Cryptococcus–Epithelial Interactions
Abstract
:1. Introduction
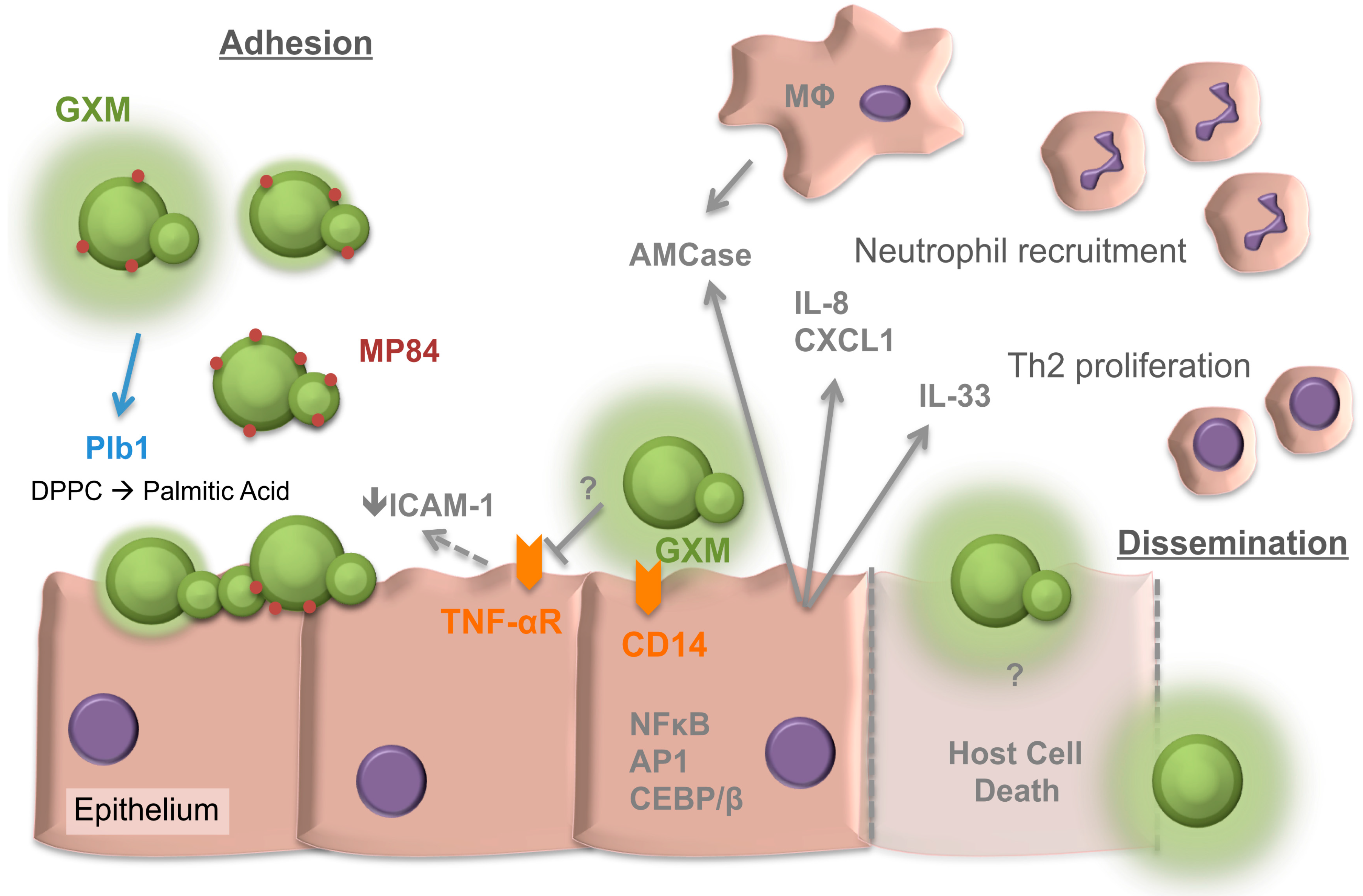
2. Adherence of Cryptococcus neoformans to the Respiratory Epithelia
3. Internalisation of Cryptococci by the Epithelia
4. The Response of the Respiratory Epithelia to Cryptococcus
5. The Cryptococcal Response to the Respiratory Niche
6. Collectins of the Respiratory Lining and Cryptococcus
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Pappas, P.G. Cryptococcal infections in non-HIV-infected patients. Trans. Am. Clin. Climatol. Assoc. 2013, 124, 61–79. [Google Scholar] [PubMed]
- Doering, T.L. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu. Rev. Microbiol. 2009, 63, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Smith, L.M.; May, R.C. New weapons in the Cryptococcus infection toolkit. Curr. Opin. Microbiol. 2016, 34, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Taborda, C.P.; Casadevall, A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur. J. Immunol. 2003, 33, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Chrisman, C.J.; Castelli, M.V.; Frases, S.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L.; Casadevall, A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Lam, J.S.; Levitz, S.M. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2006, 2, e120. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Nielsen, K.; Daou, S.; Brigitte, M.; Chretien, F.; Dromer, F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 2009, 77, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Garcia-Rodas, R.; Nosanchuk, J.D.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L.; Casadevall, A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010, 6, e1000945. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Miller, K.A.; Hotham, R.; Lewis, A.; Ogryzko, N.V.; Kamuyango, A.A.; Frost, H.; Gibson, R.H.; Stillman, E.; May, R.C.; et al. Cryptococcus neoformans Intracellular Proliferation and Capsule Size Determines Early Macrophage Control of Infection. Sci. Rep. 2016, 6, 21489. [Google Scholar] [CrossRef] [PubMed]
- Kechichian, T.B.; Shea, J.; Del Poeta, M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect. Immun. 2007, 75, 4792–4798. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Hiemstra, P.S. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004, 23, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.K.; Chong, P.P.; Ho, A.S.; Yong, P.V. The role of host microfilaments and microtubules during opsonin-independent interactions of Cryptococcus neoformans with mammalian lung cells. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lucho, V.; Ginsburg, V.; Krivan, H.C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Gal β 1–4Glc β 1–1Cer), a possible adhesion receptor for yeasts. Infect. Immun. 1990, 58, 2085–2090. [Google Scholar] [PubMed]
