Plant-Based Methods for Irrigation Scheduling of Woody Crops
Abstract
:1. Precision Irrigation
2. Irrigation Scheduling from Plant-Based Measurements
2.1. Non-Automated Methods
2.1.1. Stomatal Conductance
2.1.2. Leaf and Stem Water Potential
2.1.3. Thermal Sensing
Ground-Based Imagery
Airborne Imagery
2.1.4. NIR Spectroscopy
2.2. Automated Measurements
2.2.1. Sap Flow
2.2.2. Stem and Fruit Diameter
2.2.3. Leaf Thickness
2.2.4. Leaf Turgor Pressure
2.2.5. Stem Water Content
2.2.6. Electrical Potential
2.3. The Combined Use of Methods
2.4. An Alternative to the Signal-Intensity Approach
2.5. Choosing the Most Appropriate Method
3. Choosing the Right Production Target
4. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Smith, R.J.; Baillie, J.N. Defining precision irrigation: A new approach to irrigation management. In Proceedings of the Irrigation and Drainage Conference, Irrigation Australia 2009, Swan Hill, Australia, 18–24 October 2009; pp. 1–6. [Google Scholar]
- Fereres, E.; Goldhamer, D.A.; Parsons, L.R. Irrigation Water Management of Horticultural Crops. HortScience 2003, 38, 1036–1043. [Google Scholar]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanchez, M.C.; Domingo, R.; Castel, J.R. Review. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 8, S5–S20. [Google Scholar] [CrossRef]
- Fernández, J.E. Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Environ. Exp. Bot. 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Fernández, J.E.; Perez-Martin, A.; Torres-Ruiz, J.M.; Cuevas, M.V.; Rodriguez-Dominguez, C.M.; Elsayed-Farag, S.; Morales-Sillero, A.; García, J.M.; Hernandez-Santana, V.; Diaz-Espejo, A. A regulated deficit irrigation strategy for hedgerow olive orchards with high plant density. Plant Soil 2013, 372, 279–295. [Google Scholar] [CrossRef]
- Padilla-Díaz, C.M.; Rodriguez-Dominguez, C.M.; Hernandez-Santana, V.; Perez-Martin, A.; Fernández, J.E. Scheduling regulated deficit irrigation in a hedgerow olive orchard from leaf turgor pressure related measurements. Agric. Water Manag. 2016, 164, 28–37. [Google Scholar] [CrossRef]
- Abd-El-Rahman, A.A.; El-Sharkawi, H.M. Response of olive and almonds orchards to partial irrigation under dry-farming practices in semi-arid regions: II. Plant-soil water relations in olive during the growing season. Plant Soil 1974, 41, 13–31. [Google Scholar] [CrossRef]
- Lavee, S.; Nashef, M.; Wodner, M.; Harshemesh, H. The effect of complementary irrigation added to old olive trees (Olea europaea L.) cv. Souri on fruit characteristics, yield and oil production. Adv. Hortic. Sci. 1990, 4, 135–138. [Google Scholar]
- Proietti, P.; Nasini, L.; Ilarioni, L. Photosynthetic behavior of Spanish Arbequina and Italian Maurino olive (Olea europaea L.) cultivars under super-intensive grove conditions. Photosynthetica 2012, 50, 239–246. [Google Scholar] [CrossRef]
- Lavee, S.; Wodner, M. Factors affecting the nature of oil accumulation in fruit of olive (Olea europaea L.) cultivars. J. Hortic. Sci. 1991, 66, 583–591. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Ferguson, L.; Dunai, J. Irrigation requirements of olive trees and responses to sustained deficit irrigation. Acta Hortic. 1994, 356, 172–175. [Google Scholar] [CrossRef]
- Grattan, S.R.; Berenguer, M.J.; Connell, J.H.; Polito, V.S.; Vossen, P.M. Olive oil production as influenced by different quantities of applied water. Agric. Water Manag. 2006, 85, 133–140. [Google Scholar] [CrossRef]
- Ramos, A.F.; Santos, F.L. Yield and olive oil characteristics of a low–density orchard (cv. Cordovil) subjected to different irrigation regimes. Agric. Water Manag. 2010, 97, 363–373. [Google Scholar] [CrossRef]
- Dry, P.R.; Loveys, B.R.; Botting, D.; During, H. Effects of partial rootzone drying on grapevine vigour, yield, composition of fruit and use of water. In Proceedings of the 9th Australian Wine Industry Technical Conference, FAdelaide, Australia, 16–19 July 1995; Stockley, C.S., Sas, A.N., Johnstone, R.S., Lee, T.H., Eds.; Winetitles: Adelaide, Australia, 1996; pp. 126–131. [Google Scholar]
- Fernández, J.E.; Díaz-Espejo, A.; Infante, J.M.; Durán, P.; Palomo, M.J.; Chamorro, V.; Girón, I.F.; Villagarcía, L. Water relations and gas exchange in olive trees under regulated deficit irrigation and partial rootzone drying. Plant Soil 2006, 284, 273–291. [Google Scholar]
- Chalmers, D.J.; Mitchell, P.D.; Van Heek, L. Control of peach tree growth and productivity by regulated water supply, tree density and summer pruning. J. Am. Soc. Hortic. Sci. 1981, 106, 307–312. [Google Scholar]
- Goldhamer, D.A. Regulated deficit irrigation for California canning olives. Acta Hortic. 1999, 474, 369–372. [Google Scholar] [CrossRef]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.E. Plant-based sensing to monitor water stress: Applicability to commercial orchards. Agric. Water Manag. 2014, 142, 99–109. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.; Nicolás, E.; Nortes, P.A.; Alarcón, J.J.; Intrigliolo, D.S.; Fereres, E. Using high resolution UAV thermal imagery to assess the variability in the water status of five fruit tree species within a commercial orchard. Precis. Agric. 2013, 14, 660–678. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Goldhamer, D.A.; Zarco-Tejada, P.J.; Fereres, E. Improving the precision of irrigation in a pistachio farm using an unmanned airborne thermal system. Irrig. Sci. 2015, 33, 43–52. [Google Scholar] [CrossRef]
- Zimmermann, U.; Schneider, H.; Wegner, L.H.; Haase, A. Water ascent in tall trees: Does evolution of land plants rely on a highly metastable state? New Phytol. 2004, 162, 575–615. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Jones, H.G. Estimation of an effective soil water potential at the root surface of transpiring plants. Plant Cell Environ. 1983, 6, 671–674. [Google Scholar]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Phys. 1989, 40, 19–38. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. Plant Physiol. 1988, 88, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Costa, J.M.; Zarrouk, O.; Pinheiro, C.; Lopes, C.M.; Pereira, J.S. Controlling stomatal aperture in semi-arid regions-The dilemma of saving water or being cool? Plant Sci. 2016, 251, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.). An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Fernández, J.E.; Moreno, F.; Girón, I.F.; Blázquez, O.M. Stomatal control of water use in olive tree leaves. Plant Soil 1997, 190, 179–192. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Walcroft, A.S.; Fernández, J.E.; Hafidi, B.; Palomo, M.J.; Giron, I.F. Modeling photosynthesis in olive leaves under drought conditions. Tree Physiol. 2006, 26, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Santana, V.; Fernández, J.E.; Rodriguez-Dominguez, C.M.; Romero, R.; Diaz-Espejo, A. The dynamics of radial sap flux density reflects changes in stomatal conductance in response to soil and air water deficit. Agric. For. Meteorol. 2016, 218–219, 92–101. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Schultz, H.R. Differences in hydraulic architecture account for nearisohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Cuevas, M.V.; Torres-Ruiz, J.M.; Álvarez, R.; Jiménez, M.D.; Cuerva, J.; Fernández, J.E. Assessment of trunk diameter variation derived indices as water stress indicators in mature olive trees. Agric. Water Manag. 2010, 97, 1293–1302. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal control of transpiration. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar]
- Alarcón, J.J.; Domingo, R.; Green, S.R.; Nicolás, E.; Torrecillas, A. Estimation of hydraulic conductance within field-grown apricot using sap flow measurements. Plant Soil 2003, 251, 125–135. [Google Scholar] [CrossRef]
- Naor, A. Midday stem water potential as a plant water stress indicator for irrigation scheduling in fruit trees. Acta Hortic. 2000, 537, 447–454. [Google Scholar] [CrossRef]
