Cryptococcosis in Colombia: Compilation and Analysis of Data from Laboratory-Based Surveillance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Case Definition
2.4. Epidemiological Analysis
2.5. Statistical Analysis
2.6. Laboratory Tests and Molecular Typing
3. Results
3.1. Incidence of Cryptococcosis in Colombia, 1997–2016
3.2. General Characteristics of Cryptococcosis Patients in Colombia, 1997–2016
3.3. Risk Factors Associated with Cryptococcosis in Colombia, 1997–2016
3.4. Diagnosis of Cryptococcosis Cases in Colombia, 1997–2016
3.5. Diagnostic Images
3.6. Clinical Manifestations
3.7. Cryptococcosis Classification
3.8. Antifungal Treatment and Other Therapies
3.9. Mortality and Relapses
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwon-Chung, K.J.; Bennett, J.E.; Wickes, B.L.; Meyer, W.; Cuomo, C.A.; Wollenburg, K.R.; Bicanic, T.A.; Castañeda, E.; Chang, Y.C.; Chen, J.; et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I.; Sionov, E.; Falk, R.; Parnmen, S.; Lumbsch, H.T.; Boekhout, T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef] [PubMed]
- Aminnejad, M.; Cogliati, M.; Duan, S.; Arabatzis, M.; Tintelnot, K.; Castañeda, E.; Lazéra, M.; Velegraki, A.; Ellis, D.; Sorrell, T.C.; et al. Identification and Characterization of VNI/VNII and Novel VNII/VNIV Hybrids and Impact of Hybridization on Virulence and Antifungal Susceptibility Within the, C. neoformans/C. gattii Species Complex. PLoS ONE 2016, 20, e0163955. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, J.; Linares, M.; De Bedout, C.; Restrepo, A.; Agudelo, C.I.; Castañeda, E.; Grupo Colombiano para el Estudio de la Criptococosis. Estudio clínico y epidemiológico de la criptococosis en Colombia: Resultado de nueve años de la encuesta nacional, 1997–2005. Biomédica 2007, 27, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Escandón, P.; De Bedout, C.; Lizarazo, J.; Agudelo, C.I.; Tobón, A.; Bello, S.; Restrepo, A.; Castañeda, E.; Grupo Colombiano para el Estudio de la Criptococosis. Cryptococosis in Colombia: Results of the national surveillance program for the years 2006–2010. Biomédica 2012, 32, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Chastain, D.B.; Henao-Martínez, A.F.; Franco-Paredes, C. Opportunistic Invasive Mycoses in AIDS: Cryptococcosis, Histoplasmosis, Coccidiodomycosis, and Talaromycosis. Curr. Infect. Dis. Rep. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Viviani, M.A. Epidemiological working groups of ECMM. Mycol. Newslett. 1997, 2, 4–5. [Google Scholar]
- Yehia, B.R.; Eberlein, M.; Sisson, S.D.; Hager, D.N. Disseminated cryptococcosis with meningitis, peritonitis, and cryptococcemia in a HIV-negative patient with cirrhosis: A case report. Cases J. 2009, 28, 170. [Google Scholar] [CrossRef] [PubMed]
- Proyecciones de Población. Available online: http://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/proyecciones-de-poblacion (accessed on 10 September 2017).
- Panorama del VIH/sida en Colombia 1983–2010. Available online: http://colombia.unfpa.org/sites/default/files/pub-pdf/PANORAMA-VIH-SIDA-COLOMBIA-1983-2010.pdf (accessed on 25 January 2018).
- Country Fact Sheets-Colombia 2016. Available online: http://www.unaids.org/es/regionscountries/countries/colombia (accessed on 25 January 2018).
