Allergic Aspergillus Rhinosinusitis
Abstract
:1. Introduction
2. Historical Account
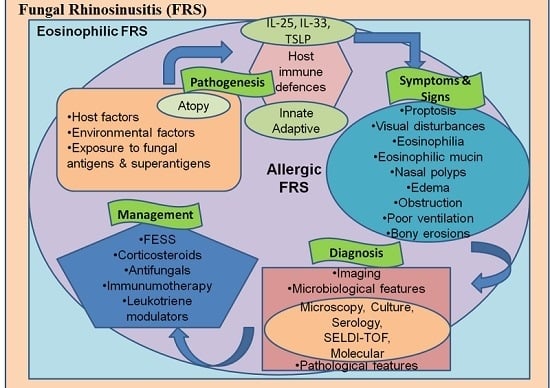
3. Classification
3.1. The Invasive Form Includes
- Acute (fulminant, necrotizing) FRS: This type is commonly seen in immunosuppressed patients (hematological malignancy, diabetes mellitus, transplant and on immunosuppressive drug) with history of less than 4 weeks. It is characterized by vascular invasion by fungal hyphae, necrotizing reaction with abundant hyphae. Occasionally bland necrosis is seen [23,26,27,28]. It is most commonly caused by fungi under Mucorales or Aspergillus species [1].
- Granulomatous invasive FRS: This form of FRS is seen in immunocompetent patients from tropical regions from Sudan to India [22,29,30]. The lesion typically presents with granuloma and sparse A. flavus hyphae with or without foreign body or giant cells. The duration of illness is more than 12 weeks and affects cheek, nose, orbit and paranasal sinuses with predominant proptosis.
- Chronic invasive FRS: This condition is seen in mildly immunosuppressed patients (diabetes, steroid therapy) and lasts for more than 12 weeks with progression at a relatively slow pace. It affects ethmoid and sphenoid sinuses commonly. Histologically, it presents with abundant fungal hyphae (commonly A. fumigatus), mixed inflammatory reaction, and occasional vascular invasion. The disease spreads to cheek; orbit-like chronic granulomatous type [23,29,31].
3.2. The Non-Invasive Fungal Rhinosinusitis (FRS) Comprises of Following Categories
- Fungal colonization: It is an asymptomatic saprobic colonization of nasal cavity or sinuses by fungi in immunocompetent hosts often after local surgery. It usually follows benign course [25].
- Fungal ball (previously known as sinus aspergilloma/mycetoma): It is defined as accumulation of dense conglomerated fungal hyphae in sinuses without invasion [32]. This condition generally affects older, immunocompetent patients (average age 64 years). Most commonly, it represents maxillary sinus colonization (followed by sphenoid sinus) by fungi with poor inflammatory reaction, often seen in adult immunocompetent females of southern France [32]. It is characterized by sinus opacification, cheesy discharge, chronic inflammatory reaction without any tissue invasion by fungi. Bone erosion is reported in 4%–17% patients. The exact pathogenesis of the condition is unclear although aerogenic and iatrogenic pathway theories are proposed [32]. According to aerogenic theory, a high burden of fungal spores make their way into sinuses through ostia while iatrogenic or odontogenic pathway is secondary to any dental procedure which causes formation of oro-antral communication. Upon microscopic examination, tightly packed hyphae are observed in alternating dense and less dense zones similar to concentric layers of onion skin which are surrounded by a dense inflammatory exudate of predominantly neutrophils. The diagnosis of fungal ball should be highly suspected in a patient of recurrent unilateral sinusitis refractory to treatment supported by CT findings of opacified sinus with central metal dense spots and microbiological and histopathological features. The isolation of fungi may fail sometime; diagnosis depends on microscopy and histopathology in those cases.
- Eosinophil-related FRS: This category suffers from confusion in defining three entities (AFRS, EMRS and EFRS) as distinct varieties.
- ➢
- AFRS: It is characterized by nasal polyposis, type I (raised IgE) and possibly type III hypersensitivity reaction, production of allergic mucin with abundant eosinophils and non-invading fungal hyphae [25]. The fungi behave as allergens in atopic host causing inflammation of sinuses thereby obstructing the sinus ostia hampering drainage [25,33,34]. Occasionally, patients with recurrent AFRS may not have nasal polyps due to previous surgery though eosinophilic mucin and hyphae are present. DeShazo removed the criterion type I hypersensitivity in defining AFRS, as some researchers did not find immediate hypersensitivity in all patients with AFRS [35].
- ➢
- EMRS: EMRS is described as a distinct entity by Ferguson [26]. It represents a systemic immune dysregulation where fungal hyphae do not play any role and are not detected in the eosinophilic mucin. It occurs in patients with asthma, aspirin sensitivity and IgG1 deficiency and is generally bilateral [25,26]. She proposed four types of eosinophil-related FRS: allergic fungal rhinosinusitis, non-allergic fungal eosinophilic rhinosinusitis, super antigen-induced eosinophilic rhinosinusitis, and aspirin-exacerbated eosinophilic rhinosinusitis [26]. The important features that distinguish EMRS from AFRS include age (young in AFRS, old in EMRS); nasal obstruction (100% in AFRS, one-third cases of EMRS); laterality (unilateral or bilateral in AFRS, bilateral in nearly all cases of EMRS); orbital involvement (common in AFRS); total IgE levels (raised in AFRS); fungal hyphae demonstration (absent in EMRS) and expression of genes for cathepsin B, sialyltransferase 1, GM2 ganglioside-activation protein and S100 calcium binding protein (absent in AFRS) [3].
- ➢
- EFRS: Ponikau et al. described this entity to characterize the patients with FRS having fungal hyphae embedded in eosinophilic mucin with or without evidence of type I hypersensitivity [22]. His group even claimed that all cases of chronic rhinosinusitis are due to fungi as etiology. Braun et al. and Polzehl et al. supported the hypothesis by demonstrating fungi in sinuses of all cases of chronic rhinosinusitis using sensitive techniques, even without atopy [36,37]. They claimed that certain fungi might be able to mount eosinophilic immune response in the absence of atopy, which was further supported by the in vitro observation of elicitation of Th1 and Th2 responses by non-atopic CRS patients in response to fungal (Alternaria species) exposure [38].
4. Controversies: Where Do We Stand?
5. Epidemiology
- ■
- Geographical variation: AFRS is reported in areas with warm, dry and humid climate [3]. The high prevalence of the disease is noted in India, North Africa, the Middle East and southeastern and southwestern parts of the US (especially Mississippi basin) [46,77,78,79,80,81,82,83,84,85]. Northern states of the US have a lower frequency of 0.4%, while Southern states reported ≥10% [79]. AFRS constitutes the highest number of cases of CRS in India accounting for 56%–79% of cases [51,73,84,86]. AFRS cases are also reported from Australia, Malaysia, and Thailand [18,87,88].
- ■
- Seasonal variation: The study from rural northern India reported a correlation of high incidence of FRS with wheat-harvesting season in winter months, when fungal spore count in the air increases due to wheat thrashing [73].
- ■
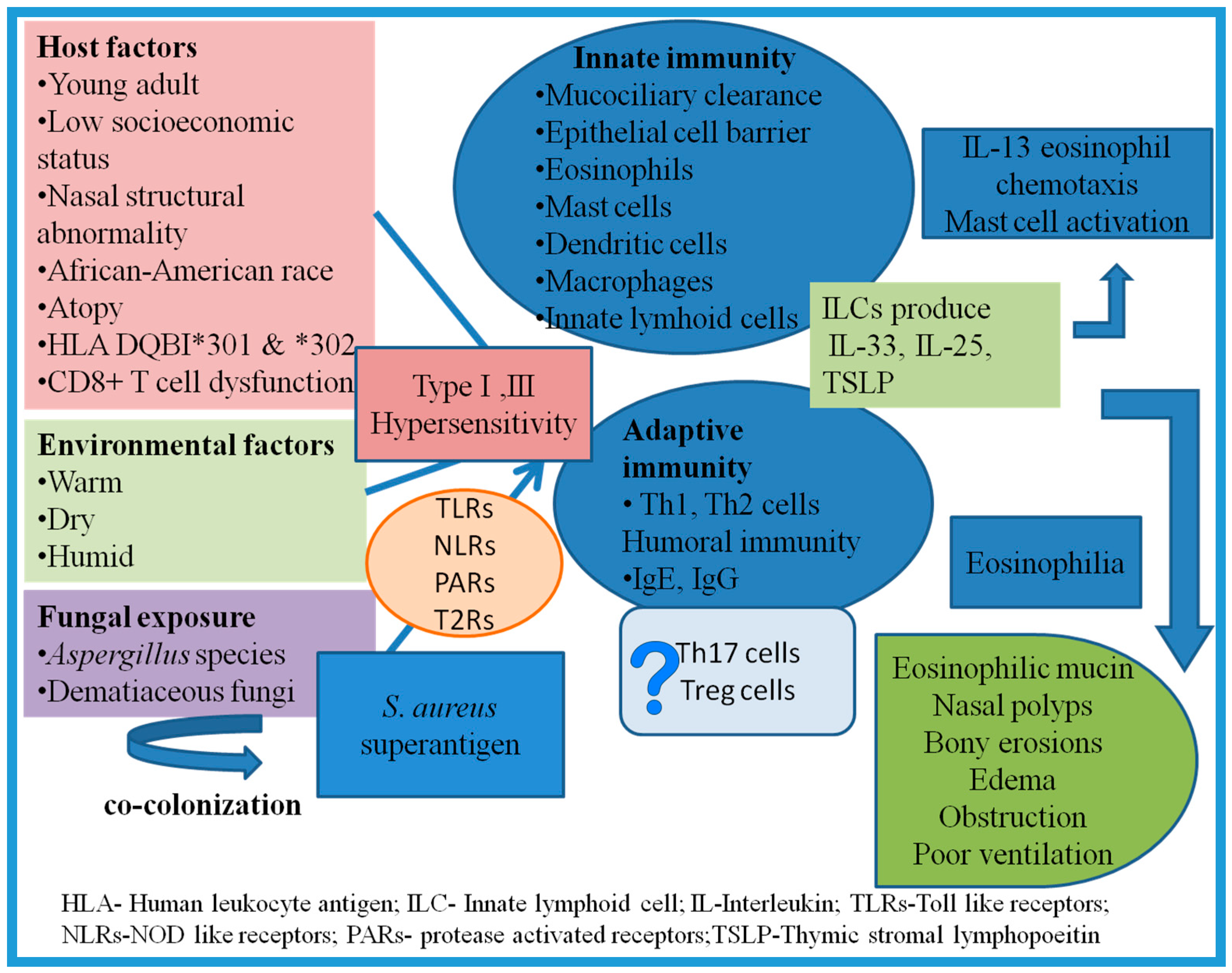
- Host factors: AFRS is observed commonly in young adult males from rural areas attributed to their work in the fields in warm climates, thus predisposing them to nasal mucosal injury and fungal colonization [73,78]. Other predisposing factors include African-American origin, structural anomalies, and low socioeconomic status. Bony erosion is 15 times more common in African-Americans with higher rate of intraorbital and intracranial extension of the lesion [89,90,91,92]. While Ghegan et al. failed to observe any correlation between bony erosion and low socioeconomic status, other studies have found a significant correlation between the bony erosion and inhabitants of low-income countries with poor housing conditions [3,92,93]. Patients with intracranial and intraorbital extension of the disease were also found to be residents of rural areas where primary healthcare was poor and patients reported to hospitals only in the later stages of the disease [94]. HLA studies have shown higher association of AFRS with DQB1*301 and *302 [95]. Other host factors include atopy, asthma, and aspirin sensitivity [26,96].
