaramillo, C.G.J.; González, V.H.; Porto, R.M.D.O.; Delange, D.M.; Fidalgo, L.M.; Piccinelli, A.L.; Campone, L.; et al. Chemical profile and anti-leishmanial activity of three Ecuadorian propolis samples from Quito, Guayaquil and Cotacachi regions. Fitoterapia 2017, 120, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Marsola, A.; Ikegaki, M.; Alencar, S.M.; Rosalen, P.L. The effect of seasons on Brazilian red propolis and its botanical source: Chemical composition and antibacterial activity. Nat. Prod. Res. 2017, 31, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- Issam, A.-A.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of benzyl isothiocyanate, carvacrol, and kaempferol with antibiotics against multidrug resistant pathogens. Proc. Int. J. Med. Microbiol. 2012, 302, 21. [Google Scholar]
- Dos Santos, L.; Hochheim, S.; Boeder, A.M.; Kroger, A.; Tomazzoli, M.M.; Dal Pai Neto, R.; de Cordova, C.M. Chemical characterization, antioxidant, cytotoxic and antibacterial activity of propolis extracts and isolated compounds from the Brazilian stingless bees Melipona quadrifasciata and Tetragonisca angustula. J. Apic. Res. 2017, 56, 543–558. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C. Propolis: A review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 2013, 24, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Danert, F.C.; Zampini, C.; Ordoñez, R.; Maldonado, L.; Bedascarrasbure, E.; Isla, M. Nutritional and functional properties of aqueous and hydroalcoholic extracts from Argentinean propolis. Nat. Prod. Commun. 2014, 9, 167–170. [Google Scholar] [PubMed]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.B.; Svetaz, L.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Tapia, A. Urban propolis from San Juan province (Argentina): Ethnopharmacological uses and antifungal activity against Candida and dermatophytes. Ind. Crop. Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Coelho, G.R.; de Senna Villar, K.; Figueiredo, C.A.; Badari, J.C.; Mendonça, R.M.Z.; Oliveira, M.I.; Mendonça, R.Z. Antiviral effects of Scaptotrigona postica propolis and their fractions. In BMC Proceedings; BioMed Central Ltd.: London, UK, 2014; Volume 8, p. 63. [Google Scholar]
- Souza, E.A.D.; Inoue, H.T.; Fernandes Júnior, A.; Veiga, N.; Orsi, R.D.O. Influence of seasonality and production method on the antibacterial activity of propolis. Acta Sci. Anim. Sci. 2014, 36, 49–53. [Google Scholar] [CrossRef]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother. Res. 2008, 22, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Končić, M.Z.; Kosalec, I. Modulation of antioxidant, chelating and antimicrobial activity of poplar chemo-type propolis by extraction procures. LWT Food Sci. Technol. 2014, 57, 530–537. [Google Scholar] [CrossRef]
- Fernandes Júnior, A.; Balestrin, E.C.; Betoni, J.E.C.; Orsi, R.D.O.; Cunha, M.D.L.R.D.; Montelli, A.C. Propolis: Anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Mem. Inst. Oswaldo Cruz 2005, 100, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Takaisi-Kikuni, N.B.; Schilcher, H. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med. 1994, 60, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.D.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella typhi and their synergism with antibiotics acting on the ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Wąsik, T.J. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [PubMed]