- Ordoñez, N.; Castañeda, E. Serotipificación de aislamientos clínicos y del medio ambiente de Cryptococcus neoformans en Colombia. Biomédica 1994, 14, 131–139. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Polacheck, I.T.; Bennet, J.E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J. Clin. Microbiol. 1982, 5, 535–537. [Google Scholar]
- Escandón, P.; Sánchez, A.; Martínez, M.; Meyer, W.; Castañeda, E. Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res. 2006, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Aanensen, D.M.; Boekhout, T.; Cogliati, M.; Diaz, M.R.; Esposto, M.C.; Fisher, M.; Gilgado, F.; Hagen, F.; Kaocharoen, S. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med. Mycol. 2009, 47, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Casali, A.K.; Goulart, L.; Rosa e Silva, L.K.; Ribeiro, A.M.; Amaral, A.A.; Alves, S.H.; Schrank, A.; Meyer, W.; Vainstein, M.H. Molecular typing of clinical and environmental Cryptococcus neoformans isolates in the Brazilian state Rio Grande do Soul. FEMS Yeast Res. 2003, 3, 405–415. [Google Scholar] [CrossRef]
- Halliday, C.L.; Bul, T.; Krockenberger, M.; Malik, R.; Ellis, D.; Carter, D. Presence of α and a Mating types in environmental and clinical collections of Cryptococcus neoformans var. gattii strains from Australia. J. Clin. Microbiol. 1999, 37, 2920–2926. [Google Scholar] [PubMed]
- Lizarazo, J.; Escandón, P.; Agudelo, C.I.; Firacative, C.; Meyer, W.; Castañeda, E. Retrospective Study of the Epidemiology and Clinical Manifestations of Cryptococcus gattii Infections in Colombia from 1997 to 2011. PLoS Negl. Trop. Dis. 2014, 8, e3272. [Google Scholar] [CrossRef] [PubMed]
- Callejas, A.; Ordoñez, N.; Rodriguez, M.C.; Castañeda, E. First isolation of Cryptococcus neoformans var. gattii, serotype C, from the environment in Colombia. Med. Mycol. 