- Merkel, G.J.; Cunningham, R.K. The interaction of Cryptococcus neoformans with primary rat lung cell cultures. J. Med. Vet. Mycol. 1992, 30, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Merkel, G.J.; Scofield, B.A. The in vitro interaction of Cryptococcus neoformans with human lung epithelial cells. FEMS Immunol. Med. Microbiol. 1997, 19, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.M.; Fonseca, F.L.; Holandino, C.; Alviano, C.S.; Nimrichter, L.; Rodrigues, M.L. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 2006, 8, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Cleare, W.; Feldmesser, M.; Glatman-Freedman, A.; Goldman, D.L.; Kozel, T.R.; Lendvai, N.; Mukherjee, J.; Pirofski, L.A.; Rivera, J.; et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 1998, 42, 1437–1446. [Google Scholar] [PubMed]
- Chen, S.C.; Wright, L.C.; Golding, J.C.; Sorrell, T.C. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem. J. 2000, 347, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ganendren, R.; Carter, E.; Sorrell, T.; Widmer, F.; Wright, L. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect. 2006, 8, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, R.; Zoellner, H.; Sorrell, T.; Wilson, C.; Donald, C.; Djordjevic, J.; Shounan, Y.; Wright, L. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 2004, 72, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.A.; Penha, L.L.; Mendonca-Previato, L.; Previato, J.O. Mannoprotein MP84 mediates the adhesion of Cryptococcus neoformans to epithelial lung cells. Front. Cell. Infect. Microbiol. 2014, 4, 106. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Carroll, S.F.; Badawy, M.; Qureshi, S.T. Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir. Res. 2008, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.M.; Fonseca, F.L.; Figueiredo, R.T.; Bozza, M.T.; Casadevall, A.; Nimrichter, L.; Rodrigues, M.L. Binding of glucuronoxylomannan to the CD14 receptor in human A549 alveolar cells induces interleukin-8 production. Clin. Vaccine Immunol. 2007, 14, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, T.; Fujikawa, K.; Nishio, Y.; Mukaida, N.; Matsushima, K.; Kishimoto, T.; Akira, S. Transcription factors NF-IL6 and NF-κ B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA 1993, 90, 10193–10197. [Google Scholar] [CrossRef] [PubMed]
- Fries, B.C.; Goldman, D.L.; Cherniak, R.; Ju, R.; Casadevall, A. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect. Immun. 1999, 67, 6076–6083. [Google Scholar] [PubMed]
- Turner, S.H.; Cherniak, R.; Reiss, E.; Kwon-Chung, K.J. Structural variability in the glucuronoxylomannan of Cryptococcus neoformans serotype A isolates determined by 13C NMR spectroscopy. Carbohydr. Res. 1992, 233, 205–218. [Google Scholar] [CrossRef]
- McFadden, D.C.; Fries, B.C.; Wang, F.; Casadevall, A. Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, R.S.; Lavrador, M.A.; Ferreira, J.C.; Candido, R.C.; Maffei, C.M. Cryptococcus neoformans var. grubii—Pathogenicity of environmental isolates correlated to virulence factors, susceptibility to fluconazole and molecular profile. Mem. Inst. Oswaldo Cruz 2010, 105, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, M.; Kress, Y.; Casadevall, A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 2001, 147, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Piehler, D.; Grahnert, A.; Eschke, M.; Richter, T.; Kohler, G.; Stenzel, W.; Alber, G. T1/ST2 promotes T helper 2 cell activation and polyfunctionality in bronchopulmonary mycosis. Mucosal Immunol. 