| No. | Compounds | Rt (min) GLC-MS | EEP | WEP | ||
|---|---|---|---|---|---|---|
| Germany | Ireland | Czech | Germany | |||
| 1 | 2-Furanmethanol | 5.33 | − | − | − | + |
| 2 | Cyclopentanedione | 6.53 | − | − | − | + |
| 3 | benzyl alcohol | 6.86 | + | + | − | − |
| 4 | Phenol | 8.12 | − | − | − | − |
| 5 | N-Benyoyloxycarbonzl-l-tyrosine | 8.87 | − | − | + | − |
| 6 | 2-Methoxyphenolacetat | 10.15 | + | − | − | − |
| 7 | 1,2,3-Propanetriol monoacetat | 10.27 | − | − | + | − |
| 8 | Phenylethanol | 10.49 | − | + | − | − |
| 9 | Phenylethanol | 10.51 | − | − | + | − |
| 10 | Benzoic acid ethyl ester | 11.7 | − | − | − | − |
| 11 | Benzoic Acid | 12.32 | + | + | + | − |
| 12 | O-Hydroxy-cinnamic acid | 12.71 | + | − | − | − |
| 13 | 2,3-Dihydro benzofuran (p-Vinylphenol) | 12.71 | − | + | − | − |
| 14 | 2,3-Dihydro-Benzofuran | 12.73 | − | − | + | − |
| 15 | 2-Hydroxy cinnamic acid | 12.76 | − | − | − | + |
| 16 | Cinnamyl alcohol | 14.16 | − | + | + | − |
| 17 | 2-Methoxy-4-vinylphenol | 14.35 | + | + | + | + |
| 18 | 4-Vinyl-2-Methoxy-phenol | 14.52 | − | + | − | + |
| 19 | Hydrocinnamic acid ethyl ester | 14.98 | − | + | − | − |
| 20 | 4-Hydroxybenzaldehyde | 15.26 | + | − | + | − |
| 21 | 4,5-Dihydroxy-2-methyl-benzaldehyde | 15.59 | + | − | − | − |
| 22 | 3-Hydroxy-4-methoxy-benzaldehyde | 15.6 | − | + | − | − |
| 23 | 2-Methyl-4,5Dihydroxybenzaldehyde | 15.62 | − | − | + | − |
| 24 | 4-Hydroxybenzaldehyde | 16.5 | − | + | − | − |
| 25 | Cinnamic acid | 16.5 | − | − | − | + |
| 26 | 4-Hydroxy-acetophenone | 16.6 | + | − | − | − |
| 27 | 4-Propyl-guajacol (=2-Methoxy-4-propyl-phenol) | 16.64 | + | − | − | − |
| 28 | Cinnamic acid ethyl ester | 16.94 | + | + | − | − |
| 29 | p-Methoxyphenyl-2-Butanone | 17.35 | − | + | − | − |
| 30 | 4-Hydroxy-3-methoxyphenyl-2-propanone | 17.8 | + | − | − | − |
| 31 | 1-Methyl-n-vanillyl-2-Phenethamine | 17.81 | − | − | + | − |
| 32 | 4-Methoxyphenyl propanoic acid ethyl ester | 19.02 | + | − | − | − |
| 33 | 3-(4-Methoxyphenyl) propionic acid ethyl ester | 19.02 | − | + | − | − |
| 34 | Dodecanoic acid ethyl ester | 19.26 | + | − | − | − |
| 35 | Guaiol | 19.39 | − | + | − | − |
| 36 | Eudesmol-Isomere | 19.89 | − | − | − | − |
| 37 | Eudesmol | 19.89 | − | + | + | − |
| 38 | Cardinol | 20 | − | − | + | − |
| 39 | Eudesm-4(14)-en-11-ol | 20.15 | − | + | + | − |
| 40 | alpha-Eudesmol | 20.23 | − | − | + | − |
| 41 | alpha-Bisabolol | 20.59 | − | + | + | − |
| 42 | 1-Hydroxy-3-(4-hydroxy-3-methoxyphenyl)2-propanone | 20.66 | − | − | + | − |
| 43 | 4-Hydroxy-methoxy-phenyl-2-propenal | 20.81 | − | − | + | − |
| 44 | Benzoic acid benzyl ester | 21.43 | + | − | + | − |
| 45 | Myristic acid ethyl ester | 22.16 | + | − | − | − |
| 46 | Salicylic acid benzyl ester | 22.31 | + | − | − | − |
| 47 | 2-Hydroxy-benzoic acid benzyl ester | 22.9 | − | − | + | − |
| 48 | 2-Methoxy-benzoic acid benzyl ester | 24.29 | − | − | + | − |
| 49 | Hexadecanoic acid | 24.36 | − | − | − | + |
| 50 | Ethyl hexadecanoate | 24.79 | − | + | − | − |
| 51 | Hexadecanoic acid ethyl ester | 24.8 | + | − | − | − |