1998, 36, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C.; Torres, G.; Rodríguez, M.C.; Escandón, P. First environmental isolation of Cryptococcus gattii serotype B, from Cúcuta, Colombia. Biomédica 2011, 31, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, J.; Escandón, P.; Agudelo, C.I.; Castañeda, E. Cryptococcosis in Colombian children and literature review. Mem. Inst. Oswaldo Cruz. 2014, 109, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Goldman, D.L. Cryptococcal Disease in HIV-Infected Children. Curr. Infect. Dis. Rep. 2016, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Severo, C.B.; Xavier, M.O.; Gazzoni, A.F.; Severo, L.C. Cryptococcosis in children. Paediatr. Respir. Rev. 2009, 10, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- de Oliveira, R.B.; Atobe, J.H.; Souza, S.A.; de Castro Lima Santos, D.W. Epidemiology of invasive fungal infections in patients with acquired immunodeficiency syndrome at a reference hospital for infectious diseases in Brazil. Mycopathologia 2014, 178, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.A.; Somayaji, R.; Myers, R.; Mody, C.H. Epidemiology and trends of cryptococcosis in the United States from 2000 to 2007: A population-based study. Int. J. STD AIDS 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Frola, C.; Guelfand, L.; Blugerman, G.; Szyld, E.; Kaufman, S.; Cahn, P.; Sued, O.; Pérez, H. Prevalence of cryptococcal infection among advanced HIV patients in Argentina using lateral flow immunoassay. PLoS ONE 2017, 12, e0178721. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, M.C.; Melhem, M.S. Cryptococcosis a review of the Brazilian experience for the disease. Rev. Inst. Med. Trop. São Paulo 2003, 45, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Illnait-Zaragozi, M.T.; Martínez-Machín, G.F.; Fernández-Andreu, C.M.; Perurena-Lancha, M.R.; Hagen, F.; Meis, J.F. Cryptococcus and cryptococcosis in Cuba. A minireview. Mycoses 2014, 57707–57717. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sorrell, T.; Nimmo, G.; Speed, B.; Currie, B.; Ellis, D.; Marriott, D.; Pfeiffer, T.; Parr, D.; Byth, K. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin. Infect. Dis. 2000, 31, 499–508. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, J.A.; Powderly, W.G.; Spec, A. Cryptococcosis Today: It Is Not All About HIV Infection. Curr. Clin. Microbiol. Rep. 2017, 4, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.F.; Chiller, T.; Greene, G.S.; Burry, J.; Govender, N.P.; Kanyama, C.; Mfinanga, S.; Lesikari, S.; Mapoure, Y.N.; Kouanfack, C.; et al. Cryptococcal meningitis: A neglected NTD? PLoS Negl. Trop. Dis. 2017, 11, e0005575. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, E.; Lizarazo, J. Protocolo de estudio y manejo de los pacientes con criptococosis. Infectio 2012, 16, 123–125. [Google Scholar] [CrossRef]
- Chetchotisakd, P.; Kumarasamy, N.; Govender, N.; Lynen, L.; Harrison, T.; Meintjes, G.; Horvath, T.; Meyers, T.; Kaplan, J.; Mohan, N.; et al.; World Health Organization Rapid Advice: Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children; World Health Organization: Geneve, Switzerland, 2011; pp. 4–5. ISBN 978-92-4-150297-9. [Google Scholar]
- Jarvis, J.N.; Harrison, T.S.; Lawn, S.D.; Meintjes, G.; Wood, R.; Cleary, S. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS ONE 2013, 8, e69288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mfinanga, S.; Chanda, D.; Kivuyo, S.L.; Guinness, L.; Bottomley, C.; Simms, V.; Chijoka, C.; Masasi, A.; Kimaro, G.; Ngowi, B.; et al. Cryptococcal meningitis screening and community-based early adherence support reduces all-cause mortality among HIV- infected people initiating antiretroviral therapy with advanced disease: A randomised-controlled trial in Tanzania and Zambia. Lancet 2015, 385, 2173–2182. [Google Scholar] [CrossRef]
- Lizarazo, J.; Peña, Y.; Chaves, O.; Omaña, R.; Huérfano, S.; Castañeda, E. Diagnóstico temprano de criptococosis e histoplasmosis en personas con VIH/sida, informe preliminar. IQEN 2002, 7, 453–458. [Google Scholar]
- Escandón, P.; Lizarazo, J.; Agudelo, C.I.; Chiller, T.; Castañeda, E. Evaluation of a rapid lateral flow immunoassay for the detection of cryptococcal antigen for the early diagnosis of cryptococcosis in HIV patients in Colombia. Med. Mycol. 2013, 51, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, D.H.; Zuluaga, A.; Tabares, A.M.; Chiller, T.; González, A.; Gómez, B.L. Evaluation of a Cryptococcal antigen Lateral Flow Assay in serum and cerebrospinal fluid for rapid diagnosis of cryptococcosis in Colombia. Rev. Inst. Med. Trop. São Paulo 2017, 59. [Google Scholar] [CrossRef]