2013, 6, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Piehler, D.; Eschke, M.; Schulze, B.; Protschka, M.; Muller, U.; Grahnert, A.; Richter, T.; Heyen, L.; Kohler, G.; Brombacher, F.; et al. The IL-33 receptor (ST2) regulates early IL-13 production in fungus-induced allergic airway inflammation. Mucosal Immunol. 2016, 9, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Flaczyk, A.; Duerr, C.U.; Shourian, M.; Lafferty, E.I.; Fritz, J.H.; Qureshi, S.T. IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J. Immunol. 2013, 191, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Voelz, K.; Lammas, D.A.; May, R.C. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 2009, 77, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Heyen, L.; Muller, U.; Siegemund, S.; Schulze, B.; Protschka, M.; Alber, G.; Piehler, D. Lung epithelium is the major source of IL-33 and is regulated by IL-33-dependent and IL-33-independent mechanisms in pulmonary cryptococcosis. Pathog. Dis. 2016, 74, ftw086. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.A.; Synguelakis, M.; Johansson, J.; Pedron, T.; Girard, R.; Chaby, R. Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide. Infect. Immun. 2003, 71, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Merkel, G.J.; Scofield, B.A. The effects of Cryptococcus neoformans-secreted antigens on tumor necrosis factor-α-induced intercellular adhesion molecule-1 expression on human lung epithelial cells. FEMS Immunol. Med. Microbiol. 2000, 29, 329–332. [Google Scholar] [CrossRef]
- Goldman, D.L.; Davis, J.; Bommarito, F.; Shao, X.; Casadevall, A. Enhanced allergic inflammation and airway responsiveness in rats with chronic Cryptococcus neoformans infection: Potential role for fungal pulmonary infection in the pathogenesis of asthma. J. Infect. Dis. 2006, 193, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.L.; Khine, H.; Abadi, J.; Lindenberg, D.J.; Pirofski, L.; Niang, R.; Casadevall, A. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 2001, 107, E66. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kadota, J.; Ishimatsu, Y.; Iwashita, T.; Tomono, K.; Kawakami, K.; Kohno, S. Th1-Th2 cytokine kinetics in the bronchoalveolar lavage fluid of mice infected with Cryptococcus neoformans of different virulences. Microbiol. Immunol. 2000, 44, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, A.G.; Narain, S.; Du, Z.; Zeng, W.Y.; Ritch, J.; Casadevall, A.; Goldman, D.L. Pulmonary cryptococcosis induces chitinase in the rat. Respir. Res. 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Love, G.L.; Boyd, G.D.; Greer, D.L. Large Cryptococcus neoformans isolated from brain abscess. J. Clin. Microbiol. 1985, 22, 1068–1070. [Google Scholar] [PubMed]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chretien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Crabtree, J.N.; Okagaki, L.H.; Wiesner, D.L.; Strain, A.K.; Nielsen, J.N.; Nielsen, K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect. Immun. 2012, 80, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- Trevijano-Contador, N.; Rueda, C.; Zaragoza, O. Fungal morphogenetic changes inside the mammalian host. Semin. Cell Dev. Biol. 2016, 57, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cheng, P.Y.; Sham, A.; Perfect, J.R.; Kronstad, J.W. Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol. Microbiol. 2008, 69, 1456–1475. [Google Scholar] [CrossRef] [PubMed]