- ■
- Agent factors: Manning and Holman reported isolation of 87% dematiaceous fungi and 13% Aspergillus species from patients with AFRS [97]. However, Montone et al. reported higher (34%) isolation of Aspergillus than dematiaceous (30%) fungi [46,98]. Aspergillus flavus is the most common isolate (upto 96%) from patients with AFRS from India and Sudan [73,78,80,81,82,84,85,87]. Similarly, A. flavus was isolated from >50% patients with AFRS in the Middle East [83].
6. Clinical Presentation
7. Pathogenesis and Immunology: Recent Concepts
7.1. Role of Atopy
7.2. Exposure to Antigens
7.3. Innate Immune Response
7.4. Adaptive Immune System
7.5. Role of Superantigens
8. Diagnosis
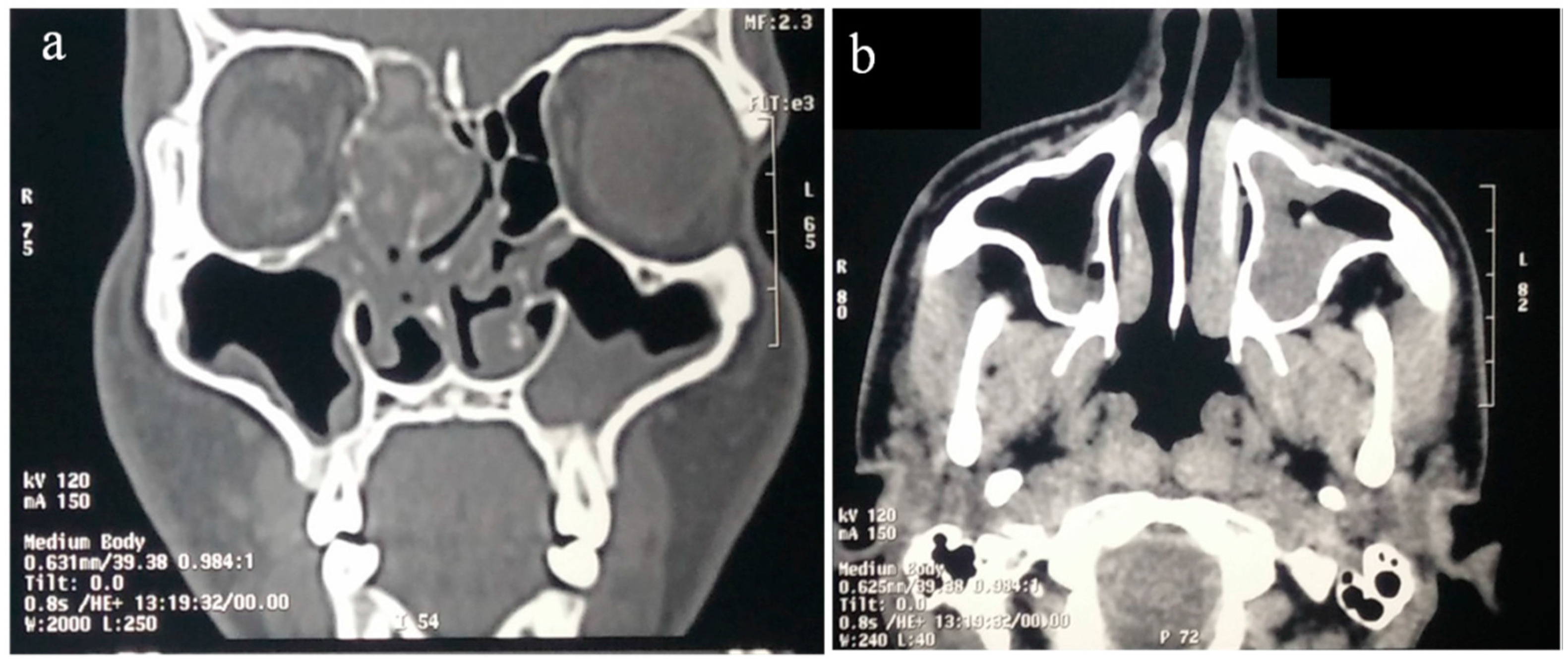
8.1. Imaging
8.2. Microbiology
- Microscopy: The eosinophilic mucin and debris of sinus contents demonstrate fungal hyphae on direct KOH mount or more sensitive calcoflour white stain.
- Culture: Culture of sinus contents shows positive results in 10%–93% of AFRS cases [117,226,227]. However, growth of fungus in culture media does not always signify AFRS, as fungi are ubiquitous and may give false-positive results. Ponikau et al. demonstrated 100% positive-culture results in both patients and controls with an average of 2.3 organisms per host [22]. A negative culture does not rule out AFRS and a positive culture may represent environmental contamination. Thus, culture results act as mere supportive evidence for AFRS.
- Serology: Type I hypersensitivity to fungi is demonstrated by either ImmunoCAP or skin prick test, the former being more specific and having higher negative predictive value [228]. It is observed that AFRS patients possess high levels of specific IgE to multiple fungi which may aid in differentiating them from other CRS cases [112]. Total IgE in these patients is often more than 1000 IU/mL [99]. The role of fungal-specific IgG in diagnosis of AFRS is uncertain as it is also elevated in other varieties of AFRS. Fungal-specific precipitins may also be observed in 85% of AFRS patients [16]. However, the role of allergy is still questionable in AFRS. All patients may not display increased IgE levels or a positive skin test [94].
- Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS): It allows protein profiling of serum and identifies AFRS patients with sensitivity of 84% and specificity of 90% [229]. However, the routine application of this technique is not yet recommended.
- Molecular test: A PCR using ITS1/ITS2 performed directly on samples from CRS patients demonstrated sensitivity of 100% confirming its superiority over culture and also allows accurate identification by sequencing [230].
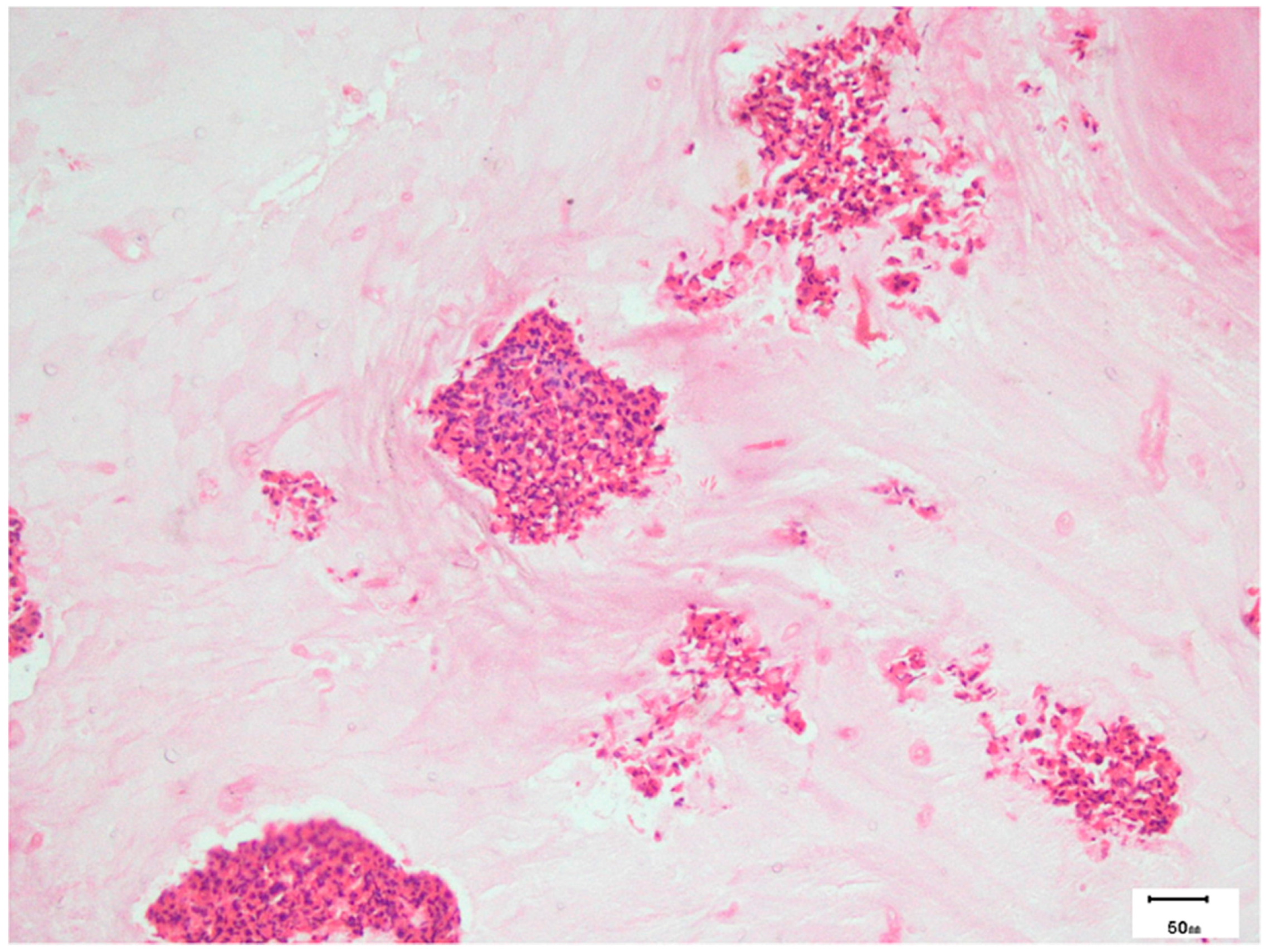
8.3. Pathology
9. Management
9.1. Surgical
9.2. Medical Therapy
- Saline irrigations: If given both pre- and postoperatively, the saline irrigations aid in softening and debriding thick mucoid secretions and improve mucociliary function of epithelium [94].