- Naor, A. Irrigation scheduling and evaluation of tree water status in deciduous orchards. Hortic. Rev. 2006, 32, 111–165. [Google Scholar]
- Garnier, E.; Berger, A. Testing water potential in peach trees as an indicator of water stress. J. Hortic. Sci. 1985, 60, 47–56. [Google Scholar] [CrossRef]
- Naor, A. The interactions of soil-and stem-water potentials with crop level, fruit size and stomatal conductance of field-grown ‘Black Amber’ Japanese plum. J. Hortic. Sci. Biotech. 2004, 79, 273–280. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Measurement of plant water status by the pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Hsiao, T.C. Measurements of plant water status. In Irrigation of Agricultural Crops. (Monograph 30); Stewart, B.A., Nielsen, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; pp. 243–279. [Google Scholar]
- Dichio, B.; Xiloyannis, C.; Sofo, A.; Montanaro, G. Osmotic regulation in leaves and roots of olive trees during a water deficit and rewatering. Tree Physiol. 2005, 26, 179–185. [Google Scholar] [CrossRef]
- Bentrup, F.W. Water ascent in trees and lianas: The cohesion-tension theory revisited in the wake of Otto Renner. Protoplasma 2017, 254, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.E.; Rodriguez-Dominguez, C.M.; Perez-Martin, A.; Zimmermann, U.; Rüger, S.; Martín-Palomo, M.J.; Torres-Ruiz, J.M.; Cuevas, M.V.; Sann, C.; Ehrenberger, W.; et al. Online-monitoring of tree water stress in a hedgerow olive orchard using the leaf patch clamp pressure probe. Agric. Water Manag. 2011, 100, 25–35. [Google Scholar] [CrossRef]
- Martinez, E.M.; Cancela, J.J.; Cuesta, T.S.; Neira, X.X. Review. Use of psychrometers in field measurements of plant material: Accuracy and handling difficulties. Span. J. Agric. Res. 2011, 9, 313–328. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, H.; Hutson, J.L.; Wang, H.; Ewenz, C.; Shang, S.; Simmons, C.T. Examination and parameterization of the root water uptake model from stem water potential and sap flow measurements. Hydrol. Process. 2013, 20, 2857–2863. [Google Scholar] [CrossRef]
- Pagay, V.; Santiago, M.; Sessoms, D.A.; Huber, E.J.; Vincent, O.; Pharkya, A.; Corso, T.N.; Lakso, A.N.; Stroock, A.D. A microtensiometer capable of measuring water potentials below −10 MPa. Lab Chip 2014. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.E.; Morales, A.; Martín-Palomo, M.J.; Muriel, J.L.; Romero, R.; Diaz-Espejo, A. Seasonal changes on hydraulic conductance of mature olive trees under different water regimes. Acta Hortic. 2009, 846, 263–269. [Google Scholar] [CrossRef]
- Fuchs, M.; Tanner, C.B. Infrared thermometry of vegetation. Agron. J. 1966, 58, 597–601. [Google Scholar] [CrossRef]
- García-Tejero, I.; Durán-Zuazo, V.H.; Arriaga, J.; Hernández, A.; Vélez, L.M.; Muriel-Fernández, J.L. Approach to assess infrared thermal imaging of almond trees under water-stress conditions. Fruits 2012, 67, 463–474. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Girona, J.; Gonzalez-Dugo, V.; Fereres, E.; Ustin, S.L.; Zarco-Tejada, P.J. Airborne thermal imagery to detect the seasonal evolution of crop water status in peach, nectarine and Saturn peach orchards. Remote Sens. 2016, 8, 39. [Google Scholar] [CrossRef]
- Grant, O.M.; Ochagavía, H.; Baluja, J.; Diago, M.P.; Tardáguila, J. Thermal imaging to detect spatial and temporal variation in the water status of grapevine (Vitis vinifera L.). J. Hortic. Sci. Biotech. 2016, 91, 43–54. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating water stress in irrigated olives: Correlation of soil water status, tree water status, and thermal imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Sela, E.; Saranga, Y.; Cohen, S.; Meron, M.; Bosak, A.; Tsipris, J.; Ostrovsky, V.; Orolov, V.; et al. Crop water status estimation using thermography: Multi-year model development using ground-based thermal images. Precis. Agric. 2015, 16, 311–329. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Coopman, R.E.; Gallego, P.P.; Ribas-Carbo, M.; Flexas, J.; Escalona, J.; Medrano, H. UAVs challenge to assess water stress for sustainable agriculture. Agric. Water Manag. 2015, 153, 9–19. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, M.V.; Fereres, E. Seasonal stability of chlorophyll fluorescence quantified from airborne hyperspectral imagery as an indicator of net photosynthesis in the context of precision agriculture. Remote Sens. Environ. 2016, 179, 89–103. [Google Scholar] [CrossRef]
- Ha, W.; Gowda, P.H.; Howell, T.A. A review of downscaling methods for remote sensing-based irrigation management: Part I. Irrig. Sci. 2013, 31, 831–850. [Google Scholar] [CrossRef]
- Ha, W.; Gowda, P.H.; Howell, T.A. A review of potential image fusion methods for remote sensing-based irrigation management: Part II. Irrig. Sci. 2013, 31, 851–869. [Google Scholar] [CrossRef]
- Bellvert, J.; Zarco-Tejada, P.J.; Girona, J.; Fereres, E. Mapping crop water stress index in a “Pinot-noir” vineyard: Comparing ground measurements with thermal remote sensing imagery from an unmanned aerial vehicle. Precis. Agric. 2014, 15, 361–376. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martínez, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in super-high density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Jones, H.G. Application of Thermal Imaging and Infrared Sensing in Plant Physiology and Ecophysiology. Adv. Bot. Res. 2004, 41, 107–163. [Google Scholar]
- Maes, W.H.; Steppe, K. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: A review. J. Exp. Bot. 2012, 63, 4671–4712. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.S.N.; García-Tejero, I.; Lopes, T.S.; Costa, J.M.; Vaz, M.; Durán-Zuazo, V.H.; Chaves, M.; Glenn, D.M.; Campostrini, E. Linking thermal imaging to physiological indicators in Carica papaya L. under different watering regime. Agric. Water Manag. 2016, 164, 148–157. [Google Scholar] [CrossRef]
- Ishimwe, R.; Abutaleb, K.; Ahmed, F. Applications of Thermal Imaging in Agriculture—A Review. Adv. Remote Sens. 2014, 3, 128–140. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, J.P.J. Canopy temperature as a drought stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Prashar, A.; Jones, H.G. Assessing drought responses using thermal infrared imaging. Methods Mol. Biol. 2016, 1398, 209–219. [Google Scholar] [PubMed]
- Mahan, J.R.; Conaty, W.; Neilsen, J.; Payton, P.; Cox, S.B. Field performance in agricultural settings of a wireless temperature monitoring system based on a low-cost infrared sensor. Comput. Electron. Agric. 2010, 71, 176–181. [Google Scholar] [CrossRef]
- O’Shaughnessy, S.A.; Hebel, M.H.; Evett, S.R.; Colaizzi, P.D. Evaluation of a wireless infrared thermometer with a narrow field of view. Comput. Electron. Agric. 2011, 76, 59–68. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Lopes, C.M.; Chaves, M.M. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol. 2012, 39, 179–189. [Google Scholar] [CrossRef]
- Conaty, W.C.; Mahan, J.R.; Neilsen, J.E.; Constable, C.A. Vapour pressure deficit aids the interpretation of cotton canopy temperature response to water deficit. Funct. Plant Biol. 2014, 41, 535–546. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Nortes, P.A.; Sánchez-Blanco, M.J.; Ortuño, M.F. Sensitivity of thermal imaging and infrared thermometry to detect water status changes in Euonymus japonica plants irrigated with saline reclaimed water. Biosyst. Eng. 2015, 133, 21–32. [Google Scholar] [CrossRef]
- Xu, J.; Lv, Y.; Liu, X.; Dalson, T.; Yang, S.; Wu, J. Diagnosing crop water stress of rice using infra-red thermal imager under water deficit condition. Int. J. Agric. Biol. 2016, 18, 565–572. [Google Scholar] [CrossRef]