- Cogliati, M. Global Molecular Epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An Atlas of the Molecular Types. Scientica 2013. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, M.; Zani, A.; Rickerts, V.; McCormick, I.; Desnos-Ollivier, M.; Velegraki, A.; Escandon, P.; Ichikawa, T.; Ikeda, R.; Bienvenue, A.L.; et al. Multilocus sequence typing analysis reveals that Cryptococcus neoformans var. neoformans is a recombinant population. Fungal Genet. Biol. 2016, 87, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Fries, B.; Cox, G.M. Cryptococcosis in AIDS. In Cryptococcus: From Human Pathogen to Model Yeast; Heitman, J., Kozel, T.R., Kwon-Chung, K.J., Perfect, J.R., Casadevall, A., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 515–526. ISBN 9781555815011. [Google Scholar]
- Bekondi, C.; Bernede, C.; Passone, N.; Minssart, P.; Kamalo, C.; Mbolidi, D.; Germani, Y. Primary and opportunistic pathogens associated with meningitis in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus seros-tatus. Int. J. Infect. Dis. 2006, 10, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.H.; Sorrell, T.C.; Allworth, A.M.; Heath, C.H.; McGregor, A.R.; Papanaoum, K.; Richards, M.J.; Gottlieb, T. Cryptococcal disease of the CNS in immunocompetent hosts: Influence of cryptococcal variety on clinical manifestations and outcome. Clin. Infect. Dis. 1995, 20, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Huston, S.M.; Li, S.S.; Stack, D.; Timm-McCann, M.; Jones, G.J.; Islam, A.; Berenger, B.M.; Xiang, R.F.; Colarusso, P.; Mody, C.H. Cryptococcus gattii is killed by dendritic cells but evades adaptive immunity by failing to induce dendritic cell maturation. J. Immunol. 2013, 191, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Chi, C.Y.; Wang, Y.J.; Tseng, S.W.; Chou, C.H.; Ho, C.M.; Lin, P.C.; Ho, M.W.; Wang, J.H. Different presentations and outcomes between HIV-infected and HIV-uninfected patients with Cryptococcal meningitis. J. Microbiol. Immunol. Infect. 