- Corticosteroids: Similar to surgical therapy, oral steroids are the mainstay of management of AFRS and have a significant role postoperatively in reducing recurrence and inflammatory markers, and ultimately improving the outcome in these patients. They may even obviate the need of revision surgery [107,189,234]. Gan et al. reviewed the available literature and found four studies (two level 2b and two level 4 studies), which looked into the benefits of oral steroids in AFRS and recommended the use of tapering doses of oral steroid [232]. The benefit of oral steroids in AFRS was first demonstrated in retrospective case series by Kupferberg et al. and Kuhn and Javer [237,238]. Woodworth et al. observed better SNOT-20 and nasal endoscopic scores and diminished levels of IL-3, IL-5, eotaxin, and monocyte chemoattractant protein-4 (MCP-4) when oral prednisolone was used [239]. Landsberg et al. demonstrated the radiologic and endoscopic benefits of preoperative administration of oral steroids in AFRS patients as compared to other CRSwNP cases, although the number of AFRS patients was low [240]. Their use in preoperative period helps in removing mechanical obstruction and that helps in viewing sinonasal anatomy during FESS [3]. Rupa et al. showed significant improvement in symptoms and polyp resolution in patients who received prednisolone after FESS as compared to placebo group [241]. Complete disease-free state was confirmed by nasal endoscopy in 100% patients who received oral steroids for 12 weeks. They recommended administration of postoperative oral steroid therapy for at least 12 weeks in AFRS patients. However, the exact dosage (0.4–1 mg/kg/day) and duration of oral therapy depend on the severity of symptoms and surgical outcome and need to be assessed in larger RCTs [232]. Ikram et al. noted the recurrence rate was reduced to 15% from 50% when surgery with medical therapy were combined [242]. Although the steroids have shown significant benefit in AFRS patients, their prolonged use is associated with adverse effects. On the contrary, topical corticosteroids possess a better safety profile and have shown benefit in the form of decreased polyp size and recurrence when added to local saline irrigation [189]. Rudmik et al. strongly recommended the use of standard topical steroids in patients with CRS supported by grade A evidence (well-designed randomized controlled trials (RCTs) exist and are strongly recommended) [232,243]. The evidence-based review by Gan et al. and European position paper from 2012 concluded that level 1a evidence (well-designed randomized controlled trials (RCTs) exist and are strongly recommended) exists for use of topical steroids in patients with CRSwNP although literature of their use is scarce [232]. The Food and Drug Administration (FDA) also approved the same. However, non-FDA-approved steroids should be used cautiously and restricted to refractory cases only [232].
- Antifungal therapy: There is a lack of evidence for any recommendation of oral or topical antifungal agents for AFRS [3,232]. It may be considered as an option in post-surgical refractory patients with a category C recommendation (recommended on the basis of observation studies in the form of case control and cohort) [3,232]. They may provide benefit in terms of reduction of symptoms, steroid dependence, and tendency of recurrences such as ABPA [232]. Patro et al. recently demonstrated a significant decrease in SNOT-20 and Lund Mackay scores, reduction in polyp size, fungal burden and opacification in AFRS patients who were given preoperative itraconazole for a month [244]. Similarly, Seiberling and Wornald et al. showed good response in 83% of patients using oral itraconazole 100 mg BD for 6 months after FESS [245]. Kupferberg et al. noted improved endoscopic scoring when oral antifungals were administered to AFRS patients while decreased recurrence (around 50%) and revision surgery (around 20%) were reported by Rains and Mineck using oral itraconazole [246]. Jen et al. also supported the benefits of a topical antifungal medication [247]. However, the benefits of antifungal use still need to be assessed over the adverse effects associated with systemic therapy. In addition, large, well-designed RCTs are required for proving the same.
- Immunotherapy: It aims at combating the activated adaptive immune response in AFRS patients. In 1998, Ferguson et al. described the role of immunotherapy in AFRS in a retrospective review of seven patients; five patients received immunotherapy before surgery and showed no improvement. However, the remaining two patients who were administered immunotherapy after the surgery showed good response, thereby suggesting the role of postoperative immunotherapy [248]. Following this, many reports supported the use of immunotherapy [249,250,251]. Mabry et al. concluded that immunotherapy resulted in decreased nasal crusting, decreased requirement of oral/topical steroids after 2 months and revision surgery up to 28 months follow up [249,250,251]. Folker et al. further noted overall improvement in endoscopic mucosal staging, quality of life and decreased need of steroid after 6–8 weeks’ postoperative immunotherapy [252]. Bassichis et al. also found similar results in addition to decreased need of revision surgery [253]. However, Marple et al. in 2002 failed to show any significant benefit of immunotherapy, thereby questioning its role in management [254]. Its use in the form of subcutaneous application is devoid of any local or systemic side effects [255]. Therefore, immunotherapy may serve as adjunct therapy in refractory cases without any unusual adverse event or formation of immune complexes although the data is limited to case reports and retrospective studies [232]. With the level of evidence C (only observation studies in the form of case control and cohort available), its recommendation is still challenging [232].
- Leukotriene modulators: There is no controlled study available regarding use of these agents in AFRS. There is only one case report of successful postoperative management of AFRS with montelukast 10 mg daily along with topical corticosteroids [256]. However, these agents have shown mixed results in other types of CRSwNP with either improved symptoms and CT scores or no benefit in comparison to steroids [233].
- Others: Anti IL-5 antibody (mepolizumab) may help to reduce polyp size and sinus opacification, as observed in a randomized controlled trial (RCT) [257]. However, the role of reslizumab in nasal polyposis is still being explored [258]. Gan et al. administered omalizumab, which binds selectively to IgE causing decrease in its levels of both serum and tissue in seven refractory cases of AFRS [259]. They observed 31% improvement in Sino-Nasal Outcome Test-22 (SNOT-22) score (52.14 decreased to 35.86) and 61% improvement in Phillpott-Javer endoscopic score (36 to 14). Omalizumab therapy also reduced the dependence of AFRS patients on corticosteroid and antifungal treatments [232]. There is also a case report of successful outcome of AFRS refractory to FESS and systemic corticosteroids with omalizumab [260]. In addition, antibacterial therapy like mupirocin has been proposed for local use to reduce both planktonic and biofilm forms of S. aureus which act as disease-modifying agents [261]. However, lack of evidence creates a dilemma about its use. Other possible therapeutic targets include TSLP inhibitors and P glycoprotein inhibitors [178,262].