- Struthers, R.; Ivanova, A.; Tits, L.; Swennen, R.; Coppin, P. Thermal infrared imaging of the temporal variability in stomatal conductance for fruit trees. Int. J. Appl. Earth Obs. 2015, 39, 9–17. [Google Scholar] [CrossRef]
- Agam, N.; Segal, E.; Peeters, A.; Levi, A.; Dag, A.; Yermiyahu, U.; Ben-Gal, A. Spatial distribution of water status in irrigated olive orchards by thermal imaging. Precis. Agric. 2014, 15, 346–359. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Rollin, E.M.; Milton, E.J. Processing of High Spectral Resolution Reflectance Data for the Retrieval of Canopy Water Content Information. Remote Sens. Environ. 1998, 65, 86–92. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.R.; Riaño, D.; Carlisle, E.; Ustin, S.; Smart, D.R. Evaluation of hyperspectral reflectance indices to detect grapevine water status in vineyards. Am. J. Enol. Viticult. 2007, 58, 302–317. [Google Scholar]
- Vila, H.; Hugalde, I.; Di Filippo, M. Estimation of leaf water potential by thermographic and spectral measurements in grapevine. RIA 2011, 37, 46–52. [Google Scholar]
- De Bei, R.; Cozzolino, D.; Sullivan, W.; Cynkar, W.; Fuentes, S.; Damberg, R.; Pech, J.; Tyerman, S. Non-destructive measurement of grapevine water potential using near infrared spectroscopy. Aust. J. Grape Wine Res. 2011, 17, 62–71. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Tardaguila, J.; Fernández-Novales, J.; Diago, M.P. Data Mining and NIR Spectroscopy in Viticulture: Applications for Plant Phenotyping under Field Conditions. Sensors 2016, 16, 236. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Wang, F.; Bao, A.; Jiapaer, G. Leaf and canopy water content estimation in cotton using hyperspectral indices and radiative transfer models. Int. J. Appl. Earth Obs. 2014, 33, 67–75. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, G. Estimation of Canopy Water Content by Means of Hyperspectral Indices Based on Drought Stress Gradient Experiments of Maize in the North Plain China. Remote Sens. 2015, 7, 15203–15223. [Google Scholar] [CrossRef]
- Cozzolino, D. Use of infrared spectroscopy for in-field measurement and phenotyping of plant properties: Instrumentation, data analysis, and examples. Appl. Spectrosc. Rev. 2014, 49, 564–584. [Google Scholar] [CrossRef]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Non-destructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Tec. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Santos, A.O.; Kaye, O. Grapevine leaf water potential based upon near infrared spectroscopy. Sci. Agric. 2009, 66, 287–292. [Google Scholar] [CrossRef]
- Edwards, W.R.N.; Becker, P.; Čermák, J. A unified nomenclature for sap flow measurements. Tree Physiol. 1996, 17, 65–67. [Google Scholar] [CrossRef]
- Lemeur, R.; Fernández, E.; Steppe, K. Symbols, SI Units and Physical Quantities within the Scope of Sap Flow Studies. Acta Hortic. 2009, 846, 21–32. [Google Scholar] [CrossRef]
- Nadezhdina, N. Sap flow index as an indicator of plant water status. Tree Physiol. 1999, 19, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.E.; Palomo, M.J.; Díaz-Espejo, A.; Clothier, B.E.; Green, S.R.; Girón, I.F.; Moreno, F. Heat-pulse measurements of sap flow in olives for automating irrigation: Tests, root flow and diagnostics of water stress. Agric. Water Manag. 2001, 51, 99–123. [Google Scholar] [CrossRef]
- Fernández, J.E.; Green, S.R.; Caspari, H.W.; Diaz-Espejo, A.; Cuevas, M.V. The use of sap flow measurements for scheduling irrigation in olive, apple and Asian pear trees and in grapevines. Plant Soil 2008a, 305, 91–104. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Cermak, J. Automatic control unit for irrigation systems based on sensing the plant water status. An. Inst. Super. Agron. 1997, 46, 149–157. [Google Scholar]
- Fernández, J.E.; Romero, R.; Montaño, J.C.; Diaz-Espejo, A.; Muriel, J.L.; Cuevas, M.V.; Moreno, F.; Girón, I.F.; Palomo, M.J. Design and testing of an automatic irrigation controller for fruit tree orchards, based on sap flow measurements. Aus. J. Agric. Res. 2008b, 59, 589–598. [Google Scholar] [CrossRef]
- Escalona, J.; Flexas, J.; Medrano, H. Drought effects on water flow, photosynthesis and growth of potted grapevines. Vitis 2002, 41, 57–62. [Google Scholar]
- Ortuño, M.F.; García-Orellana, Y.; Conejero, W.; Ruiz-Sánchez, M.C.; Alarcón, J.J.; Torrecillas, A. Stem and leaf water potentials, gas exchange, sap flow and trunk diameter fluctuations for detecting water stress in lemon trees. Trees 2006, 20, 1–8. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Performance of various water stress indicators for prediction of fruit size response to deficit irrigation in plum. Agric. Water Manag. 2006a, 83, 173–180. [Google Scholar] [CrossRef]
- Fernández, J.E.; Moreno, F.; Martín-Palomo, M.J.; Cuevas, M.V.; Torres-Ruiz, J.M.; Moriana, A. Combining sap flow and trunk diameter measurements to assess water needs in mature olive orchards. Environ. Exp. Bot. 2011b, 72, 330–338. [Google Scholar] [CrossRef]
- Cuevas, M.V.; Martín-Palomo, M.J.; Diaz-Espejo, A.; Torres-Ruiz, J.M.; Rodriguez-Dominguez, C.M.; Perez-Martin, A.; Fernández, J.E. Assessing water stress in a hedgerow olive orchard from sap flow and trunk diameter measurements. Irrig. Sci. 2013, 31, 729–746. [Google Scholar] [CrossRef]
- Green, S.R.; Clothier, B.E.; Jardine, B. Theory and practical application of heat-pulse to measure sap flow. Agron. J. 2003, 95, 1371–1379. [Google Scholar] [CrossRef]
- Fernández, J.E.; Durán, P.J.; Palomo, M.J.; Diaz-Espejo, A.; Chamorro, V.; Girón, I.F. Calibration of sap flow estimated by the compensation heat pulse method in olive, plum and orange trees: Relationships with xylem anatomy. Tree Physiol. 2006b, 26, 719–728. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Nadezhdin, V.; Ferreira, M.I.; Pitacco, A. Variability with xylem depth in sap flow in trunks and branches of mature olive trees. Tree Physiol. 2007, 27, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Shibata, E. Diurnal changes in branch diameter as indicator of water status of Hinoki cypress, Chamaecyparis obtuse. Trees 2001, 15, 315–318. [Google Scholar] [CrossRef]
- Sevanto, S.; Vesala, T.; Perämäki, M.; Nikinmaa, E. Time lags for xylem and stem diameter variations in a Scots pine tree. Plant Cell Environ. 2002, 25, 1071–1077. [Google Scholar] [CrossRef]
- Čermák, J.; Kučera, J.; Baurerle, W.L.; Phillip, N.; Hinckley, M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007, 27, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, T.M.; Bruckerhoff, D.N. The effect of drought on water relations and stem shrinkage of Quercus alba. Can. J. Bot. 1975, 53, 62–72. [Google Scholar] [CrossRef]
- Brough, D.W.; Jones, H.G.; Grace, J. Diurnal changes in water content of the stems of apple trees, as influenced by irrigation. Plant Cell Environ. 1986, 9, 1–7. [Google Scholar]
- Čermák, J.; Nadezhdina, N. Sapwood as the scaling parameter—Defining according to xylem water content or radial pattern of sap flow? Ann. Sci. Forest. 1998, 55, 509–521. [Google Scholar] [CrossRef]
- Zweifel, R.; Item, H.; Häsler, R. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees 2000, 15, 50–75. [Google Scholar] [CrossRef]
- Fernández, J.E.; Cuevas, M.V. Irrigation scheduling from stem diameter variations: A review. Agric. Forest Meteorol. 2010, 150, 135–151. [Google Scholar] [CrossRef]
- Ortuño, M.F.; Conejero, W.; Moreno, F.; Moriana, A.; Intrigliolo, D.S.; Biel, C.A.; Mellisho, C.A.D.; Pérez-Pastor, A.; Domingo, R.; Ruiz-Sánchez, M.C.A.; et al. Could trunk diameter sensors be used in woody crops for irrigation scheduling? A review of current knowledge and future perspectives. Agric. Water Manag. 2010, 97, 1–11. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Temporal dynamics of stem expansion and contraction in savanna trees: Withdrawal and recharge of stored water. Tree Physiol. 2008, 28, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lassoie, J.P. Stem dimensional fluctuations in Douglas-fir stem in response to tree water status. Forest Sci. 1979, 25, 132–144. [Google Scholar]