2012, 45, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, J.; Chaves, O.; Peña, Y.; Escandón, P.; Agudelo, C.I.; Castañeda, E. Comparación de los hallazgos clínicos y de supervivencia entre pacientes VIH positivos y VIH negativos con criptococosis meníngea en un hospital del tercer nivel. Acta Med. Colomb. 2012, 37, 49–61. [Google Scholar]
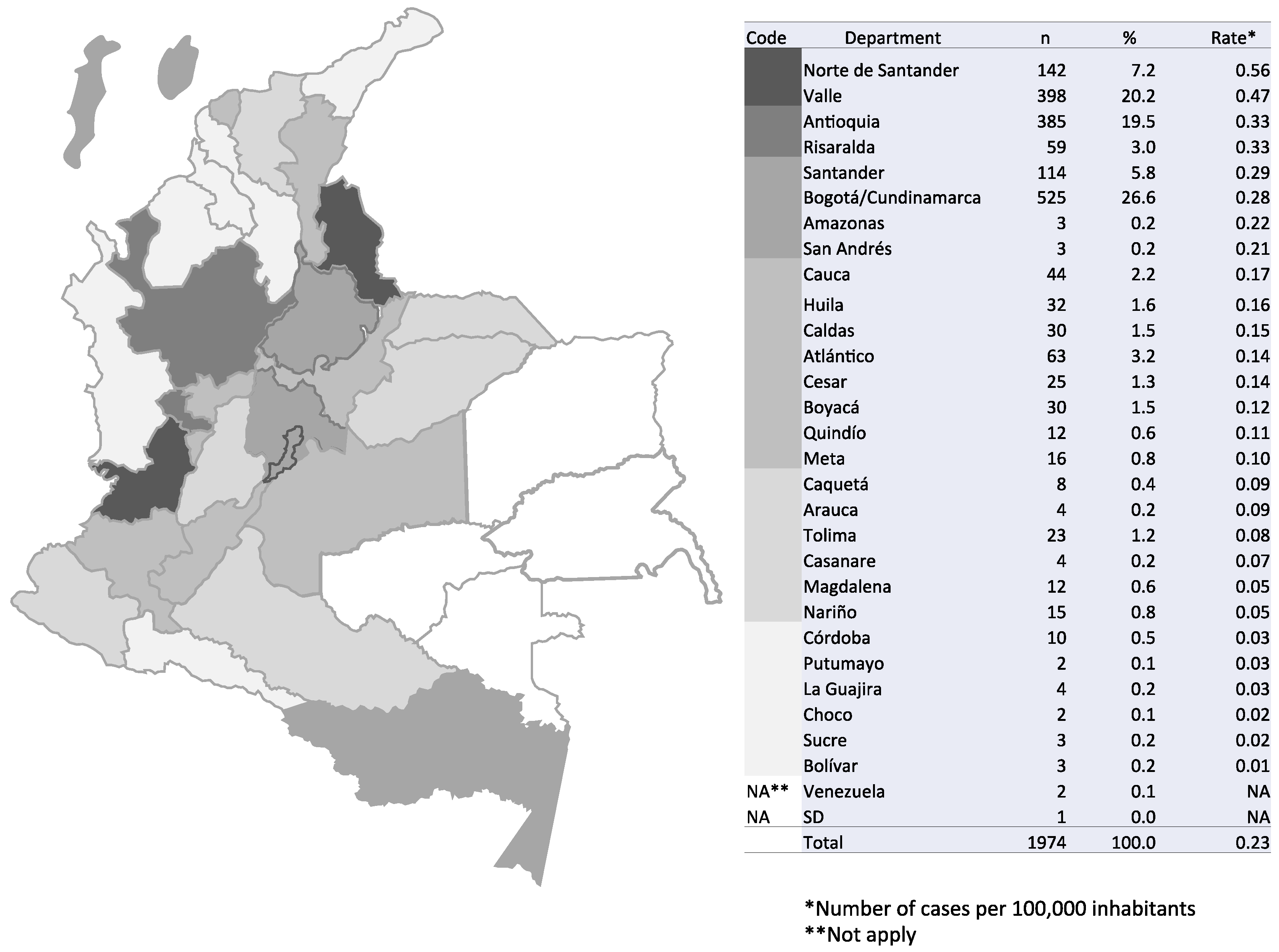
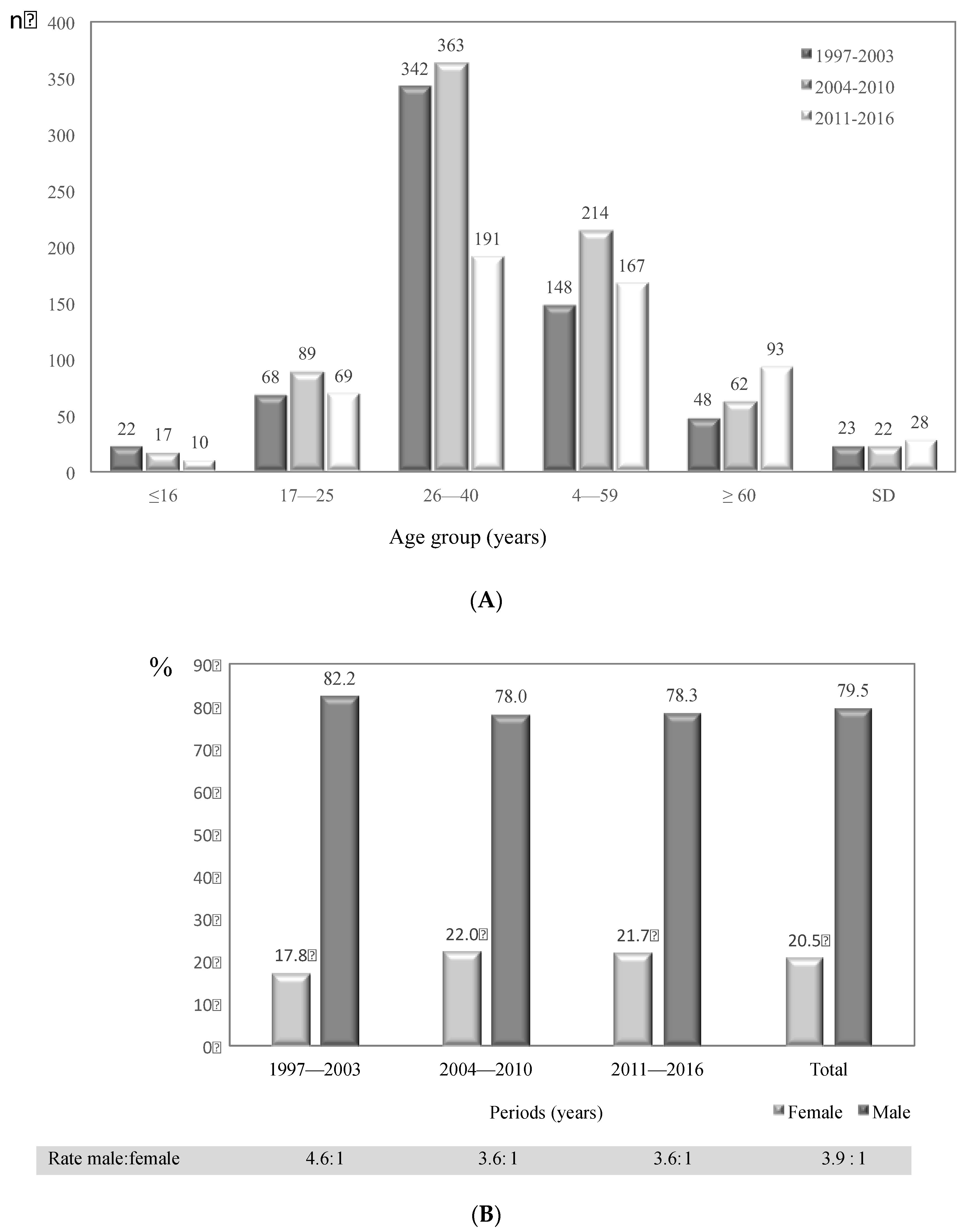
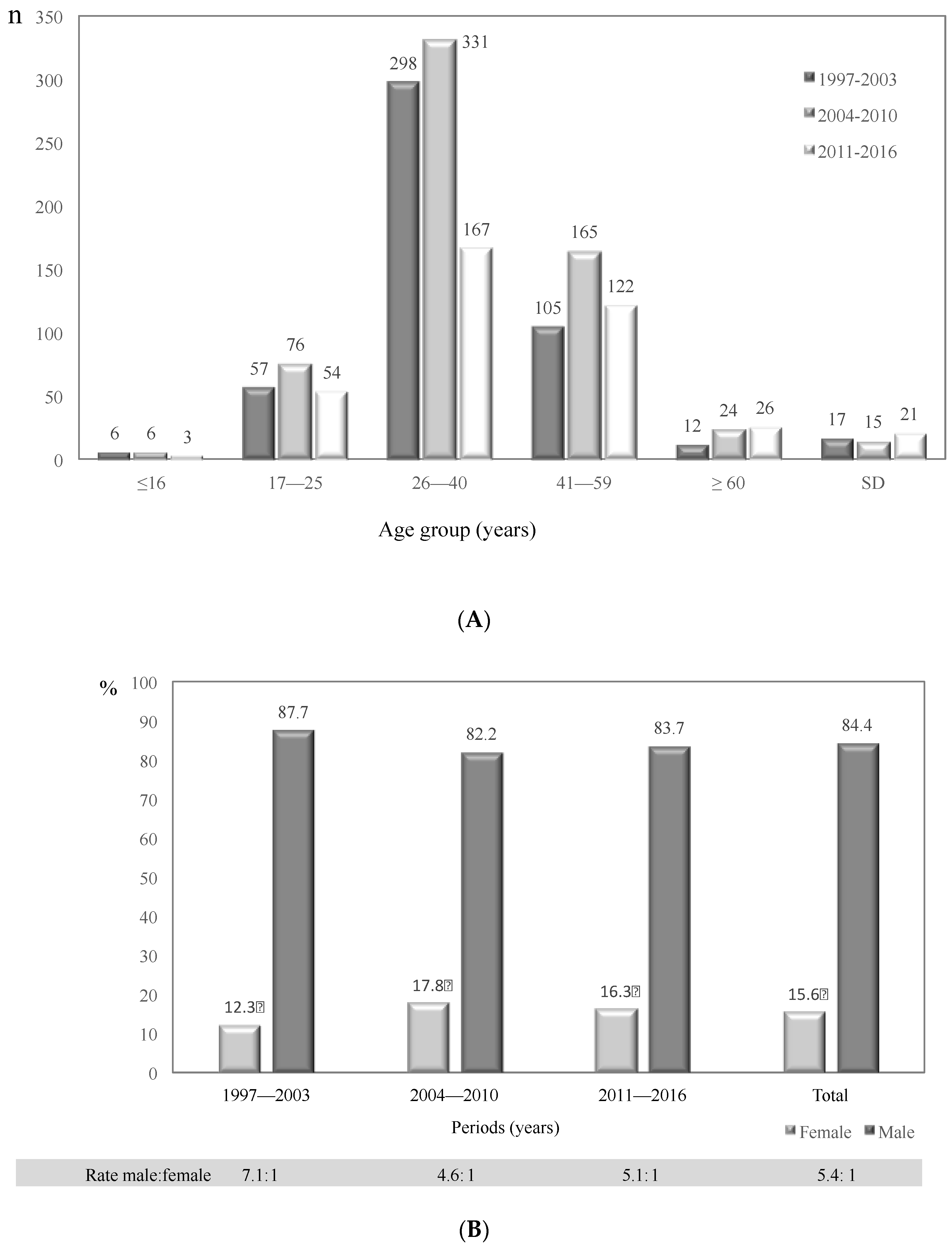
| Risk Factor | 1997–2003 | 2004–2010 | 2011–2016 | Total | |
|---|---|---|---|---|---|
| n | n | % | |||
| AIDS | 495 | 617 | 393 | 1505 | 76.2 |
| Corticosteroids | 19 | 32 | 22 | 73 | 3.7 |
| Autoimmune disease | 2 | 10 | 8 | 20 | 1.0 |
| Transplantation | 8 | 2 | 8 | 18 | 0.9 |
| Tumor | 12 | 7 | 7 | 26 | 1.3 |
| Diabetes | 3 | 4 | 11 | 18 | 0.9 |
| Cirrhosis | 1 | 2 | 1 | 4 | 0.2 |
| Chronic renal failure | 2 | 2 | 6 | 10 | 0.5 |
| Unknown or No risk factor | 88 | 80 | 80 | 248 | 12.6 |
| Others | 21 | 9 | 22 | 52 | 2.6 |
| Total | 651 | 765 | 558 | 1974 | 100.0 |
| Age Group | 1997–2003 | 2004–2010 | 2011–2016 | Total | Total * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | |
| ≤16 | 3 | 50.0 | 3 | 50.0 | 4 | 66.7 | 2 | 33.3 | 3 | 100.0 | 0 | 0.0 | 10 | 66.7 | 5 | 33.3 | 15 |
| 17–25 | 44 | 77.2 | 13 | 22.8 | 59 | 77.6 | 17 | 22.4 | 45 | 83.3 | 9 | 16.7 | 148 | 79.1 | 39 | 20.9 | 187 |
| 26–40 | 262 | 91.0 | 36 | 9.0 | 276 | 83.4 | 55 | 16.6 | 141 | 84.4 | 26 | 15.6 | 679 | 85.3 | 117 | 14.7 | 796 |
| 41–59 | 98 | 93.3 | 7 | 6.7 | 139 | 84.2 | 26 | 15.8 | 102 | 83.6 | 20 | 16.4 | 339 | 86.5 | 53 | 13.5 | 392 |
| ≥60 | 12 | 100.0 | 0 | 0.0 | 19 | 79.2 | 5 | 20.8 | 23 | 88.5 | 3 | 11.5 | 54 | 87.1 | 8 | 12.9 | 62 |
| SD | 15 | 88.2 | 2 | 11.8 | 10 | 66.7 | 5 | 33.3 | 15 | 71.4 | 6 | 28.6 | 40 | 75.5 | 13 | 24.5 | 53 |
| Total | 434 | 87.7 | 61 | 12.3 | 507 | 82.2 | 110 | 17.8 | 329 | 83.7 | 64 | 16.3 | 1270 | 84.4 | 235 | 15.6 | 1505 |