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chatterjee, S.S.; Chakrabarti, A. Epidemiology and medical mycology of fungal rhinosinusitis. Otorhinolaryngol. Clin. An. Int. J. 2009, 1, 1–13. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Panda, N.K. Overview of fungal rhinosinusitis. Indian J. Otolaryngol. Head Neck Surg. 2004, 56, 251–258. [Google Scholar] [PubMed]
- Rodrigues, J.; Caruthers, C.; Azmeh, R.; Dykewicz, M.S.; Slavin, R.G.; Knutsen, A.P. The spectrum of allergic fungal diseases of the upper and lower airways. Expert Rev. Clin. Immunol. 2016, 12, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.J. Preliminary report on aspergillus mycosis of the antrum maxillare. John Hopkins Hosp. Bull. 1893, 4, 9–10. [Google Scholar]
- Schubert, P. Zur Casulshk der. Asperglllus mycosen. Dtsch Arch. Khn Med. 1885, 36, 162–179. [Google Scholar]
- Oppe, W. Zur KentnIss der schmalnelmykosen belden Menschen. Zbl Allg Path 1987, 8, 301–306. [Google Scholar]
- Baker, R.D. Mucormycosis: A new disease? JAMA 1957, 163, 805–808. [Google Scholar] [CrossRef]
- McGill, T.J.; Simpson, G.; Healy, G.B. Fulminant aspergillosis of the nose and paranasal sinuses: A new clinical entity. Laryngoscope 1980, 90, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Hora, J.F. Primary aspergillosis of the paranasal sinuses and associated areas. Laryngoscope 1965, 75, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Milosev, B.; El-Mahgoub, S.; Aal, O.A.; El-Hassan, A.M. Primary aspergilloma of paranasal sinuses in the Sudan. A review of seventeen cases. Br. J. Surg 1969, 56, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Finby, N.; Begg, C.F. Aspergilloma of sinus. N. Y. J. Med. 1972, 72, 493–495. [Google Scholar]
- Safirstein, B.H. Allergic bronchopulmonary aspergillosis with obstruction of the upper respiratory tract. Chest 1976, 70, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.W.; Johnston, A.L.D. Allergic aspergillosis of the maxillary sinuses. Thorax 1981, 36, 710. [Google Scholar]
- Katzenstein, A.L.A.; Sale, S.R.; Greenberger, P.A. Allergic Aspergillus sinusitis: A newly recognized form of sinusitis. J. Allergy Clin. Immunol. 1983, 72, 89–93. [Google Scholar] [CrossRef]
- Manning, S.C.; Vuitch, F.; Weinberg, A.G.; Brown, O.E. Allergic aspergillosis: A newly recognized form of sinusitis in the pediatric population. Laryngoscope 1989, 99, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Waxman, J.E.; Spector, J.G.; Sale, S.R.; Katzenstein, A.L. Allergic Aspergillus sinusitis: Concepts in diagnosis and treatment of a new clinical entity. Laryngoscope 1987, 97, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Allphin, A.L.; Strauss, M.; Abdul-Karim, F.W. Allergic fungal sinusitis: Problems in diagnosis and treatment. Laryngoscope 1991, 101, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.M.; Hogan, P.G.; Benn, R.A.; Gatenby, P.A. Allergic fungal sinusitis presenting as a paranasal sinus tumour. Intern. Med. J. 1989, 19, 351–353. [Google Scholar] [CrossRef]
- Slavin, R.G. Sinusitis: Viral, bacterial, or fungal and what is the role of staph? Allergy Asthma Proc. 2006, 27, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.W. Allergic fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 2011, 44, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Cody, D.T.; Neel, H.B.; Ferreiro, J.A.; Roberts, G.D. Allergic fungal sinusitis: The Mayo clinic experience. Laryngoscope 1994, 104, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Ponikau, J.U.; Sherris, D.A.; Kern, E.B.; Homburger, H.A.; Frigas, E.; Gaffey, T.A.; Roberts, G.D. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin. Proc. 1999, 74, 877–884. [Google Scholar] [CrossRef] [PubMed]
- DeShazo, R.D.; Chapin, K.; Swain, R.E. Fungal sinusitis. N. Engl. J. Med. 1997, 337, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Das, A.; Panda, N.K. Controversies surrounding the categorization of fungal sinusitis. Med. Mycol. 2009, 47, S299–S308. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Denning, D.W.; Ferguson, B.J.; Ponikau, J.; Buzina, W.; Kita, H.; Marple, B.; Panda, N.; Vlaminck, S.; Kauffmann-Lacroix, C.; et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope 2009, 119, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J. Eosinophilic mucin rhinosinusitis: A distinct clinicopathological entity. Laryngoscope 2000, 110, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J. Mucormycosis of the nose and paranasal sinuses. Otolaryngol. Clin. N. Am. 2000, 33, 349–365. [Google Scholar] [CrossRef]
- Adelson, R.T.; Marple, B.F. Fungal rhinosinusitis: State-of-the-art diagnosis and treatment. J. Otolaryngol. 2005, 34, S18–S23. [Google Scholar] [PubMed]
- Veress, B.; Malik, O.A.; El-Tayeb, A.A.; El-Daoud, S.; Mahgoub, E.S.; El-Hassan, A.M. Further observations on the primary paranasal aspergillus granuloma in the Sudan: A morphological study of 46 cases. Am. J. Trop. Med. Hyg. 1973, 22, 765–772. [Google Scholar] [PubMed]
- DeShazo, R.D.; O’Brien, M.; Chapin, K.; Soto-Aguilar, M.; Gardner, L.; Swain, R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Milroy, C.M.; Blanshard, J.D.; Lucas, S.; Michaels, L. Aspergillosis of the nose and paranasal sinuses. J. Clin. Pathol. 1989, 42, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, P.; Weber, R. Fungus balls of the paranasal sinuses: A review. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Hejjaoui, A.; Horst, V.; Michel, F.B.; Bousquet, J. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J. Allergy Clin. Immunol. 1990, 85, 460–472. [Google Scholar] [CrossRef]
- Marple, B.F. Allergic fungal rhinosinusitis: Current theories and management strategies. Laryngoscope 2001, 111, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- DeShazo, R.D.; Swain, R.E. Diagnostic criteria for allergic fungal sinusitis. J. Allergy Clin. Immunol. 1995, 96, 24–35. [Google Scholar] [CrossRef]
- Braun, H.; Buzina, W.; Freudenschuss, K.; Beham, A.; Stammberger, H. Eosinophilic fungal rhinosinusitis: A common disorder in Europe? Laryngoscope 2003, 113, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Polzehl, D.; Weschta, M.; Podbielski, A.; Riechelmann, H.; Rimek, D. Fungus culture and PCR in nasal lavage samples of patients with chronic rhinosinusitis. J. Med. Microbiol. 2005, 54, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ponikau, J.U.; Sherris, D.A.; Congdon, D.; Frigas, E.; Homburger, H.A.; Swanson, M.C.; Gleich, G.J.; Kita, H. Chronic rhinosinusitis: An enhanced immune response to ubiquitous airborne fungi. J. Allergy Clin. Immunol. 2004, 114, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, F.A.; Georgalas, C.; Fokkens, W.J. Fungus as the cause of chronic rhinosinusitis: The case remains unproven. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 43–49. [Google Scholar] [PubMed]
- DeShazo, R.D. The fungal conundrum. Ann. Allergy. Asthma Immunol. 2006, 96, 256–257. [Google Scholar] [PubMed]
- Ebbens, F.A.; Fokkens, W.J. The mold conundrum in chronic rhinosinusitis: Where do we stand today? Curr. Allergy Asthma Rep. 2008, 8, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; van Drunen, C.; Georgalas, C.; Ebbens, F. Role of fungi in pathogenesis of chronic rhinosinusitis: The hypothesis rejected. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Ponikau, J.U.; Sherris, D.A.; Kern, E.B.; Gaffey, T.A.; Kephart, G.; Kita, H. Detection of fungal organisms in eosinophilic mucin using a fluorescein-labeled chitin-specific binding protein. Otolaryngol. Head Neck Surg. 2002, 127, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Mathers, P.H.; Ramadan, H.H. Detection of fungi in the sinus mucosa using polymerase chain reaction. Otolaryngol. Head Neck Surg. 2006, 134, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Choi, J.H.; Jeon, H.G.; Cha, H.E.; Hwang, Y.J.; Chuang, Y.S. Comparison between polymerase chain reaction and fungal culture for the detection of fungi in patients with chronic sinusitis and normal controls. Acta Otolaryngol. 2005, 125, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Panda, N.K.; Chakrabarti, A.; Das, A.; Bapuraj, R.J. Allergic fungal rhinosinusitis: An attempt to resolve the diagnostic dilemma. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kale, P.; Rudramurthy, S.M.; Panda, N.K.; Das, A.; Chakrabarti, A. The inflammatory response of eosinophil-related fungal rhinosinusitis varies with inciting fungi. Med. Mycol. 2015, 53, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S.; Goetz, D.W. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J. Allergy Clin. Immunol. 1998, 102, 387–394. [Google Scholar] [CrossRef]
- Thakar, A.; Sarkar, C.; Dhiwakar, M.; Bahadur, S.; Dahiya, S. Allergic fungal sinusitis: Expanding the clinicopathologic spectrum. Otolaryngol. Head Neck Surg. 2004, 130, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Klapper, S.R.; Lee, A.G.; Patrinely, J.R.; Stewart, M.; Alford, E.L. Orbital involvement in allergic fungal sinusitis. Ophthalmology 1997, 104, 2094–2100. [Google Scholar] [CrossRef]
- Das, A.; Bal, A.; Chakrabarti, A.; Panda, N.; Joshi, K. Spectrum of fungal rhinosinusitis; Histopathologist’s perspective. Histopathology 2009, 54, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.; Adusumilli, V.; Corey, J.P. Fungal sinusitis: Progression of disease in immunosuppression—A case report. Ear Nose Throat J. 1998, 77, 207–210. [Google Scholar] [PubMed]
- Sarti, E.J.; Lucente, F.E. Aspergillosis of the paranasal sinuses. Ear Nose Throat J. 1988, 67, 824, 826–831. [Google Scholar]
- Rowe-Jones, J. Paranasal aspergillosis—A spectrum of disease. J. Laryngol. Otol. 1993, 107, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Rowe-Jones, J.M.; Moore-Gillon, V. Destructive noninvasive paranasal sinus aspergillosis: Component of a spectrum of disease. J. Otolaryngol. 1994, 23, 92–96. [Google Scholar] [PubMed]
- Uri, N.; Cohen-Kerem, R.; Elmalah, I.; Doweck, I.; Greenberg, E. Classification of fungal sinusitis in immunocompetent patients. Otolaryngol. Head Neck Surg. 2003, 129, 372–378. [Google Scholar] [CrossRef]