| 52 | 2,4-Di-tert-butylphenyl benzoate | 25.46 | − | + | + | − |
| 53 | Benzyl cinnamate | 25.62 | + | + | + | − |
| 54 | 4-Hydroxy cinnamic acid | 25.82 | − | + | + | − |
| 55 | 9-Octadecenoic acid ethal ester | 26.86 | + | − | − | − |
| 56 | Ethyl-9-octadecenoate | 26.86 | − | + | − | − |
| 57 | 4-Hydroxy-3-methoxy cinnamic acid | 26.98 | − | + | + | − |
| 58 | Stearic acid ethyl ester | 27.2 | − | − | − | + |
| 59 | 4-Hydroxy-methoxy cinnamic acid | 27.55 | − | + | + | − |
| 60 | 2′,6′-dihydroxy-4′-methoxy-chalcone | 28 | + | + | + | + |
| 61 | Tricosane | 28.57 | − | + | − | − |
| 62 | Cinnamyl cinnamate | 29.21 | + | + | − | − |
| 63 | 4-Acetyl-benzoic acid phenylmethyl ester | 29.74 | + | − | − | − |
| 64 | Benzyl-4-acetylbenzoate | 29.74 | − | + | + | − |
| 65 | Dihydrochrysin | 29.84 | − | + | + | − |
| 66 | Pentacosane | 30.68 | − | + | − | − |
| 67 | 4′,5-Dihydroxy-7-methoxy flavone | 31.59 | − | − | + | − |
| 68 | Chrysin | 31.93 | − | + | + | − |
| 69 | Heptacosane | 32.64 | − | + | − | − |
| 70 | Methyl pentacosanoate | 33.38 | − | + | − | − |
| 71 | Nonacosane | 34.46 | − | + | − | − |
| HPLC analysis | ||||||
| 1 | Caffeic acid | 17.26 | + | − | + | − |
| 2 | trans-p-Coumaric acid | 19.66 | − | − | + | − |
| 3 | Pinocembrin | 24.42 | + | + | + | − |
| 4 | Cinnamic acid | 26.06 | − | − | + | + |
| 5 | Galangin | 34.41 | − | + | + | − |
| Microorganisms | ATCC NO. | EEP (mg/mL) | WEP (mg/mL) | Vancomycin (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Germany | Ireland | Czech | Germany | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| Staphylococcus aureus Amme | 29213 | 1.2 | 5 | 0.3 | 0.6 | 0.6 | 2.5 | 1.2 | 5 | 0.2 | 1.6 |
| Staphylococcus aureus BAA | 977 | 1.2 | 5 | 0.6 | 1.2 | 0.6 | 1.2 | 1.2 | 5 | 0.8 | 1.6 |
| Staphylococcus saprophyticus | 15305 | 1.2 | >5 | 0.3 | 0.6 | 1.2 | 2.5 | 1.2 | 5 | 1.6 | 1.6 |
| Staphylococcus aureus | 25923 | 1.2 | 2.5 | 0.08 | 0.1 | 0.3 | 0.6 | 1.2 | 2.5 | 0.8 | 1.6 |
| Staphylococcus epidermidis | 14990 | 0.6 | 1.2 | 0.6 | 1.2 | 0.6 | 1.2 | 1.2 | 5 | 0.8 | 3.1 |
| MRSA/NCTC | 10442 | 0.3 | 0.6 | 1.2 | >5 | 0.6 | 1.2 | 1.2 | >5 | 1.6 | 1.6 |
| VRE VanB | 51299 | 2.5 | 5 | 5 | >5 | 2.5 | 5 | 2.5 | >5 | 25 | >50 |
| Streptococcus pyogenes | 12344 | 0.6 | 1.2 | 0.08 | 0.6 | 0.08 | 0.1 | 0.6 | 2.5 | 0.4 | 1.6 |
| Streptococcus pneumoniae | 49619 | 0.3 | 0.6 | 0.08 | 0.1 | 0.08 | 0.1 | 0.6 | 2.5 | NT | NT |
| Streptococcus oralis | 35037 | 0.3 | 0.6 | 0.1 | 0.3 | 0.1 | 0.3 | 1.2 | 5 | 0.8 | 0.8 |
| Streptococcus agalactia | 27956 | 0.6 | 1.2 | 0.1 | 0.3 | 0.3 | 0.6 | 0.6 | 5 | 0.4 | 0.4 |
| Streptococcus thermophilus | 19258 | 0.3 | 0.6 | 0.04 | 0.08 | 0.08 | 0.1 | 0.3 | 0.6 | 0.4 | 0.8 |
| Bacillus subtilis | 6051 | 0.3 | 0.6 | 0.08 | 0.3 | 0.3 | 0.6 | 2.5 | 5 | 0.4 | 0.8 |
| Enterococcus casseliflavus | 70032 | 2.5 | >5 | 0.6 | 1.2 | 1.2 | 2.5 | 5 | >5 | 12.5 | >50 |
| Microorganisms | EEP (mg/mL) | WEP (mg/mL) | Streptomycin (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC NO. | Germany | Ireland | Czech | Germany | |||||||
| MC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| K. pneumoniae | 700603 | 5 | >5 | 0.6 | >5 | 1.2 | >5 | 2.5 | 2.5 | 1.6 | 3.1 |
| Klebsiella pneumoniae * | 800877 | >5 | NT | >5 | NT | >5 | NT | 1.2 | 2.5 | 25 | 50 |