| Diagnostic Method | Study Period | Total | |||
|---|---|---|---|---|---|
| 1997–2003 | 2004–2010 | 2011–2016 | |||
| n | n | % | |||
| Culture | 612 | 738 | 551 | 1901 | 96.3 |
| + direct examination | 359 | 397 | 202 | 958 | 48.5 |
| + antigenemia + direct examination | 180 | 231 | 130 | 541 | 27.4 |
| only culture | 55 | 75 | 175 | 305 | 15.5 |
| + antigenemia | 18 | 35 | 44 | 97 | 4.9 |
| Antigenemia | 32 | 17 | 6 | 55 | 2.8 |
| only antigenemia | 25 | 10 | 5 | 40 | 2.0 |
| + direct examination | 7 | 7 | 1 | 15 | 0.8 |
| Direct examination | 7 | 10 | 1 | 18 | 0.9 |
| Total | 651 | 765 | 558 | 1974 | 100.0 |
| Clinical Presentation | AIDS Condition | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | SD | ||||||
| n | % | n | % | n | % | n | % | |
| Neurocryptococcosis/meningitis | 1218 | 80.9 | 319 | 82.2 | 64 | 79.0 | 1601 | 81.0 |
| Disseminated | 206 | 13.7 | 42 | 10.8 | 14 | 17.3 | 262 | 13.3 |
| Pulmonary | 46 | 3.1 | 14 | 3.6 | 2 | 2.5 | 62 | 3.1 |
| Cutaneous | 6 | 0.4 | 1 | 0.3 | 1 | 1.2 | 8 | 0.4 |
| Ganglion | 3 | 0.2 | 0 | 0.0 | 0 | 0.0 | 3 | 0.2 |
| Oropharyngeal | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 |
| Peritoneal | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 |
| No classified | 22 | 1.5 | 12 | 3.1 | 0 | 0.0 | 34 | 1.7 |
| Total | 1505 | 100.0 | 388 | 100.0 | 81 | 100.0 | 1974 | 100.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escandón, P.; Lizarazo, J.; Agudelo, C.I.; Castañeda, E. Cryptococcosis in Colombia: Compilation and Analysis of Data from Laboratory-Based Surveillance. J. Fungi 2018, 4, 32. https://doi.org/10.3390/jof4010032
Escandón P, Lizarazo J, Agudelo CI, Castañeda E. Cryptococcosis in Colombia: Compilation and Analysis of Data from Laboratory-Based Surveillance. Journal of Fungi. 2018; 4(1):32. https://doi.org/10.3390/jof4010032
Chicago/Turabian StyleEscandón, Patricia, Jairo Lizarazo, Clara Inés Agudelo, and Elizabeth Castañeda. 2018. "Cryptococcosis in Colombia: Compilation and Analysis of Data from Laboratory-Based Surveillance" Journal of Fungi 4, no. 1: 32. https://doi.org/10.3390/jof4010032