- Lu-Myers, Y.; Deal, A.M.; Miller, J.D.; Thorp, B.D.; Sreenath, S.B.; McClurg, S.M.; Senior, B.A.; Zanation, A.M.; Ebert, C.S., Jr. Comparison of socioeconomic and demographic factors in patients with chronic rhinosinusitis and allergic fungal rhinosinusitis. Otolaryngol. Head Neck Surg. 2015, 153, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, D.L. Allergic fungal rhinitis and rhinosinusitis. Proc. Am. Thorac. Soc. 2010, 7, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Ebbens, F.; van Drunen, C.M. Fungus: A Role in pathophysiology of chronic rhinosinusitis, disease modifier, A treatment target, or no role at all? Immunol. Allergy Clin. N. Am. 2009, 29, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Marple, B.F. Fungus and chronic rhinosinusitis: Weighing the evidence. Otolaryngol. Head Neck Surg. 2010, 143, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Rank, M.A.; Adolphson, C.R.; Kita, H. Antifungal therapy for chronic rhinosinusitis: The controversy persists. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Porter, P.C.; Ongeri, V.; Luong, A.; Kheradmand, F.; Corry, D.B. Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol. 2011, 32, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Foreman, A.; Psaltis, A.J.; Tan, L.W.; Wormald, P.J. Characterization of bacterial and fungal biofilms in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2009, 23, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.D.; Ramakrishnan, V.; Palmer, J.N. Biofilms. Otolaryngol. Clin. N. Am. 2010, 43, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Ebbens, F.A.; Scadding, G.K.; Badia, L.; Hellings, P.W.; Jorissen, M.; Mullol, J.; Cardesin, A.; Bachert, C.; van Zele, T.P.J.; Dijkgraaf, M.G.W.; et al. Amphotericin B nasal lavages: Not a solution for patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2006, 118, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, I.; Fittler, A.; Mayer, A.; Patzko, A.; Fonay, F.; Pytel, J.; Botz, L. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis. Can recidive polyposis be prevented? Amphotericin B-Tartalmu Orrspray Posztoperativ Alk 2008, 149, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.L.; Su, M.C.; Shiao, J.Y.; Tseng, H.C.; Hsin, C.H.; Lin, J.F.; Jiang, R.S. Amphotericin B irrigation for the treatment of chronic rhinosinusitis without nasal polyps: A randomized, placebo-controlled, double-blind study. Am. J. Rhinol. 2008, 22, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ponikau, J.U.; Sherris, D.A.; Weaver, A.; Kita, H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: A randomized, placebo-controlled, double-blind pilot trial. J. Allergy Clin. Immunol. 2005, 115, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Weschta, M.; Rimek, D.; Formanek, M.; Polzehl, D.; Podbielski, A.; Riechelmann, H. Topical antifungal treatment of chronic rhinosinusitis with nasal polyps: A randomized, double-blind clinical trial. J. Allergy Clin. Immunol. 2004, 113, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.W.; Kuhn, F.A.; Hamilos, D.L.; Zinreich, S.J.; Butler, D.; Warsi, G.; Poster, P.J.; Tavakkol, A. Treatment of chronic rhinosinusitis with high-dose oral terbinafine: A double blind, placebo-controlled study. Laryngoscope 2005, 115, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Sacks, P.L.; Harvey, R.J.; Rimmer, J.; Gallagher, R.M.; Sacks, R. Topical and systemic antifungal therapy for the symptomatic treatment of chronic rhinosinusitis. Cochrane Database Syst. Rev. 2011, 36, 2183. [Google Scholar]
- Schiller, J.S.; Lucas, J.W.; Ward, B.W.; Peregoy, J.A. Summary health statistics for U.S. Adults: National health interview survey, 2012. Vital Heal. Stat. 2012, 10, 1–171. [Google Scholar]
- Chakrabarti, A.; Rudramurthy, S.M.; Panda, N.; Das, A.; Singh, A. Epidemiology of chronic fungal rhinosinusitis in rural India. Mycoses 2015, 58, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Pleis, J.R.; Ward, B.W.; Lucas, J.W. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat. 2010, 10, 1–207. [Google Scholar]
- Bachert, C.; Holtappels, G. Pathophysiology of chronic rhinosinusitis, pharmaceutical therapy options. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2015, 94, S32–S63. [Google Scholar]
- Thorp, B.D.; McKinney, K.A.; Rose, A.S.; Ebert, C.S. Allergic fungal sinusitis in children. Otolaryngol. Clin. N. Am. 2012, 45, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Lanza, D.C.; Dhong, H.J.; Tantilipikorn, P.; Tanabodee, J.; Nadel, D.M.; Kennedy, D.W. Fungus and chronic rhinosinusitis: From bench to clinical understanding. Ann. Otol. Rhinol. Laryngol. 2006, 115, 27–34. [Google Scholar]
- Chakrabarti, A.; Sharma, S.C.; Chandler, J. Epidemiology and pathogenesis of paranasal sinus mycoses. Otolaryngol. Head Neck Surg. 1992, 107, 745–750. [Google Scholar] [PubMed]
- Ferguson, B.J.; Barnes, L.; Bernstein, J.M.; Brown, D.; Clark, C.E.; Cook, P.R.; DeWitt, W.S.; Graham, S.M.; Gordon, B.; Javer, A.R.; et al. Geographic variation in allergic fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 2000, 33, 441–449. [Google Scholar] [CrossRef]
- Chhabra, A.; Handa, K.K.; Chakrabarti, A.; Mann, S.B.; Panda, N. Allergic fungal sinusitis: Clinicopathological characteristics. Mycoses 1996, 39, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Panda, N.K.; Sharma, S.C.; Chakrabarti, A.; Mann, S.B.S. Paranasal sinus mycoses in north India. Mycoses 1998, 41, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Dhiwakar, M.; Thakar, A.; Bahadur, S.; Sarkar, C.; Banerji, U.; Handa, K.K.; Chhabra, S.K. Preoperative diagnosis of allergic fungal sinusitis. Laryngoscope 2003, 113, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Taj-Aldeen, S.J.; Hilal, A.A.; Schell, W.A. Allergic fungal rhinosinusitis: A report of 8 cases. Am. J. Otolaryngol. 2004, 25, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Michael, R.C.; Michael, J.S.; Ashbee, R.H.; Mathews, M.S. Mycological profile of fungal sinusitis: An audit of specimens over a 7-year period in a tertiary care hospital in Tamil Nadu. Indian J. Pathol. Microbiol. 2008, 51, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Rupa, V.; Jacob, M.; Mathews, M.S.; Job, A.; Kurien, M.; Chandi, S.M. Clinicopathological and mycological spectrum of allergic fungal sinusitis in South India. Mycoses 2002, 45, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Prateek, S.; Banerjee, G.; Gupta, P.; Singh, M.; Goel, M.M.; Verma, V. Fungal rhinosinusitis: A prospective study in a university hospital of Uttar Pradesh. Indian J. Med. Microbiol. 2013, 31, 266–269. [Google Scholar] [PubMed]
- Aeumjaturapat, S.; Saengpanich, S.; Isipradit, P.; Keelawat, S. Eosinophilic mucin rhinosinusitis: Terminology and clinicopathological presentation. J. Med. Assoc. Thail. 2003, 86, 420–424. [Google Scholar] [PubMed]
- Goh, B.S.; Singh Gendeh, B.; Mohamed Rose, I.; Pit, S.; Abdul Samad, S. Prevalence of allergic fungal sinusitis in refractory chronic rhinosinusitis in adult Malaysians. Otolaryngol. Head Neck Surg. 2005, 133, 27–31. [Google Scholar] [CrossRef] [PubMed]
- White, L.C.; Jang, D.W.; Yelvertan, J.C.; Kountakis, S.E. Bony erosion patterns in patients with allergic fungal sinusitis. Am. J. Rhinol. Allergy 2015, 29, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.K.; Rogers, G.A.; Ghegan, M.D.; Harvey, R.J.; DelGaudio, J.M.; Schlosser, R.J. Radiologic staging system for allergic fungal rhinosinusitis (AFRS). Otolaryngol. Head Neck Surg. 2009, 140, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Zinreich, S.J.; Kennedy, D.W.; Malat, J.; Curtin, H.D.; Epstein, J.I.; Huff, L.C.; Kumar, A.J.; Johns, M.E.; Rosenbaum, A.E. Fungal sinusitis: Diagnosis with CT and MR imaging. Radiology 1988, 169, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ghegan, M.D.; Lee, F.S.; Schlosser, R.J. Incidence of skull base and orbital erosion in allergic fungal rhinosinusitis (AFRS) and non-AFRS. Otolaryngol. Head Neck Surg. 2006, 134, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.K.; Ghegan, M.D.; Gorham, E.; Schlosser, R.J. Socioeconomic factors in the diagnosis of allergic fungal rhinosinusitis. Otolaryngol. Head Neck Surg. 2008, 138, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Loftus, P.A.; Wise, S.K. Allergic fungal rhinosinusitis: The latest in diagnosis and management. Adv. Otorhinolaryngol. 2016, 79, 13–20. [Google Scholar] [PubMed]
- Schubert, M.S.; Hutcheson, P.S.; Graff, R.J.; Santiago, L.; Slavin, R.G. HLA-DQB1*03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. J. Allergy Clin. Immunol. 2004, 114, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J. Definitions of fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 2000, 33, 227–235. [Google Scholar] [CrossRef]
- Manning, S.C.; Holman, M. Further evidence for allergic pathophysiology in allergic fungal sinusitis. Laryngoscope 1998, 108, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Montone, K.T.; Livolsi, V.A.; Feldman, M.D.; Palmer, J.; Chiu, A.G.; Lanza, D.C.; Kennedy, D.W.; Loevner, L.A.; Nachamkin, I. Fungal rhinosinusitis: A retrospective microbiologic and pathologic review of 400 patients at a single university medical center. Int. J. Otolaryngol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.; Amedee, R.G. Allergic fungal rhinosinusitis: A review. Ochsner J. 2011, 11, 271–275. [Google Scholar] [PubMed]
- Patro, S.K.; Verma, R.K.; Panda, N.K.; Chakrabarti, A. Understanding paediatric allergic fungal sinusitis: Is it more aggressive? Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1876–1880. [Google Scholar] [CrossRef] [PubMed]
- Kupferberg, S.B.; Bent, J.P. Allergic fungal sinusitis in the pediatric population. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Marple, B.F.; Gibbs, S.R.; Newcomer, M.T.; Mabry, R.L. Allergic fungal sinusitis-induced visual loss. Am. J. Rhinol. 1999, 13, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Al-Radadi, A.M.; Alnoury, K.I. Optic chiasma involvement secondary to allergic fungal rhinosinusitis. J. Pak. Med. Assoc. 2011, 61, 704–707. [Google Scholar] [PubMed]