- Antonova, G.F.; Cherkashin, V.P.; Stasova, V.V.; Varaksina, T.N. Daily dynamics in xylem cell radial growth of Scots pine (Pinus sylvestris L.). Trees 1995, 10, 24–30. [Google Scholar] [CrossRef]
- Herzog, K.M.; Häsler, R.; Thum, R. Diurnal changes in the radius of a subalpine Norway spruce stem: Their relation to the sap flow and their use to estimate transpiration. Trees 1995, 10, 94–101. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Fereres, E. Irrigation scheduling protocols using continuously recorded trunk diameter measurements. Irrig. Sci. 2001, 20, 115–125. [Google Scholar] [CrossRef]
- Gallardo, M.; Thompson, R.B.; Valdez, L.C.; Fernández, M.D. Response of stem diameter variations to water stress in greenhouse-grown vegetable crops. J. Hortic. Sci. Biotech. 2006, 81, 483–495. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Dodd, I.C.; Domingo, R.; Pérez-Pastor, A. Early morning fluctuations in trunk diameter are highly sensitive to water stress in nectarine trees. Irrig. Sci. 2016, 34, 117–128. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Evaluation of grapevine water status from trunk diameter variations. Irrig. Sci. 2007, 26, 49–59. [Google Scholar] [CrossRef]
- Moriana, A.; Fereres, E. Plant indicators for scheduling irrigation of young olive trees. Irrig. Sci. 2002, 21, 83–90. [Google Scholar]
- Ortuño, M.F.; Alarcón, J.J.; Nicolás, E.; Torrecillas, A. Interpreting trunk diameter changes in young lemon trees under deficit irrigation. Plant Sci. 2004, 167, 275–280. [Google Scholar] [CrossRef]
- Nortes, P.A.; Pérez-Pastor, A.; Egea, G.; Conejero, W.; Domingo, R. Comparison of changes in stem diameter and water potential values for detecting water stress in young almond trees. Agric. Water Manag. 2005, 77, 296–307. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Crop load affects maximum daily trunk shrinkage of plum trees. Tree Physiol. 2007b, 27, 89–96. [Google Scholar] [CrossRef]
- Pérez-López, D.; Moriana, A.; Rapoport, H.; Olmedilla, M.; Ribas, F. New approach for using trunk growth rate and endocarp development in the irrigation scheduling of young olive orchards. Sci. Hortic. 2008, 115, 244–251. [Google Scholar] [CrossRef]
- Moriana, A.; Orgaz, F.; Pastor, M.; Fereres, E. Yield responses of a mature olive orchard to water deficits. J. Am. Soc. Hortic. Sci. 2003, 128, 425–431. [Google Scholar]
- Intrigliolo, D.S.; Castel, J.R. Continuous measurement of plant and soil water status for irrigation scheduling in plum. Irrig. Sci. 2004, 23, 93–102. [Google Scholar] [CrossRef]
- De Swaef, T.; Mellisho, C.D.; Baert, A.; De Schepper, V.; Torrecillas, A.; Conejero, W.; Steppe, K. Model-assisted evaluation of crop load effects on stem diameter variations and fruit growth in peach. Trees 2014, 28, 1607–1622. [Google Scholar] [CrossRef]
- De Swaef, T.; De Schepper, V.; Vandegehuchte, M.W.; Steppe, K. Stem diameter variations as a versatile research tool in ecophysiology. Tree Physiol. 2015, 35, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Silber, A.; Naor, A.; Israeli, Y.; Assouline, S. Combined effect of irrigation regime and fruit load on the patterns of trunk-diameter variation of ‘Hass’ avocado at different phenological periods. Agric Water Manag. 2013, 129, 87–94. [Google Scholar] [CrossRef]
- Böhmerle, K. Die Pfister’sche Zuwachsuhr. Zentralblatt für das gesamte Forstwesen 1883, 9, 83–93. [Google Scholar]
- Friedrich, J. Zuwachsmesser. Zentralblatt für das Gesamte Forstwesen 1890, 16, 174–179. [Google Scholar]
- Fritts, D.C. An evaluation of three techniques for measuring radial tree growth. Bull. Ecol. Soc. Am. 1961, 42, 54–55. [Google Scholar]
- Kozlowski, T.T.; Winget, C.H. Diurnal and seasonal variations in radii of tree stems. Ecology 1964, 45, 149–155. [Google Scholar] [CrossRef]
- Holmes, J.W.; Shim, S.Y. Diurnal changes in stem diameter of Canary Island pine trees caused by soil water stress and varying microclimate. J. Exp. Bot. 1968, 19, 219–232. [Google Scholar] [CrossRef]
- Ueda, M.; Yoshikawa, K.; Okitu, J. Measurement of diurnal changes in stem and branch diameter using strain gauges. J. For. Res. 1996, 1, 139–142. [Google Scholar] [CrossRef]
- Pelloux, G.; Lorendeau, J.Y.; Huguet, J.G. Pepista: Translation of plants behaviour by the measurement of diameters of stem or fruits as a self-adjusted method for irrigation scheduling. In Proceedings of the 3rd International Congress for Computer Technology, Frankfurt-sur-le-Main, Bad-Soden, Germany, 1990; pp. 229–235. [Google Scholar]
- Bussi, C.; Huguet, J.G.; Besset, J.; Girard, T. Irrigation scheduling of an early maturing peach cultivar using tensiometers and diurnal changes in stem diameter. Fruits 1999, 54, 57–66. [Google Scholar]
- Moriana, A.; Corell, M.; Girón, I.F.; Conejero, W.; Morales, D.; Torrecillas, A.; Moreno, F. Regulated deficit irrigation based on threshold values of trunk diameter fluctuation indicators in table olive trees. Sci. Hortic. 2013, 164, 102–111. [Google Scholar] [CrossRef]
- Girón, I.F.; Corell, M.; Martín-Palomo, M.J.; Galindo, A.; Torrecillas, A.; Moreno, F.; Moriana, A. Feasibility of trunk diameter fluctuations in the scheduling of regulated deficit irrigation for table olive trees without reference trees. Agric. Water Manag. 2015, 161, 114–126. [Google Scholar] [CrossRef]
- Girón, I.F.; Corell, M.; Martín-Palomo, M.J.; Galindo, A.; Torrecillas, A.; Moreno, F.; Moriana, A. Limitations and usefulness of maximum daily shrinkage (MDS) and trunk growth rate (TGR) indicators in the irrigation scheduling of table olive trees. Agric. Water Manag. 2016, 164, 38–45. [Google Scholar] [CrossRef]
- Agüero-Alcaras, L.M.; Rousseaux, M.C.; Searles, P.S. Responses of several soil and plant indicators to post-harvest regulated deficit irrigation in olive trees and their potential for irrigation scheduling. Agric. Water Manag. 2016, 171, 10–20. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Pérez-Sarmiento, F.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Using midday stem water potential for scheduling deficit irrigation in mid–late maturing peach trees under Mediterranean conditions. Irrig. Sci. 2016a, 34, 161–173. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Pérez-Sarmiento, F.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Reference values of maximum daily trunk shrinkage for irrigation scheduling in mid-late maturing peach trees. Agric. Water Manag. 2016b, 171, 31–39. [Google Scholar] [CrossRef]
- Higgs, K.H.; Jones, H.G. A microcomputer-based system for continuous measurement and recording fruit diameter in relation to environmental factors. J. Exp. Bot. 1984, 160, 1646–1655. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Zhang, Y.; Fu, C.; Xing, B.; Li, W.; Qian, J.; Li, S.; Wang, H.; Fan, X.; Yan, Y.; Wang, Y.; Yang, X. Apple Fruit Diameter and Length Estimation by Using the Thermal and Sunshine Hours Approach and Its Application to the Digital Orchard Management Information System. PLoS ONE 2015, 10, e0120124. [Google Scholar] [CrossRef] [PubMed]
- Manfrini, L.; Pierpaoli, E.; Zibordi, M.; Morandi, B.; Muzzi, E.; Losciale, P.; Corelli-Grapadelli, L. Monitoring strategies for precise production of high quality fruit and yield in Apple in Emilia-Romagna. Chem. Eng. Trans. 2015, 44. [Google Scholar]
- Morandi, B.; Manfrini, L.; Zibordi, M.; Noferini, M.; Fiori, G.; Corelli-Grappadelli, L. A Low-cost Device for Accurate and Continuous Measurements of Fruit Diameter. HortScience 2007, 42, 1380–1382. [Google Scholar]
- Tyree, M.T.; Cameron, S.I. A new technique for measuring oscillatory and diurnal changes in leaf thickness. Can. J. For. Res. 1977, 7, 540–544. [Google Scholar] [CrossRef]