| Klebsiella pneumoniae * | 809273 | 1.2 | 2.5 | 0.6 | 1.2 | 1.2 | 2.5 | >5 | NT | 25 | 50 |
| Klebsiella oxytoca | 700324 | 2.5 | >5 | 1.2 | >5 | 2.5 | >5 | 2.5 | 2.5 | 3.1 | 6.2 |
| Escherichia coli | 25922 | 5 | >5 | 1.2 | >5 | 0.6 | >5 | 2.5 | 2.5 | 3.1 | 6.2 |
| Escherichia coli O157:H7 | 35150 | 5 | >5 | 0.6 | >5 | 0.6 | >5 | 1.2 | 2.5 | 6.2 | 12.5 |
| Pseudomonas aeruginosa | 27853 | 2.5 | >5 | 0.6 | >5 | 1.2 | >5 | 2.5 | 5 | 3.1 | 12.5 |
| Salmonella choleraesuis | 554 | >5 | NT | >5 | NT | >5 | NT | 2.5 | 5 | 6.2 | 25 |
| Shigella flexneri | 29903 | 2.5 | >5 | 0.3 | >5 | 0.6 | >5 | 2.5 | 2.5 | 3.1 | 3.1 |
| Haemophilus influenzae | 49747 | 2.5 | >5 | 0.6 | 1.2 | 1.2 | 2.5 | 2.5 | 5 | NT | NT |
| Acinetobacter baumannii | BAAm 747 | >5 | NT | 5 | >5 | 5 | 5 | 1.2 | 0.6 | 3.1 | 12.5 |
| Burkholderia cepacia | 25416 | 5 | >5 | 1.2 | 5 | 1.2 | 5 | 1.2 | 2.5 | >50 | >50 |
| Enterobacter cloacae | 700323 | >5 | NT | >5 | NT | >5 | NT | 2.5 | >5 | 25 | 50 |
| Yersinia enterocolitis | 9610 | 2.5 | >5 | 1.2 | 5 | 1.2 | 5 | 1.2 | 2.5 | 25 | 25 |
| Microorganisms | EEP (mg/mL) | WEP (mg/mL) | Nystatin (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC No. | Germany | Ireland | Czech | Germany | |||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | ||
| Candida albicans | 90028 | 5 | >5 | 0.6 | 0.6 | 0.6 | 1.2 | 2.5 | 5 | 0.2 | 0.4 |
| Candida albicans * | 105366 | 5 | >5 | 0.3 | 0.3 | 1.2 | 2.5 | 2.5 | >5 | 0.2 | 0.4 |
| Candida glabrata MYA | 2950 | 5 | >5 | 0.3 | 0.6 | 0.6 | 0.6 | 5 | >5 | 0.2 | 0.4 |
| Candida glabrata * | 105410 | >5 | >5 | 0.6 | 1.2 | 2.5 | 5 | 5 | >5 | 6.2 | 12.5 |
| Candida glabrata * | 105413 | >5 | >5 | 0.1 | 0.1 | 0.6 | 1.2 | 2.5 | 2.5 | 6.2 | 12.5 |
| Candida parapsilosis | 22019 | 1.2 | >5 | 0.3 | 0.6 | 0.6 | 0.6 | 2.5 | 5 | 0.4 | 0.8 |
| Candida parapsilosis * | 105328 | >5 | >5 | 0.6 | 0.6 | 2.5 | 2.5 | 2.5 | >5 | 1.3 | 2.5 |
| Candida tropicalis | 9968 | 5 | >5 | 0.2 | 0.3 | 0.6 | 1.2 | 5 | >5 | 0.8 | 1.6 |
| Candida krusei | 90878 | >5 | >5 | 0.6 | 0.6 | 1.2 | 2.5 | 1.2 | 2.5 | 25 | 25 |
| Microorganisms | ATCC NO. | Agent | MIC (µg/mL) | FIC | FICI | Interpretation | |
|---|---|---|---|---|---|---|---|
| Alone | Combination | ||||||
| MRSA | 10442 | EEP | 600 | 150 | 0.3 | 0.38 | Synergy |
| Vancomycin | 1.6 | 0.2 | 0.1 | ||||
| EEP | 300 | 75 | 0.3 | 0.5 | |||
| Oxacillin | 50 | 12.5 | 0.3 | ||||
| E. faecalis | 51299 | EEP | 2500 | 312.5 | 0.1 | 0.4 | Synergy |
| Vancomycin | 25 | 6.25 | 0.3 | ||||
| S. pneumoniae | 49619 | EEP | 80 | 20 | 0.3 | 0.5 | Synergy |
| Levofloxacin | 0.4 | 0.1 | 0.3 | ||||
| H. influenza | 49747 | EEP | 600 | 150 | 0.3 | 0.5 | Synergy |
| Levofloxacin | 0.008 | 0.002 | 0.3 | ||||
| S. pyogenes | 12344 | EEP | 80 | 20 | 0.3 | 0.5 | Synergy |
| Vancomycin | 0.4 | 0.1 | 0.3 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. https://doi.org/10.3390/medicines5010002
AL-Ani I, Zimmermann S, Reichling J, Wink M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines. 2018; 5(1):2. https://doi.org/10.3390/medicines5010002
Chicago/Turabian StyleAL-Ani, Issam, Stefan Zimmermann, Jürgen Reichling, and Michael Wink. 2018. "Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics" Medicines 5, no. 1: 2. https://doi.org/10.3390/medicines5010002