- Illing, E.A.; Dunlap, Q.; Woodworth, B.A. Outcomes of pressure-induced cranial neuropathies from allergic fungal rhinosinusitis. Otolaryngol. Head Neck Surg. 2015, 152, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.C.; Schaefer, S.D.; Close, L.G.; Vuitch, F. Culture-positive allergic fungal sinusitis. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, J.F.; Eastwood, J.D.; Kilani, R.K. Intracranial abscess as a complication of allergic fungal sinusitis. J. Neuroimaging 2014, 24, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Patadia, M.O.; Welch, K.C. Role of immunotherapy in allergic fungal rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.; Macardle, P. CD8+ T cells implicated in the pathogenesis of allergic fungal rhinosinusitis. Allergy Rhinol. 2014, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S. Allergic fungal sinusitis: Pathophysiology, diagnosis and management. Med. Mycol. 2009, 47, S324–S330. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Nair, S.; Smith, W.; Kette, F.; Gillis, D.; Wormald, P.J. Role of local immunoglobulin E production in the pathophysiology of noninvasive fungal sinusitis. Laryngoscope 2004, 114, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.; Kette, F.E.; Smith, W.B.; Wormald, P.J.; Macardle, P.J. Fungal-specific humoral response in eosinophilic mucus chronic rhinosinusitis. Laryngoscope 2005, 115, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.E.; Hunsaker, D.H. Fungus-specific IgG and IgE in allergic fungal rhinosinusitis. Otolaryngol. Head Neck Surg. 2002, 127, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Feger, T.A.; Rupp, N.T.; Kuhn, F.A.; Ford, J.L.; Dolen, W.K. Local and systemic eosinophil activation in allergic fungal sinusitis. Ann. Allergy Asthma Immunol. 1997, 79, 221–225. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Hauser, L.J.; Frank, D.N. The sinonasal bacterial microbiome in health and disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, R.R.; Rupp, N.T.; Kuhn, F.A.; Phillips, A.E.; Dolen, W.K. Allergenic fungi in allergic fungal sinusitis. Ann. Allergy Asthma Immunol. 1997, 79, 431–435. [Google Scholar] [CrossRef]
- Knowles, M.R.; Boucher, R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.A.; Peiser, C.; Dinh, Q.T.; Matthias, J.; Eynott, P.R.; Heppt, W.; Carlstedt, I.; Witt, C.; Fischer, A.; Chung, K.F. Distribution of respiratory mucin proteins in human nasal mucosa. Laryngoscope 2003, 113, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.C.; Cope, E.; Chen, B.; Leid, J.G.; Cohen, N.A. Regulation of murine sinonasal cilia function by microbial secreted factors. Int. Forum Allergy Rhinol. 2012, 2, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Amitani, R.; Taylor, G.; Elezis, E.N.; Llewellyn-Jones, C.; Mitchell, J.; Kuze, F.; Cole, P.J.; Wilson, R. Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect. Immun. 1995, 63, 3266–3271. [Google Scholar] [PubMed]
- Blount, A.; Zhang, S.; Chestnut, M.; Hixon, B.; Skinner, D.; Sorscher, E.J.; Woodworth, B.A. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope 2011, 121, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; Cho, K.; Kim, D.W.; Han, D.H.; Khalmuratova, R.; Kim, S.W.; Jeon, S.Y.; Min, Y.G.; Lee, C.H.; Rhee, C.S.; et al. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am. J. Respir. Crit. Care Med. 2012, 185, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.M.; Abraham, V.; Spielman, D.; Kolls, J.K.; Rubenstein, R.C.; Conner, G.E.; Cohen, N.A.; Kreindler, J.L. IL-17A induces Pendrin expression and chloride-bicarbonate exchange in human bronchial epithelial cells. PLoS ONE 2014, 9, e103263. [Google Scholar] [CrossRef] [PubMed]
- Nofziger, C.; Vezzoli, V.; Dossena, S.; Schonherr, T.; Studnicka, J.; Nofziger, J.; Vanoni, S.; Stephan, S.; Silva, M.E.; Meyer, G.; et al. STAT6 links IL-4/IL-13 stimulation with pendrin expression in asthma and chronic obstructive pulmonary disease. Clin. Pharmacol. Ther. 2011, 90, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Nofziger, C.; Dossena, S.; Suzuki, S.; Izuhara, K.; Paulmichl, M. Pendrin function in airway epithelia. Cell. Physiol. Biochem. 2011, 28, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Lu, X.; Purkey, M.R.; Homma, T.; Choi, A.W.; Carter, R.; Suh, L.; Norton, J.; Harris, K.E.; Conley, D.B.; et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2015, 753, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Investig. 2014, 124, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Cohen, N.A. Sinonasal solitary chemosensory cells “taste” the upper respiratory environment to regulate innate immunity. Am. J. Rhinol. Allergy 2014, 28, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Wooding, S.; Ricci, D.; Jorde, L.B.; Drayna, D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum. Mutat. 2005, 26, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 2015, 136, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Den Beste, K.A.; Hoddeson, E.K.; Parkos, C.A.; Nusrat, A.; Wise, S.K. Epithelial permeability alterations in an in vitro air-liquid interface model of allergic fungal rhinosinusitis. Int. Forum Allergy Rhinol. 2013, 3, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pothoven, K.L.; Norton, J.E.; Hulse, K.E.; Suh, L.A.; Carter, R.G.; Rocci, E.; Harris, K.E.; Shintani-Smith, S.; Conley, D.B.; Chandra, R.K.; et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J. Allergy Clin. Immunol. 2015, 136. [Google Scholar] [CrossRef] [PubMed]
- Rudack, C.; Steinhoff, M.; Mooren, F.; Buddenkotte, J.; Becker, K.; Von Eiff, C.; Sachse, F. PAR-2 activation regulates IL-8 and GRO-α synthesis by NF-κB, but not RANTES, IL-6, eotaxin or TARC expression in nasal epithelium. Clin. Exp. Allergy 2007, 37, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-activated receptors: Contribution to physiology and disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.M.; Chen, H.C.; Pochard, P.; Eisenbarth, S.C.; Herrick, C.A.; Bottomly, H.K. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J. Immunol. 2010, 184, 3535–3544. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Kale, S. Airway epithelial cells: Barrier and much more. Indian J. Allergy Asthma Immunol. 2013, 27, 95. [Google Scholar] [CrossRef]
- Ramanathan, M.; Lee, W.-K.; Dubin, M.G.; Lin, S.; Spannhake, E.W.; Lane, A.P. Sinonasal epithelial cell expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am. J. Rhinol. 2007, 21, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Tengroth, L.; Arebro, J.; Kumlien Georén, S.; Winqvist, O.; Cardell, L.-O. Deprived TLR9 expression in apparently healthy nasal mucosa might trigger polyp-growth in chronic rhinosinusitis patients. PLoS ONE 2014, 9, e105618. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.F.; Tomee, J.F.; van de Riet, M.A.; Timmerman, A.J.; Borger, P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000, 105, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Ebert, C.S.; McKinney, K.A.; Urrutia, G.; Wu, M.; Rose, A.S.; Fleischman, G.M.; Thorp, B.; Senior, B.A.; Zanation, A.M. Expression of protease-activated receptors in allergic fungal rhinosinusitis. Int. Forum Allergy Rhinol. 2014, 4, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, F.; Amadesi, S.; Dabbagh, K.; Lewis, D.E.; Knott, P.; Bunnett, N.W.; Gater, P.R.; Geppetti, P.; Bertrand, C.; Stevens, M.E. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J. Immunol. 2002, 169, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Schleimer, R.; Kern, R.C. The etiology and pathogenesis of chronic rhinosinusitis: A review of current hypotheses. Curr. Allergy Asthma Rep. 2015, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Månsson, A.; Bogefors, J.; Cervin, A.; Uddman, R.; Cardell, L.O. NOD-like receptors in the human upper airways: A potential role in nasal polyposis. Allergy 2011, 66, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bogefors, J.; Rydberg, C.; Uddman, R.; Fransson, M.; Månsson, A.; Benson, M.; Adner, M.; Cardell, L.O. Nod1, Nod2 and Nalp3 receptors, new potential targets in treatment of allergic rhinitis? Allergy 2010, 65, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Saijo, S.; Iwakura, Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int. Immunol. 2011, 23, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Marr, K.A. Impact of Aspergillus fumigatus in allergic airway diseases. Clin. Transl. Allergy 2011, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.M.; Klein, B.S. Fungal glycan interactions with epithelial cells in allergic airway disease. Curr. Opin. Microbiol. 2013, 16, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [PubMed]
- Hayden, M.S.; West, A.P.; Ghosh, S. SnapShot: NF-κB signaling pathways. Cell 2006, 127, 1286–1287. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Q.; Li, Y.; Li, P.; Zhang, G.; Li, Y. Involvement of mitogen-activated protein kinases and nuclear factor κB pathways in signaling COX-2 expression in chronic rhinosinusitis. Inflamm. Res. 2009, 58, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases—Regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [PubMed]
- Herlaar, E.; Brown, Z. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today 1999, 5, 439–447. [Google Scholar] [CrossRef]
- Takeno, S.; Hirakawa, K.; Ueda, T.; Furukido, K.; Osada, R.; Yajin, K. Nuclear factor-κB activation in the nasal polyp epithelium: Relationship to local cytokine gene expression. Laryngoscope 2002, 112, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Homma, T.; Norton, J.E.; Sha, Q.; Siebert, J.; Gupta, D.S.; Schroeder, J.W., Jr.; Schleimer, R.P. Suppression of epithelial STAT1 activation by extracts of Aspergillus fumigatus. Am. J. Respir. Cell. Mol. Biol. 2014, 53, 1–33. [Google Scholar]
- Wolk, K.; Kunz, S.; Witte, E.; Friedrich, M.; Asadullah, K.; Sabat, R. IL-22 increases the innate immunity of tissues. Immunity 2004, 21, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.-A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; McAllister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Schleh, C.; Rothen-Rutishauser, B.M.; Blank, F.; Lauenstein, H.D.; Nassimi, M.; Krug, N.; Braun, A.; Erpenbeck, V.J.; Gehr, P.; Hohlfeld, J.M. Surfactant Protein D modulates allergen particle uptake and inflammatory response in a human epithelial airway model. Respir. Res. 2012, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodworth, B.A.; Lathers, D.; Neal, J.G.; Skinner, M.; Richardson, M.; Young, M.R.; Schlosser, R.J. Immunolocalization of surfactant protein A and D in sinonasal mucosa. Am. J. Rhinol. 2006, 20, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Tewfik, M.A.; Latterich, M.; DiFalco, M.R.; Samaha, M. Proteomics of nasal mucus in chronic rhinosinusitis. Am. J. Rhinol. 2007, 21, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, V.C.; Spector, S.L.; Ganz, T.; Cole, A.M. Lysozyme levels in the nasal secretions of patients with perennial allergic rhinitis and recurrent sinusitis. Ann. Allergy Asthma Immunol. 2004, 93, 288–292. [Google Scholar] [CrossRef]