- Búrquez, A. Leaf thickness and water deficit in plants: A tool for field studies. J. Exp. Bot. 1987, 38, 109–114. [Google Scholar] [CrossRef]
- McBurney, T. The relationship between leaf thickness and plant water potential. J. Exp. Bot. 1992, 43, 327–335. [Google Scholar] [CrossRef]
- Seelig, H.D.; Wolterb, A.; Schröder, F.G. Leaf thickness and turgor pressure in bean during plant desiccation. Sci. Hortic. 2015, 184, 55–62. [Google Scholar] [CrossRef]
- Seelig, H.D.; Stoner, R.J.; Linden, J.C. Irrigation control of cowpea plants using the measurement of leaf thickness under greenhouse conditions. Irrig. Sci. 2012, 30, 247–257. [Google Scholar] [CrossRef]
- Sharon, Y.; Bravdo, B. A fully-automated orchard irrigation system based on continuous monitoring of turgor potential with a leaf sensor. Acta Hortic. 2001, 562, 55–61. [Google Scholar] [CrossRef]
- Nadler, A.; Raveh, E.; Yermiyahu, U.; Green, S.R. Stress induced water content variations in mango stem by time domain reflectometry. Soil Sci. Soc. Am. J. 2006, 70, 510–520. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Buckley, T.N.; Egea, G.; de Cires, A.; Hernandez-Santana, V.; Martorell, S.; Diaz-Espejo, A. Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ. 2016, 39, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N.; Mott, K.A.; Farquhar, G.D. A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ. 2003, 26, 1767–1785. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Sussmilch, F.C.; Brodribb, T.J. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ. 2016, 39, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lintilhac, P.M.; Wei, C.; Tanguay, J.J.; Outwater, J.O. Ball tonometry: A rapid, non-destructive method for measuring cell turgor pressure in thin-walled plant cells. J. Plant Growth Regul. 2000, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, D.; Reuss, R.; Westhoff, M.; Gessner, P.; Bauer, W.; Bamberg, E.; Bentrup, F.-W.; Zimmermann, U. A novel, non-invasive, online monitoring, versatile and easy plant-based probe for measuring leaf water status. J. Exp. Bot. 2008, 59, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, U.; Bitter, R.; Ribeiro-Marchiori, P.E.; Rüger, S.; Ehrenberger, W.; Sukhorukov, V.L.; Schüttler, A.; Vasconcelos-Ribeiro, R. A non-invasive plant-based probe for continuous monitoring of water stress in real time: A new tool for irrigation scheduling and deeper insight into drought and salinity stress physiology. Theor. Exp. Plant Physiol. 2013, 25, 2–11. [Google Scholar] [CrossRef]
- Ehrenberger, W.; Rüger, S.; Rodriguez-Dominguez, C.M.; Díaz-Espejo, A.; Fernández, J.E.; Moreno, J.; Zimmermann, D.; Sukhorukov, L.; Zimmermann, U. Leaf patch clamp pressure probe measurements on olive leaves in a nearly turgorless state. Plant Biol. 2012, 14, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Rüger, S.; Ehrenberger, W.; Arend, M.; Geßner, P.; Zimmermann, G.; Zimmermann, D.; Bentrup, F.W.; Nadler, A.; Raveh, E.; Sukhorukov, V.L.; Zimmermann, U. Comparative monitoring of temporal and spatial changes in tree water status using the non-invasive leaf patch clamp pressure probe and the pressure bomb. Agric. Water Manag. 2010a, 98, 232–290. [Google Scholar] [CrossRef]
- Westhoff, M.; Zimmermann, D.; Zimmermann, G.; Gessner, P.; Wegner, L.H.; Bentrup, F.W.; Zimmermann, U. Distribution and function of epistomatal mucilage plugs. Protoplasma 2009, 235, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Rüger, S.; Netzer, Y.; Westhoff, M.; Zimmermann, D.; Reuss, R.; Ovadya, S.; Gessner, P.; Zimmermann, G.; Schwartz, A.; Zimmermann, U. Remote monitoring of leaf turgor pressure of grapevines subjected to different irrigation treatments using the leaf patch clamp pressure probe. Aust. J. Grape Wine Res. 2010, 16, 405–412. [Google Scholar] [CrossRef]
- Zimmermann, U.; Rüger, S.; Shapira, O.; Westhoff, M.; Wegner, L.H.; Reuss, R.; Geßner, P.; Zimmermann, G.; Israeli, Y.; Zhou, A.; et al. Effects of environmental parameters and irrigation on the turgor pressure of banana plants measured using the non-invasive, online monitoring leaf patch clamp pressure probe. Plant Biol. 2010, 12, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gimeno, M.A.; Castiella, M.; Rüger, S.; Intrigliolo, D.S.; Ballester, C. Evaluating the usefulness of continuous leaf turgor pressure measurements for the assessment of Persimmon tree water status. Irrig. Sci. 2017, 35, 159–167. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Kool, D.; Agam, N.; van Halsema, G.E.; Yermiyahu, U.; Yafe, A.; Presnov, E.; Erel, R.; Majdop, A.; Zipori, I.; et al. Whole-tree water balance and indicators for short-term drought stress in non-bearing ‘Barnea’ olives. Agric. Water Manag. 2010, 98, 124–133. [Google Scholar] [CrossRef]
- Aissaoui, F.; Chehab, H.; Bader, B.; Ben-Salem, A.; M’barki, N.; Laamari, S.; Chihaoui, B.; Mahjoub, Z.; Boujnah, D. Early water stress detection on olive trees (Olea europaea L. cvs ‘chemlali’ and ‘Chetoui’) using the leaf patch clamp pressure probe. Comput. Electron. Agric. 2016, 131, 20–28. [Google Scholar] [CrossRef]
- Marino, G.; Pernice, F.; Marra, F.P.; Caruso, T. Validation of an online system for the continuous monitoring of tree water status for sustainable irrigation managements in olive (Olea europaea L.). Agric. Water Manag. 2016, 177, 298–307. [Google Scholar] [CrossRef]
- Lee, K.M.; Driever, S.M.; Heuvelink, E.; Rüger, S.; Zimmermann, U.; de Gelder, A.; Marcelis, L.F. Evaluation of diel patterns of relative changes in cell turgor of tomato plants using leaf patch clamp pressure probes. Physiol. Plant. 2012, 146, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Burch, D.; Ehrenberger, W.; Bitter, R.; Rüger, S.; Mason, J.; Rodin, J.; Materne, M.; Zimmermann, U.; Spangenberg, G. A novel crop water analysis system: Identification of drought-tolerant genotypes in Brassica napus using the non-invasive magnetic turgor pressure probes. Plant Breeding 2014. [Google Scholar] [CrossRef]
- Bramley, H.; Ehrenberger, W.; Zimmermann, U.; Palta, J.A.; Rüger, S.; Siddique, K.H.S. Non-invasive pressure probes magnetically clamped to leaves to monitor the water status of wheat. Plant Soil 2013, 369, 257–268. [Google Scholar] [CrossRef]
- Bramley, H.; Bitter, R.; Zimmermann, G.; Zimmermann, U. Simultaneous recording of diurnal changes in leaf turgor pressure and stem water status of bread wheat reveal variation in hydraulic mechanisms in response to drought. Funct. Plant Biol. 2015, 42, 1001–1009. [Google Scholar] [CrossRef]
- Calbo, A.G.; Ferreira, M.D.; Pessoa, J.D.C. A leaf lamina compression method for estimating turgor pressure. HortScience 2010, 45, 418–423. [Google Scholar]
- Aroca, R.V.; Calbo, A.G. An automatic and portable Wiltmeter leaf turgor measurement device. Comput. Electron. Agric. 2016, 121, 222–233. [Google Scholar] [CrossRef]
- Aroca, R.V.; Calbo, A.G. Protótipo de Wiltmeter R2: Para a leitura da pressão de turgescência celular de folhas no campo. In Proceedings of the Simpósio Nacional de Instrumentação Agropecuária, São Carlos, SP, Brasil, 18–20 Novembre 2014; pp. 205–208. [Google Scholar]
- Fernández, J.E.; Clothier, B.E.; van Noordwijk, M. Water Uptake. In Root Methods. A Handbook; Smit, A.L., Bengough, A.G., Engels, C., van Noordwijk, M., Pellerin, S., van de Geijn, S.C., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 2000; pp. 460–507. [Google Scholar]
- Topp, G.C.; Davis, J.L. Time-Domain reflectometry (TDR) and its application to irrigation scheduling. Adv. Irrig. 1985, 3, 107–127. [Google Scholar]
- Gibbs, R.D. Shrinkage studies II. The seasonal distribution of water and gas in trees. Can. J. Res. 1930, 2, 425–439. [Google Scholar] [CrossRef]
- Reynolds, E.R.C. Transpiration as related to internal water content. Nature (London) 1965, 208, 1002. [Google Scholar] [CrossRef]