- Liew, K.L.; Jee, J.M.; Yap, I.; Yong, P.V. In Vitro Analysis of Metabolites Secreted during Infection of Lung Epithelial Cells by Cryptococcus neoformans. PLoS ONE 2016, 11, e0153356. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.; Nicola, A.M.; Nieves, E.; Paes, H.C.; Williamson, P.R.; Silva-Pereira, I.; Casadevall, A. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. mBio 2013, 5, e00986-13. [Google Scholar] [CrossRef] [PubMed]
- Carreto-Binaghi, L.; Aliouat, E.M.; Taylor, M.L. Surfactant proteins, SP-A and SP-D, in respiratory fungal infections: their role in the inflammatory response. Respir. Res. 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Malhotra, R.; Sim, R.B.; Holmskov, U.; Bancroft, G.J. Binding of host collectins to the pathogenic yeast Cryptococcus neoformans: Human surfactant protein D acts as an agglutinin for acapsular yeast cells. Infect. Immun. 1995, 63, 3360–3366. [Google Scholar] [PubMed]
- Van de Wetering, J.K.; Coenjaerts, F.E.; Vaandrager, A.B.; van Golde, L.M.; Batenburg, J.J. Aggregation of Cryptococcus neoformans by surfactant protein D is inhibited by its capsular component glucuronoxylomannan. Infect. Immun. 2004, 72, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.S.; Zaas, A.K.; Reidy, M.F.; Perfect, J.R.; Wright, J.R. Cryptococcus neoformans is resistant to surfactant protein A mediated host defense mechanisms. PLoS ONE 2007, 2, e1370. [Google Scholar] [CrossRef] [PubMed]
- Geunes-Boyer, S.; Oliver, T.N.; Janbon, G.; Lodge, J.K.; Heitman, J.; Perfect, J.R.; Wright, J.R. Surfactant protein D increases phagocytosis of hypocapsular Cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect. Immun. 2009, 77, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Geunes-Boyer, S.; Beers, M.F.; Perfect, J.R.; Heitman, J.; Wright, J.R. Surfactant protein D facilitates Cryptococcus neoformans infection. Infect. Immun. 2012, 80, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, M.; Kress, Y.; Novikoff, P.; Casadevall, A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 2000, 68, 4225–4237. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Croudace, J.E.; Lammas, D.A.; May, R.C. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 2006, 16, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Casadevall, A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 2006, 16, 2161–2165. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Fonseca, F.L.; Frases, S.; Casadevall, A.; Nimrichter, L. The still obscure attributes of cryptococcal glucuronoxylomannan. Med. Mycol. 2009, 47, 783–788. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor-Smith, L.M. Cryptococcus–Epithelial Interactions. J. Fungi 2017, 3, 53. https://doi.org/10.3390/jof3040053
Taylor-Smith LM. Cryptococcus–Epithelial Interactions. Journal of Fungi. 2017; 3(4):53. https://doi.org/10.3390/jof3040053
Chicago/Turabian StyleTaylor-Smith, Leanne M. 2017. "Cryptococcus–Epithelial Interactions" Journal of Fungi 3, no. 4: 53. https://doi.org/10.3390/jof3040053