- Seshadri, S.; Lin, D.C.; Rosati, M.; Carter, R.G.; Norton, J.E.; Suh, L.; Kato, A.; Chandra, R.K.; Harris, K.E.; Chu, H.W.; et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Prince, A. Innate immunity in the respiratory epithelium. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, A.J.; Bruhn, M.A.; Ooi, E.H.; Tan, L.W.; Wormald, P.J. Nasal mucosa expression of lactoferrin in patients with chronic rhinosinusitis. Laryngoscope 2007, 117, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Tarran, R.; Redinbo, M.R. Mammalian short palate lung and nasal epithelial clone 1 (SPLUNC1) in pH-dependent airway hydration. Int. J. Biochem. Cell. Biol. 2014, 52, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Tieu, D.D.; Peters, A.T.; Carter, R.T.; Suh, L.; Conley, D.B.; Chandra, R.; Norton, J.; Grammer, L.C.; Harris, K.E.; Kato, A.; et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2010, 125, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Lane, A.P. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2007, 136, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wijkstrom-Frei, C.; El-Chemaly, S.; Ali-Rachedi, R.; Gerson, C.; Cobas, M.A.; Forteza, R.; Salathe, M.; Conner, G.E. Lactoperoxidase and human airway host defense. Am. J. Respir. Cell. Mol. Biol. 2003, 29, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.; Mjösberg, J.; Spits, H. Th1- and Th2-like subsets of innate lymphoid cells. Immunol. Rev. 2013, 252, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.M.; Trifari, S.; Crellin, N.K.; Peters, C.P.; van Drunen, C.M.; Piet, B.; Fokkens, W.J.; Cupedo, T.; Spits, H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 2011, 12, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.; Fakhri, S.; Citardi, M.J.; Porter, P.C.; Corry, D.B.; Kheradmand, F.; Liu, Y.J.; Luong, A. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2013, 188, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.G.; Sabat, R.; Dumoutier, L.; Renauld, J.C.; Willart, M.; Lambrecht, B.; Teixeira, M.M.; Charron, S.; Fick, L.; Erard, F.; et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am. J. Respir. Crit. Care Med. 2011, 183, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hirose, K.; Kawashima, S.; Niwa, Y.; Wakashin, H.; Iwata, A.; Tokoyoda, K.; Renauld, J.C.; Iwamoto, I.; Nakayama, T.; et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J. Allergy Clin. Immunol. 2011, 128, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, D.R.; Poposki, J.A.; Tan, B.K.; Comeau, M.R.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Norton, J.; Harris, K.E.; Grammer, L.C.; et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2013, 132, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.S.; Bleier, B.S. Influence of P-Glycoprotein function on chronic rhinosinusitis/nasal polyps pathophysiology. Adv. Otorhinolaryngol. 2016, 79, 38–47. [Google Scholar] [PubMed]
- Olze, H.; Förster, U.; Zuberbier, T.; Morawietz, L.; Luger, E.O. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology 2006, 44, 145–150. [Google Scholar] [PubMed]
- Stevens, W.W.; Ocampo, C.J.; Berdnikovs, S.; Sakashita, M.; Mahdavinia, M.; Suh, L.; Takabayashi, T.; Norton, J.E.; Hulse, K.E.; Conley, D.B.; et al. Cytokines in chronic rhinosinusitis role in eosinophilia and aspirin-exacerbated respiratory disease. Am. J. Respir. Crit. Care Med. 2015, 192, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Kojima, Y.; Koyanagi, A.; Yokoi, H.; Saito, T.; Kawano, K.; Furukawa, M.; Kusunoki, T.; Ikeda, K. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope 2009, 119, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Cihakova, D. Alternatively activated macrophages in infection and autoimmunity. J. Autoimmun. 2009, 33, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K. Evaluation of classical, alternative, and regulatory functions of bone marrow-derived macrophages. Methods Mol. Biol. 2013, 1032, 79–89. [Google Scholar] [PubMed]
- Stevens, D.A.; Moss, R.B.; Kurup, V.P.; Knutsen, A.P.; Greenberger, P.; Judson, M.A.; Denning, D.W.; Crameri, R.; Brody, A.S.; Light, M.; et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—State of the art: Cystic fibrosis foundation consensus conference. Clin. Infect. Dis. 2003, 37, S225–S264. [Google Scholar] [CrossRef] [PubMed]
- Ayers, C.M.; Schlosser, R.J.; O’Connell, B.P.; Atkinson, C.; Mulligan, R.M.; Casey, S.E.; Bleier, B.S.; Wang, E.W.; Sansoni, E.R.; Kuhlen, J.L.; et al. Increased presence of dendritic cells and dendritic cell chemokines in the sinus mucosa of chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Int. Forum Allergy Rhinol. 2011, 1, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, J.K.; Bleier, B.S.; O’Connell, B.; Mulligan, R.M.; Wagner, C.; Schlosser, R.J. Vitamin D3 correlates inversely with systemic dendritic cell numbers and bone erosion in chronic rhinosinusitis with nasal polyps and allergic fungal rhinosinusitis. Clin. Exp. Immunol. 2011, 164, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Shahangian, A.; Schlosser, R.J. Role of Vitamin D in pathogenesis of chronic sinusitis with nasal polyposis. Adv. Otorhinolaryngol. 2016, 79, 86–90. [Google Scholar] [PubMed]
- Plonk, D.P.; Luong, A. Current understanding of allergic fungal rhinosinusitis and treatment implications. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Fujiwara, T.; Kariya, S.; Higaki, T.; Haruna, T.; Matsushita, O.; Noda, Y.; Makihara, S.; Kanai, K.; Noyama, Y.; et al. Cellular responses to Staphylococcus aureus α-toxin in chronic rhinosinusitis with nasal polyps. Allergol. Int. 2014, 63, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.R.; Silva, I.D.C.G.; Weckx, L.L.M. Inflammatory genes in nasal polyposis. Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.L.; Kraft, M. IL-13 in asthma and allergic disease: Asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol. 2012, 130, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Van Zele, T.; Perez-Novo, C.; Van Bruaene, N.; Holtappels, G.; DeRuyck, N.; Van Cauwenberge, P.; Bachert, C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J. Allergy Clin. Immunol. 2008, 122, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, M.; Suh, L.A.; Carter, R.G.; Stevens, W.W.; Norton, J.E.; Kato, A.; Tan, B.K.; Kern, R.C.; Conley, D.B.; Chandra, R.; et al. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation Asians suggest genetic regulation of eosinophilia. J. Allergy Clin. Immunol. 2015, 135, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Van Zele, T.; Gevaert, P.; Holtappels, G.; Van Cauwenberge, P.; Bachert, C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin. Exp. Allergy 2007, 37, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Peters, A.; Suh, L.; Carter, R.; Harris, K.E.; Chandra, R.; Conley, D.; Grammer, L.C.; Kern, R.; Schleimer, R.P. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2008, 121, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Hulse, K.E.; Norton, J.E.; Suh, L.; Zhong, Q.; Mahdavinia, M.; Simon, P.; Kern, R.C.; Conley, D.B.; Chandra, R.K.; Tan, B.K.; et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J. Allergy Clin. Immunol. 2013, 131, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Fang, S.Y. Tissue-specific immunoglobulin E in maxillary sinus mucosa of allergic fungal sinusitis. Rhinology 2008, 46, 226–230. [Google Scholar] [PubMed]
- Wise, S.K.; Ahn, C.N.; Lathers, D.M.R.; Mulligan, R.M.; Schlosser, R.J. Antigen-specific IgE in sinus mucosa of allergic fungal rhinosinusitis patients. Am. J. Rhinol. 2008, 22, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.N.; Wise, S.K.; Lathers, D.M.R.; Mulligan, R.M.; Harvey, R.J.; Schlosser, R.J. Local production of antigen-specific IgE in different anatomic subsites of allergic fungal rhinosinusitis patients. Otolaryngol. Head Neck Surg. 2009, 141, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pratt, E.; Collins, A.M.; Sewell, W.A.; Harvey, R.J. Antigen selection in IgE antibodies from individuals with chronic rhinosinusitis with nasal polyps. Am. J. Rhinol. Allergy 2010, 24, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S. A superantigen hypothesis for the pathogenesis of chronic hypertrophic rhinosinusitis, allergic fungal sinusitis, and related disorders. Ann. Allergy. Asthma Immunol. 2001, 87, 181–188. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Seethala, R.; Wood, W.A. Eosinophilic bacterial chronic rhinosinusitis. Laryngoscope 2007, 117, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.W.; Wenaas, A.; Luong, A.; Citardi, M.J.; Fakhri, S. Staphylococcus aureus prevalence in allergic fungal rhinosinusitis vs. other subsets of chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2013, 3, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Zhang, N.; Patou, J.; van Zele, T.; Gevaert, P. Role of staphylococcal superantigens in upper airway disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.M.; Kansal, R. Superantigen hypothesis for the early development of chronic hyperplastic sinusitis with massive nasal polyposis. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Van Bruaene, N.; Pérez-Novo, C.A.; Basinski, T.M.; Van Zele, T.; Holtappels, G.; De Ruyck, N.; Schmidt-Weber, C.; Akdis, C.; Van Cauwenberge, P.; Bachert, C.; et al. T-cell regulation in chronic paranasal sinus disease. J. Allergy Clin. Immunol. 2008, 121, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Zaravinos, A.; Soufla, G.; Bizakis, J.; Spandidos, D.A. Expression analysis of VEGFA, FGF2, TGFβ1, EGF and IGF1 in human nasal polyposis. Oncol. Rep. 2008, 19, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, T.; Kato, A.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Carter, R.; Norton, J.; Grammer, L.C.; Cho, S.H.; Tan, B.K.; et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am. J. Respir. Crit. Care Med. 2013, 187, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, T.; Kato, A.; Peters, A.T.; Hulse, K.E.; Suh, L.A.; Carter, R.; Norton, J.; Grammer, L.C.; Tan, B.K.; Chandra, R.K.; et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2013, 132, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Bent, J.P.; Kuhn, F.A. Diagnosis of allergic fungal sinusitis. Otolaryngol. Head Neck Surg. 1994, 111, 580–588. [Google Scholar] [CrossRef]