- Constantz, J.; Murphy, F. Monitoring storage moisture in trees using time domain reflectometry. J. Hydrol. 1990, 119, 31–42. [Google Scholar] [CrossRef]
- Nadler, A.; Tyree, M.T. Substituting stem’s water content by electrical conductivity for monitoring water status changes. Soil Sci. Soc. Am. J. 2008, 72, 1006–1013. [Google Scholar] [CrossRef]
- Nadler, A.; Yermiyahu, U.; Nasser, A.; Bark, M.; Green, S.R. Detecting Water Stress in Trees Using Stem Electrical Conductivity Measurements. Soil Sci. Soc. Am. J. 2008, 72, 1014–1024. [Google Scholar] [CrossRef]
- Coskun, A.; Konukcu, F. Detecting water stress in irrigation time in viticulture from stem’s water content and electrical conductivity measurements. Acta Hortic. 2014, 1038, 511–516. [Google Scholar] [CrossRef]
- Dodd, I.C. Root-to-shoot signalling: Assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant Soil 2005, 274, 251–270. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Manzi, M.; Ross, J.J.; Brodribb, T.J.; Gómez-Cadenas, A. Uprooting an abscisic acid paradigm: Shoots are the primary source. Plant Signal Behav. 2016b, 11, e1169359. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Huang, L.; Wang, C.; Hou, R.; Xu, Z.; Qiao, X. Review Research progress on electrical signals in higher plants. Prog. Nat. Sci. 2009, 19, 531–541. [Google Scholar] [CrossRef]
- Fromm, J.; Eschrich, W. Electric signals released from roots of willow (Salix viminalis L.) change transpiration and photosynthesis. J. Plant Physiol. 1993, 141, 673–680. [Google Scholar] [CrossRef]
- Fromm, J.; Fei, H. Electrical signaling and gas exchange in maize plants of drying soil. Plant Sci. 1998, 132, 203–213. [Google Scholar] [CrossRef]
- Gil, P.; Gurovich, L.; Schaffer, B. The electrical response of fruit trees to soil water availability and diurnal light–dark cycles. Plant Signal Behav. 2008, 3, 1026–1029. [Google Scholar] [CrossRef]
- Gurovich, L.; Hermosilla, P. Electric signaling in fruit trees in response to water applications and light–darkness conditions. J. Plant Physiol. 2009, 166, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gil, P.; Gurovich, L.; Schaffer, B.; García, N.; Iturriaga, R. Electrical signaling, stomata conductance, ABA and ethylene content in avocado trees in response to root hypoxia. Plant Signal Behav. 2009, 4, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, P.; Gurovich, L. Evidence for the transmission of information through electricpotentials in injured avocado trees. J. Plant Physiol. 2011, 168, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Gibert, D.; Le Mouël, J.; Lambs, L.; Nicollin, F.; Perrier, F. Sap flow and daily electric potential variations in a tree trunk. Plant Sci. 2006, 171, 572–584. [Google Scholar] [CrossRef]
- Volkov, A.; Ranatunga, R. Plants as environmental biosensors. Plant Signal Behav. 2006, 1, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A. Plant Electrophysiology. Signaling and Responses; Springer Verlag: Berlin, Germany, 2012. [Google Scholar]
- Wang, Z.; Leng, Q.; Huang, L.; Zhao, L.; Xu, Z.; Hou, R.; Wang, C. Monitoring system for electrical signals in plants in the greenhouse and its applications. Biosyst. Eng. 2009, 3, 1–11. [Google Scholar] [CrossRef]
- Rios-Rojas, L.; Tapia, F.; Gurovich, L. Electrophysiological assessment of water stress in fruit-bearing woody plants. J. Plant Physiol. 2014, 171, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Rojas, L.; Morales-Moraga, D.; Alcalde, J.; Gurovich, L.A. Use of plant woody species electrical potential for irrigation scheduling. Plant Signal Behav. 2015, 10, e976487. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; Nikinmaa, E.; Riikonen, A.; Daley, M.; Pettijohn, J.C.; Mikkelsen, T.N.; Phillips, N.; Holbrook, N.M. Linking xylem diameter variations with sap low measurements. Plant Soil 2008, 305, 77–90. [Google Scholar] [CrossRef]
- Steppe, K.; De Pauw, D.J.W.; Lemeur, R.; Vanrolleghem, P.A. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol. 2006, 26, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; De Pauw, D.J.W.; Lemeur, R. A step towards new irrigation scheduling strategies using plant-based measurements and mathematical modelling. Irrig. Sci. 2008, 26, 505–517. [Google Scholar] [CrossRef]
- Drew, D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Steppe, K.; De Pauw, D.J.W.; Lemeur, R. Validation of a dynamic stem diameter variation model and the resulting seasonal chnages in calibrated parameter values. Ecol. Model. 2008, 218, 247–259. [Google Scholar] [CrossRef]
- Motisi, A.; Rossi, F.; Consoli, S.; Papa, R.; Minacapilli, M.; Rallo, G.; Cammalleri, C.; D’Urso, G. Eddy covariance and sap flow measurement of energy and mass exchanges of woody crops in a Mediterranean environment. Acta Hortic. 2012, 951, 121–127. [Google Scholar] [CrossRef]
- Cammalleri, C.; Rallo, G.; Agnese, C.; Ciraolo, G.; Minacapilli, M.; Provenzano, G. Combined use of eddy covariance and sap flow techniques for partition of ET fluxes and water stress assessment in an irrigated olive orchard. Agric. Water Manag. 2013, 120, 89–97. [Google Scholar] [CrossRef]
- Cassiani, G.; Boaga, J.; Vanella, D.; Perri, M.T.; Consoli, S. Monitoring and modelling of soil–plant interactions: The joint use of ERT, sap flow and eddy covariance data to characterize the volume of an orange tree root zone. Hydrol. Earth Syst. Sci. 2015, 19, 2213–2225. [Google Scholar] [CrossRef]
- Ferreira, M.I.; Silvestre, J.; Conceição, N.; Malheiro, C. Crop and stress coefficients in rainfed and deficit irrigation vineyards using sap flow techniques. Irrig. Sci. 2012, 30, 433–447. [Google Scholar] [CrossRef]
- Paço, T.A.; Ferreira, M.I.; Rosa, R.D.; Paredes, P.; Rodrigues, G.C.; Conceição, N.; Pacheco, C.A.; Pereira, L.S. The dual crop coefficient approach using a density factor to simulate the evapotranspiration of a peach orchard: SIMDualKc model versus eddy covariance measurements. Irrig. Sci. 2012, 30, 115–126. [Google Scholar] [CrossRef]
- Paço, T.A.; Pôças, I.; Cunha, M.; Silvestre, J.C.; Santos, F.L.; Paredes, P.; Pereira, L.S. Evapotranspiration and crop coefficients for a super intensive olive orchard. An application of SIMDualKc and METRIC models using ground and satellite observations. J. Hydrol. 2014, 519, 2067–2080. [Google Scholar]
- Centeno, A.; Baeza, P.; Lissarrague, J.R. Relationship between soil and plant water status in wine grapes under various water deficit regimes. Horttechnology 2010, 20, 585–593. [Google Scholar]
- Cancela, J.J.; Fandiño, M.; Rey, B.J.; Martínez, E.M. Automatic irrigation system based on dual crop coefficient, soil and plant water status for Vitis vinifera (cv Godello and cv Mencía). Agric. Water Manag. 2015, 151, 52–63. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Ehrenberger, W.; Sann, C.; Rüger, S.; Sukhorukov, V.; Martín-Palomo, M.J.; Diaz-Espejo, A.; Cuevas, M.V.; Torres-Ruiz, J.M.; Perez-Martin, A.; et al. Concomitant measurements of stem sap flow and leaf turgor pressure in olive trees using the leaf patch clamp pressure probe. Agric. Water Manag. 2012, 114, 50–58. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Buckley, T.N.; Sperry, J.S.; Cuevas, M.V.; De Cires, A.; Elsayed-Farag, S.; Martin-Palomo, M.J.; Muriel, J.L.; Perez-Martin, A.; Rodriguez-Dominguez, C.M.; et al. Steps toward an improvement in process-based models of water use by fruit trees: A case study in olive. Agric. Water Manag. 2012, 114, 37–49. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Fereres, E. Irrigation scheduling of almond trees with trunk diameter sensors. Irrig. Sci. 2004, 23, 11–19. [Google Scholar] [CrossRef]
- Conejero, W.; Alarcón, J.J.; García-Orellana, Y.; Nicolás, E.; Torrecillas, A. Evaluation of sap flow and trunk diameter sensors for irrigation scheduling in early maturing peach trees. Tree Physiol. 2007, 27, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- García-Orellana, Y.; Ruiz-Sánchez, M.C.; Alarcón, J.J.; Conejero, W.; Ortuño, M.F.; Nicolás, E.; Torrecillas, A. Preliminary assessment of the feasibility of using maximum daily trunk shrinkage for irrigation scheduling in lemon trees. Agric. Water Manag. 2007, 89, 167–171. [Google Scholar] [CrossRef]
- Naor, A.; Cohen, S. Sensitivity and variability of maximum trunk shrinkage, midday stem water potential and transpiration rate in response to withholding irrigation from field-grown apple trees. HortScience 2003, 38, 547–551. [Google Scholar]
- Pereira, A.R.; Green, S.R.; Villa-Nova, N.A. Penman-Monteith reference evapotranspiration adapted to estimate irrigated tree transpiration. Agric. Water Manag. 2006, 83, 153–161. [Google Scholar] [CrossRef]
- Testi, L.; Villalobos, F.J.; Orgaz, F.; Fereres, E. Water requirements of olive orchards: I. Simulation of daily evapotranspiration for scenario analysis. Irrig. Sci. 2006, 24, 69–76. [Google Scholar] [CrossRef]
- Orgaz, F.; Testi, L.; Villalobos, F.J.; Fereres, E. Water requirements of olive orchards-II: Determination of crop coefficients for irrigation scheduling. Irrig. Sci. 2006, 24, 77–84. [Google Scholar] [CrossRef]
- Green, S.R.; Kirkham, M.B.; Clothier, B.E. Root uptake and transpiration: From measurements and models to sustainable irrigation. Agric. Water Manag. 2006, 86, 165–176. [Google Scholar] [CrossRef]
- Egea, G.; Diaz-Espejo, A.; Fernández, J.E. Soil moisture dynamics in a hedgerow olive orchard underwell-watered and deficit irrigation regimes: Assessment, predictionand scenario analysis. Agric. Water Manag. 2016, 164, 197–211. [Google Scholar] [CrossRef]
- Foster, T.; Brozović, N.; Butler, A.P.; Neale, C.M.U.; Raes, D.; Steduto, P.; Fereres, E.; Hsiao, T.C. AquaCrop-OS: An open source version of FAO’s crop water productivity model. Agric. Water Manag. 2017, 181, 18–22. [Google Scholar] [CrossRef]
- Egea, G.; Fernández, J.E.; Alcon, F. Financial assessment of adopting irrigation technology for plant-based regulated deficit irrigation scheduling in super high-density olive orchards. Agric. Water Manag. 2017, 187, 47–56. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Fereres, E.; Mata, M.; Girona, J.; Cohen, M. Sensitivity of continuous and discrete plant and soil water status monitoring in peach trees subjected to deficit irrigation. J. Am. Soc. Hortic. Sci. 1999, 124, 437–444. [Google Scholar]
- Intrigliolo, D.S.; Castel, J.R. Usefulness of diurnal trunk shrinkage as a water stress indicator in plum trees. Tree Physiol. 2006b, 26, 303–311. [Google Scholar] [CrossRef]
- Galindo, A.; Rodríguez, P.; Mellisho, C.D.; Torrecillas, E.; Moriana, A.; Cruz, Z.N.; Conejero, W.; Moreno, F.; Torrecillas, A. Assessment of discretely measured indicators and maximum daily trunk shrinkage for detecting water stress in pomegranate trees. Agric. For. Meteorol. 2013, 180, 58–65. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Steduto, P.; Fereres, E. A systematic and quantitative approach to improve water use efficiency in agriculture. Irrig. Sci. 2007, 25, 209–231. [Google Scholar] [CrossRef]
- Fernández, J.E.; Diaz-Espejo, A.; D’Andria, R.; Sebastiani, L.; Tognetti, R. Potential and limitations of improving olive orchard design and management through modelling. Plant Biosyst. 2008c, 142, 130–137. [Google Scholar]
- Steduto, P.; Hsiao, T.C.; Fereres, E. On the conservative behavior of biomass water productivity. Irrig. Sci. 2007, 25, 189–207. [Google Scholar] [CrossRef]
- Perry, C. Accounting for water use: Terminology and implications for saving water and increasing production. Agric. Water Manag. 2011, 98, 1840–1846. [Google Scholar] [CrossRef]
- Molden, D. A Water-productivity framework for understanding and action. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; Kijne, J.W., Barker, R., Molden, D., Eds.; International Water Management Institute: Colombo, Sri Lanka, 2003; pp. 1–18. [Google Scholar]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Evett, S.R.; Schwartz, R.C.; Casanova, J.J.; Heng, L.K. Soil water sensing for water balance, ET and WUE. Agric. Water Manag. 2012, 104, 1–9. [Google Scholar] [CrossRef]
- Pereira, L.S.; Cordery, I.; Iacovides, I. Improved indicators of water use performance and productivity for sustainable water conservation and saving. Agric. Water Manag. 2012, 108, 39–51. [Google Scholar] [CrossRef]
- Bouman, B.A.M. A conceptual framework for the improvement of crop water productivity at different spatial scales. Agric. Syst. 2007, 93, 43–60. [Google Scholar] [CrossRef]
- Ali, M.H.; Talukder, M.S.U. Increasing water productivity in crop production—A synthesis. Agric. Water Manag. 2008, 95, 1201–1213. [Google Scholar] [CrossRef]
- Flexas, J.; Galmés, J.; Gallé, A.; Gulías, J.; Pou, A.; Ribas-Carbo, M.; Tomàs, M.; Medrano, H. Improving water use efficiency in grapevines: Potential physiological targets for biotechnological improvement. Aust. J. Grape Wine Res. 2010, 16, 106–121. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.A.; Pérez-Urrestarazu, L.; Camacho-Poyato, E.; Montesinos, P. The paradox of irrigation scheme modernization: More efficient water use linked to higher energy demand. Span. J. Agric. Res. 2011, 9, 1000–1008. [Google Scholar] [CrossRef]
- Alcon, F.; Egea, G.; Nortes, P.A. Financial feasibility of implementing regulated and sustained deficit irrigation in almond orchards. Irrig. Sci. 2013, 31, 931–941. [Google Scholar] [CrossRef]
- Berbel, J.; Mateos, L. Does investment in irrigation technology necessarily generate rebound effects? A simulation analysis based on agro-economic model. Agric. Syst. 2014, 128, 25–34. [Google Scholar] [CrossRef]
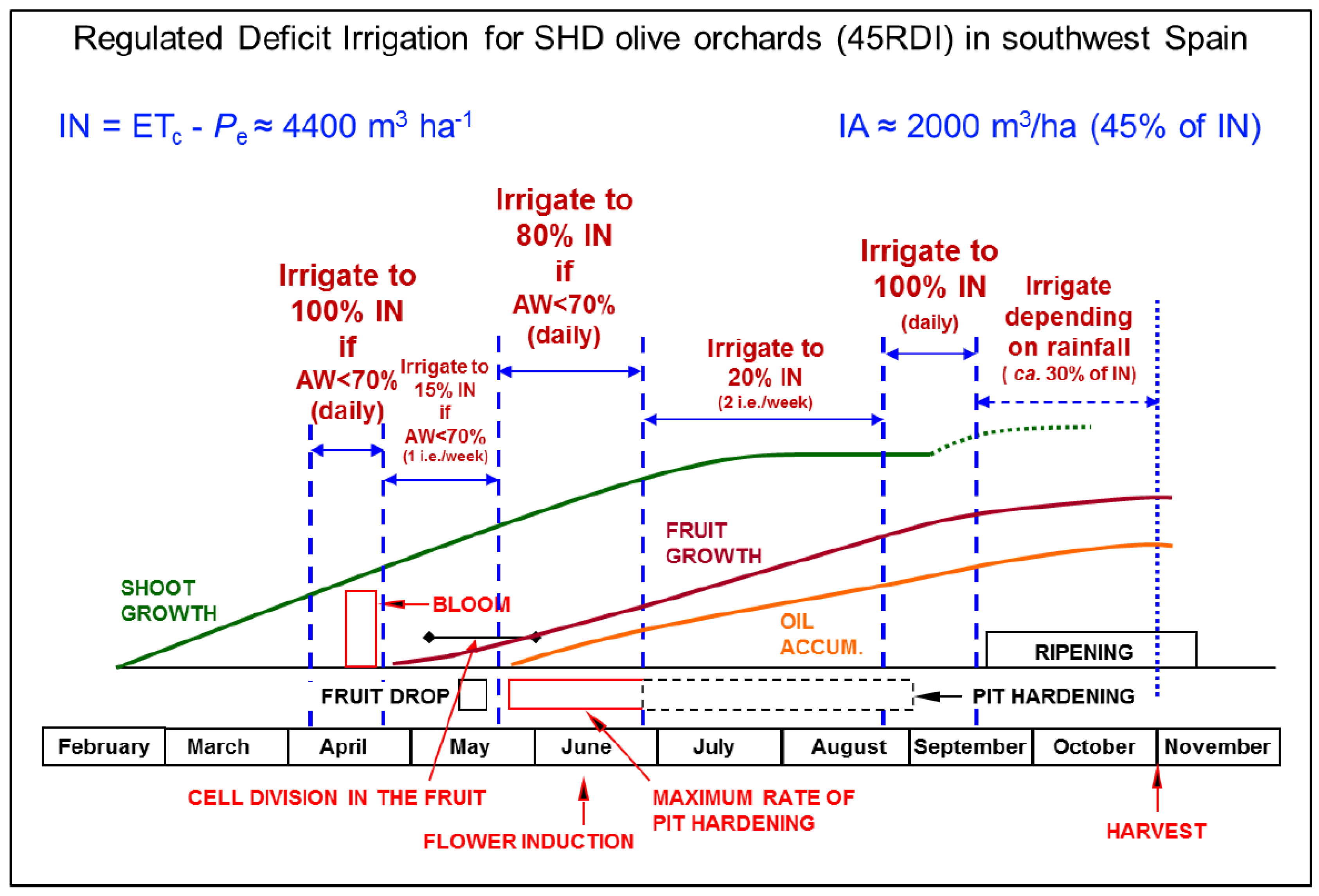


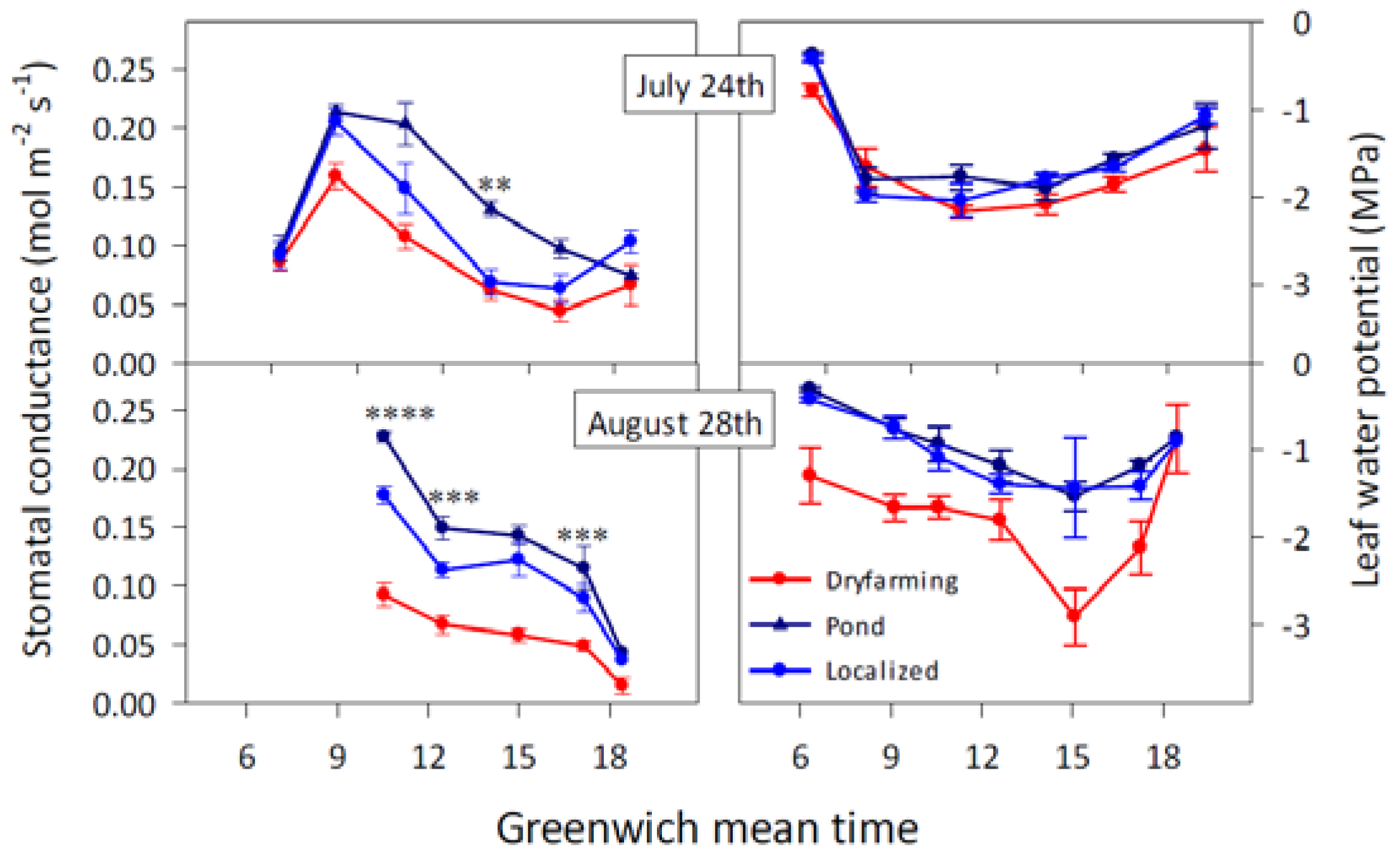
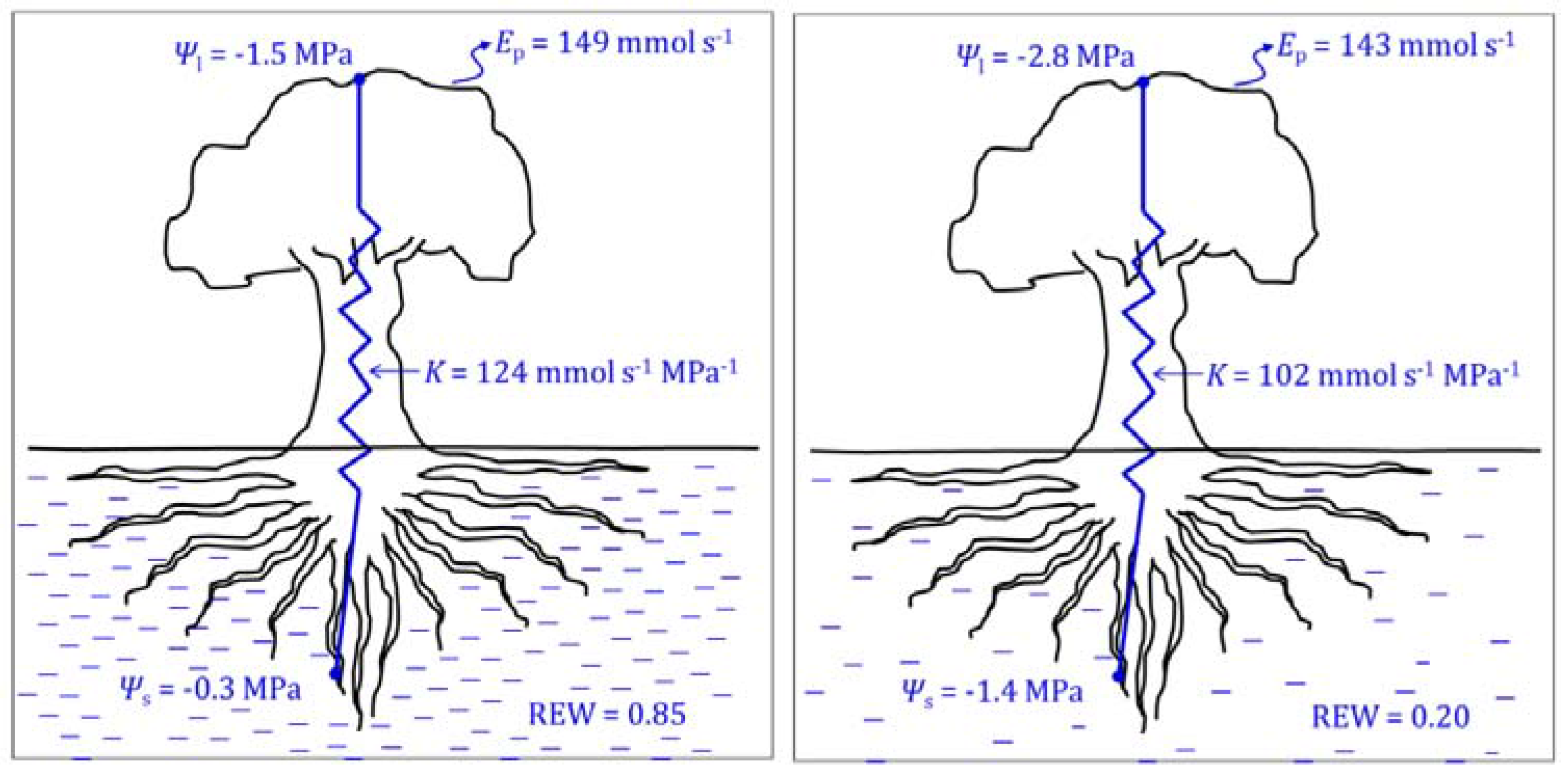





| Strategy | Definition and Remarks | Reference Paper |
|---|---|---|
| Supplementary or complementary irrigation | A single irrigation event is applied when a fixed threshold for water stress is achieved. Just 2 or 3 irrigation events in the irrigation season. Used when water for irrigation is very scarce or in temperate regions with low evapotranspiration rates or high rainfall. It can lead to substantial increases in crop performance, as compared to dry-farming, if the irrigation events are applied at the right moments of the growing cycle. | Abd-Eel-Rahman and El-Sharkawi [8]; Lavee et al. [9]; Proietti et al. [10] |
| Low frequency deficit irrigation (LFDI) | The soil is left to dry until the readily available water is consumed. Irrigation is then applied, until field capacity. This is repeated several times along the irrigation season. | Lavee and Wodner [11] |
| Sustained deficit irrigation (SDI) | A fixed fraction of the crop water needs is applied all throughout the irrigation season. Irrigation is applied daily or 2–4 times per week. | Goldhamer et al. [12]; Grattan et al. [13]; Ramos and Santos [14] |
| Partial root zone drying (PRD) | Similar to SDI, but water is applied to half of the root zone, switching to the other half every 2–3 weeks. | Dry et al. [15]; Fernández et al. [16] |
| Regulated deficit irrigation (RDI) | Irrigation amounts equal or close to the crop water needs are supplied at the phenological stages most sensitive to drought. Irrigation in those periods is applied daily, or at least several times per week. For the rest of the crop cycle, irrigation is drastically reduced (not only the dose is reduced but also the frequency, to one or two irrigation events per week) or even withheld. | Chalmers et al. [17]; Goldhamer [18]; Fernández et al. [6] |
| Measurements Related To | Water Stress Indicator | Symbol or Abbreviation | Units | Remarks |
|---|---|---|---|---|
| Leaf ↔ air gas exchange | Stomatal conductance | gs | mol m−2 s−1 | Recorded with a porometer or an IRGA in plants with waterproof cuticles. |