- Kuhn, F.R.S., Jr. Allergic fungal sinusitis: Diagnosis and treatment. Curr. Opin. Otolaryngol. Head 2003, 11, 1–5. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology 2012, 50, 1–298. [Google Scholar]
- Meltzer, E.O.; Hamilos, D.L.; Hadley, J.A.; Lanza, D.C.; Marple, B.F.; Nicklas, R.A.; Bachert, C.; Baraniuk, J.; Baroody, F.M.; Benninger, M.S.; et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. J. Allergy Clin. Immunol. 2004, 114, 155–212. [Google Scholar] [CrossRef] [PubMed]
- Loury, M.C.; Leopold, D.A.; Schaefer, S.D. Allergic Aspergillus sinusitis. Arch. Otolaryngol. Head Neck Surg. 1993, 119, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.M.; Javer, A.R.; Clark, A. Allergic fungal rhinosinusitis—A new staging system. Rhinology 2011, 49, 318–323. [Google Scholar] [PubMed]
- Hopkins, C.; Browne, J.P.; Slack, R.; Lund, V.; Brown, P. The Lund-Mackay staging system for chronic rhinosinusitis: How is it used and what does it predict? Otolaryngol. Head Neck Surg. 2007, 137, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar] [PubMed]
- Lund, V.J.; Kennedy, D.W. Staging for rhinosinusitis. Otolaryngol. Head Neck Surg. 1997, 117, S35–S40. [Google Scholar] [CrossRef]
- De Araújo Neto, S.A.; Baracat, E.C.E.; Felipe, L.F. A new score for tomographic opacification of paranasal sinuses in children. Braz. J. Otorhinolaryngol. 2010, 76, 491–498. [Google Scholar]
- Manning, S.C.; Merkel, M.; Kriesel, K.; Vuitch, F.; Marple, B. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope 1997, 107, 170–176. [Google Scholar] [CrossRef] [PubMed]
- McClay, J.E.; Marple, B.; Kapadia, L.; Biavati, M.J.; Nussenbaum, B.; Newcomer, M.; Manning, S.; Booth, T.; Schwade, N. Clinical presentation of allergic fungal sinusitis in children. Laryngoscope 2002, 112, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Aribandi, M.; McCoy, V.A.; Bazan, C. Imaging features of invasive and noninvasive fungal sinusitis: A review. Radiographics 2007, 27, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, C.; Shah, A. Allergic Aspergillus sinusitis and its association with allergic bronchopulmonary aspergillosis. Asia Pac. Allergy 2011, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Callejas, C.A.; Douglas, R.G. Fungal rhinosinusitis: What every allergist should know. Clin. Exp. Allergy 2013, 43, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.C.; Mabry, R.L.; Schaefer, S.D.; Close, L.G. Evidence of IgE-mediated hypersensitivity in allergic fungal sinusitis. Laryngoscope 1993, 103, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Calabria, C.W.; Dietrich, J.; Hagan, L. Comparison of serum-specific IgE (ImmunoCAP) and skin-prick test results for 53 inhalant allergens in patients with chronic rhinitis. Allergy Asthma Proc. 2009, 30, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Maeso, P.A.; Becker, A.M.; Prosser, J.D.; Adam, B.L.; Kountakis, S.E. Proteomics blood testing to distinguish chronic rhinosinusitis subtypes. Laryngoscope 2008, 118, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Comacle, P.; Belaz, S.; Jegoux, F.; Ruaux, C.; Le Gall, F.; Gangneux, J.P.; Robert-Gangneux, F. Contribution of molecular tools for the diagnosis and epidemiology of fungal chronic rhinosinusitis. Med. Mycol. 2016, 54, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S. Allergic fungal sinusitis: Pathogenesis and management strategies. Drugs 2004, 64, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Gan, E.C.; Thamboo, A.; Rudmik, L.; Hwang, P.H.; Ferguson, B.J.; Javer, A.R. Medical management of allergic fungal rhinosinusitis following endoscopic sinus surgery: An evidence-based review and recommendations. Int. Forum Allergy Rhinol. 2014, 4, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.K.; Hosemann, W. Comprehensive review on endonasal endoscopic sinus surgery. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2015, 14, Doc08.23. [Google Scholar] [PubMed]
- Cain, R.B.; Lal, D. Update on the management of chronic rhinosinusitis. Infect. Drug Resist. 2013, 6, 1–14. [Google Scholar] [PubMed]
- Soler, Z.M.; Sauer, D.; Mace, J.; Smith, T.L. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol. Head Neck Surg. 2010, 142, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Ishinaga, H.; Takeuchi, K. Pathogenesis of eosinophilic chronic rhinosinusitis. J. Inflamm. 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kupferberg, S.B.; Bent, J.P.; Kuhn, F.A. Prognosis for allergic fungal sinusitis. Otolaryngol. Head Neck Surg. 1997, 117, 35–41. [Google Scholar] [CrossRef]
- Kuhn, F.A.; Javer, A.R. Allergic fungal sinusitis: A four-year follow-up. Am. J. Rhinol. 2000, 14, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, B.A.; Joseph, K.; Kaplan, A.P.; Schlosser, R.J. Alterations in eotaxin, monocyte chemoattractant protein-4, interleukin-5, and interleukin-13 after systemic steroid treatment for nasal polyps. Otolaryngol. Head Neck Surg. 2004, 131, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, R.; Segev, Y.; DeRowe, A.; Landau, T.; Khafif, A.; Fliss, D.M. Systemic corticosteroids for allergic fungal rhinosinusitis and chronic rhinosinusitis with nasal polyposis: A comparative study. Otolaryngol. Head Neck Surg. 2007, 136, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Rupa, V.; Jacob, M.; Mathews, M.S.; Seshadri, M.S. A prospective, randomised, placebo-controlled trial of postoperative oral steroid in allergic fungal sinusitis. Eur. Arch. Otorhinolaryngol. 2010, 267, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Abbas, A.; Suhail, A.; Onali, M.A.; Akhtar, S.; Iqbal, M. Management of allergic fungal sinusitis with postoperative oral and nasal steroids: A controlled study. Ear Nose Throat J. 2009, 88, E8–E11. [Google Scholar] [PubMed]
- Rudmik, L.; Hoy, M.; Schlosser, R.J.; Harvey, R.J.; Welch, K.C.; Lund, V.; Smith, T.L. Topical therapies in the management of chronic rhinosinusitis: An evidence-based review with recommendations. Int. Forum Allergy Rhinol. 2013, 3, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Patro, S.K.; Verma, R.K.; Panda, N.K.; Chakrabarti, A.; Singh, P. Efficacy of preoperative itraconazole in allergic fungal rhinosinusitis. Am. J. Rhinol. Allergy 2013, 149, 299–304. [Google Scholar] [CrossRef]
- Seiberling, K.; Wormald, P.J. The role of itraconazole in recalcitrant fungal sinusitis. Am. J. Rhinol. Allergy 2009, 23, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Rains, B.M.; Mineck, C.W. Treatment of allergic fungal sinusitis with high-dose itraconazole. Am. J. Rhinol. 2003, 17, 1–8. [Google Scholar] [PubMed]
- Jen, A.; Kacker, A.; Huang, C.; Anand, V. Fluconazole nasal spray in the treatment of allergic fungal sinusitis: A pilot study. Ear Nose Throat J. 2004, 83, 694–695. [Google Scholar]
- Ferguson, B.J. What role do systemic corticosteroids, immunotherapy, and antifungal drugs play in the therapy of allergic fungal rhinosinusitis? Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Mabry, R.L.; Marple, B.F.; Folker, R.J.; Mabry, C.S. Immunotherapy for allergic fungal sinusitis: Three years’ experience. Otolaryngol. Head Neck Surg. 1998, 119, 648–651. [Google Scholar] [CrossRef]
- Mabry, R.L.; Manning, S.C.; Mabry, C.S. Immunotherapy in the treatment of allergic fungal sinusitis. Otolaryngol. Head Neck Surg. 1997, 116, 31–35. [Google Scholar] [CrossRef]
- Mabry, R.L.; Mabry, C.S. Allergic fungal sinusitis: The role of immunotherapy. Otolaryngol. Clin. N. Am. 2000, 33, 433–440. [Google Scholar] [CrossRef]
- Folker, R.J.; Marple, B.F.; Mabry, R.L.; Mabry, C.S. Treatment of allergic fungal sinusitis: A comparison trial of postoperative immunotherapy with specific fungal antigens. Laryngoscope 1998, 108, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Bassichis, B.A.; Marple, B.F.; Mabry, R.L.; Newcomer, M.T.; Schwade, N.D. Use of immunotherapy in previously treated patients with allergic fungal sinusitis. Otolaryngol. Head Neck Surg. 2001, 125, 487–490. [Google Scholar] [CrossRef]
- Marple, B.; Newcomer, M.; Schwade, N.; Mabry, R. Natural history of allergic fungal rhinosinusitis: A 4- to 10-year follow-up. Otolaryngol. Head Neck Surg. 2002, 127, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Greenhaw, B.; deShazo, R.D.; Arnold, J.; Wright, L. Fungal immunotherapy in patients with allergic fungal sinusitis. Ann. Allergy Asthma Immunol. 2011, 107, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.S. Antileukotriene therapy for allergic fungal sinusitis. J. Allergy Clin. Immunol. 2001, 108, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Van Bruaene, N.; Cattaert, T.; Van Steen, K.; Van Zele, T.; Acke, F.; De Ruyck, N.; Blomme, K.; Sousa, A.R.; Marshall, R.P.; et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J. Allergy Clin. Immunol. 2011, 128, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corren, J. Anti-interleukin-5 antibody therapy in asthma and allergies. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Gan, E.C.; Habib, A.-R.R.; Rajwani, A.; Javer, A.R. Omalizumab therapy for refractory allergic fungal rhinosinusitis patients with moderate or severe asthma. Am. J. Otolaryngol. 2015, 36, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.O., 2nd; Coop, C.A. Novel treatment of allergic fungal sinusitis using omalizumab. Allergy Rhinol. 2014, 5, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Psaltis, A.; Tan, L.W.; Wormald, P.J. The efficacy of topical antibiofilm agents in a sheep model of rhinosinusitis. Am. J. Rhinol. 2014, 22, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kato, A. Immunopathology of chronic rhinosinusitis. Allergol. Int. 2015, 64, 121–130. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakrabarti, A.; Kaur, H. Allergic Aspergillus Rhinosinusitis. J. Fungi 2016, 2, 32. https://doi.org/10.3390/jof2040032
Chakrabarti A, Kaur H. Allergic Aspergillus Rhinosinusitis. Journal of Fungi. 2016; 2(4):32. https://doi.org/10.3390/jof2040032
Chicago/Turabian StyleChakrabarti, Arunaloke, and Harsimran Kaur. 2016. "Allergic Aspergillus Rhinosinusitis" Journal of Fungi 2, no. 4: 32. https://doi.org/10.3390/jof2040032