| gsmax | mol m−2 s−1 | Maximum stomatal conductance, i.e., gs values at the time of maximum stomatal opening. | ||
| Leaf conductance | gl | mol m−2 s−1 | Recorded with a porometer or an IRGA in plants with leaf cuticles permeable to water. | |
| glmax | mol m−2 s−1 | Maximum leaf conductance, i.e., gl values at the time of maximum stomatal opening. | ||
| Net CO2 assimilation or net photosynthesis | A, PN | µmol m−2 s−1 | Net amount of CO2 assimilated per square meter of leaf and per second at the time of the measurement. | |
| Plant water status | Predawn water potential | Ψpd | MPa | |
| Leaf water potential | Ψl | MPa | ||
| Midday leaf water potential | Midday Ψl | MPa | Ψl measured at the time of the day when minimum values are recorded. | |
| Stem water potential | Ψstem | MPa | ||
| Midday stem water potential | Midday Ψstem | MPa | Ψstem measured at the time of the day when minimum values are recorded. | |
| Xylem water potential | Ψx | MPa | Water potential of a branch or trunk, measured with Scholander-type chambers or with microtensiometers. | |
| Balancing pressure | Pb | MPa | Name given by Zimmerman et al. [23] to the output of plant water potential measured with a Scholander-type chamber. | |
| Canopy temperature | Canopy temperature | Tc | °C | |
| Temperature difference between the canopy and the surrounding air | ΔTcanopy-air, Tc − Ta | °C | ||
| Crop water stress index | CWSI | - | Idso et al. [24] stated that its value ranges from 0 (no water stress) to 1 (maximum water stress). | |
| NIR spectroscopy | Estimated Ψl or Ψstem | Ψl or Ψstem | MPa | Leaf or canopy spectral measurements are correlated with values of Ψl or Ψstem made with a Scholander-type chamber, to derive an observed Ψ vs. predicted Ψ relationship. |
| Sap flow | Sap flux density | Jp or Jv | m3 m−2 s−1 | “Flow” points to matter, heat or momentum that is in motion. “Flux” expresses the amount of a substance passing through a given surface per unit of time. “Flux density” is the flux per unit of surface. |
| Sap flux | Qp | m3 s−1 | Total sap flux per plant. Often assumed as equal to total plant transpiration (Ep). | |
| Stem diameter | Maximum daily stem diameter | MXSD | µm | |
| Minimum daily stem diameter | MNSD | µm | ||
| Maximum daily shrinkage | MDS | µm | Difference between the MXSD and the MNSD measured on the day. | |
| Daily recovery | DR | µm | Difference between the MXSD measured on a day and the MNSD measured on the previous day. | |
| Daily growth | DG | µm | Difference between the MXSD measured on a day and the MXSD measured on the previous day. | |
| Stem growth rate | SGR | µm | Average DG for n days. The value of n usually ranges from 3 to 6. | |
| Cumulative growth | CG | µm | The summatory of DG for n days. | |
| Early daily shrinkage | EDS | µm | Stem shrinkage measured between 09.00 and 12.00 hours solar time. | |
| Fruit diameter | Fruit diameter | D | mm | |
| Leaf thickness | Leaf thickness | LT | µm | |
| Leaf turgor pressure | Leaf turgor pressure | Pc | kPa | |
| Pressure signal | Pp | kPa | The output pressure signal provided by the LPCP probe. | |
| Stem water content | Volumetric stem water content | θstem | cm3 cm−3 | Derived from measurements with the TDR method. |
| Stem electrical conductivity | σstem | mS m−1 | Derived from measurements with the TDR method. | |
| Electrical potential | Voltage difference between the leaf zone and the base of the stem | ΔVL-S | mV | |
| Electrical potential | EP | mV | ||
| Relative intensity of the signal | ϕex | % | ||
| Recovery time signal | τ | S |
| Characteristic | Definition | Remarks |
|---|---|---|
| Variability or noise | Accounts for the plant-to-plant variability | Quantified by the coefficient of variation (CV). It must be considered relative to the signal strength. It may increase with water stress. |
| Signal strength or signal intensity, also called signal value or just signal | Signal intensity = actual WSI value/reference WSI value | A high signal value means that the water stress indicator (WSI) responds intensively even to mild water deficits. If the signal strength is sufficient, the noise caused by a high tree-to-tree variability may not be as critical. The actual WSI value is that derived from records made in representative plants, usually under deficit irrigation. See Fernández and Cuevas [113] for details on different approaches to obtain the reference WSI value. |
| Sensitivity, or the signal:noise ratio | Sensitivity = signal intensity/CV | The sensitivity can be calculated if the WSI is based on absolute data, but not when values are relative. See Fernández and Cuevas [113] for alternatives. |
| Earliness | Response of the WSI to the onset of water stress | The WSI responds early to water stress if high signal intensity values are recorded soon after the onset of plant water stress. |
| Reliability | Accounts for the capacity of the sensor and related equipment to perform their required functions under stated conditions for a specified period of time. | All the methods mentioned in Section 2.2 yield reliable WSI. |
| Robustness | Accounts for the capacity of the sensor and related equipment to cope with variations in their operating environment with minimal damage, alteration, or loss of functionality. | All the sensors and related systems mentioned in Section 2.2 are able to work under field conditions for large periods of time. |
| The system must be inexpensive and easy to install, operate and maintain. |
| The system must be reliable and robust, capable of working under field conditions for the whole irrigation season. |
| The system must allow for both automated and continuous collection of clean data and data transmission, i.e., it must be suitable for working properly in electromagnetic-polluted environments and in areas with low cover for data transmission. |
| The system must have low power requirements (e.g., batteries fed with solar panels). |
| The water stress indicator derived from the collected records must be highly sensitive, i.e., it must show a high signal: noise ratio. |
| The indicator must also show an early response to the onset of water stress. |
| The indicator must be related to a variable of economic importance, such as crop yield or fruit quality. |
| The indicator, or the raw sensor outputs in case they are used without any further data processing, must be easy to interpret. |
| If the indicator or raw outputs are not easy to interpret, the system must be provided with an application for visual readouts, graphs, historical records, and other tools to facilitate data interpretation. |
| The system should be easily implemented with an application for the combined use of the chosen indicator with a weather prediction system. This will improve the user capacity for adjusting the timing and intensity of irrigation under changing weather conditions. |
| The system should be easily combined with methods to define areas with characteristic water-stress behaviour within the orchard, such as airborne imagery. This allows for precise irrigation in large, highly variable orchards. |
| The indicator must be suitable for automatic irrigation scheduling and control. In this case, the system should be suitable to be implemented with expert systems, alarms, and other tools for an early detection and lower impact of malfunctions. |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, J.E. Plant-Based Methods for Irrigation Scheduling of Woody Crops. Horticulturae 2017, 3, 35. https://doi.org/10.3390/horticulturae3020035
Fernández JE. Plant-Based Methods for Irrigation Scheduling of Woody Crops. Horticulturae. 2017; 3(2):35. https://doi.org/10.3390/horticulturae3020035
Chicago/Turabian StyleFernández, José Enrique. 2017. "Plant-Based Methods for Irrigation Scheduling of Woody Crops" Horticulturae 3, no. 2: 35. https://doi.org/10.3390/horticulturae3020035





