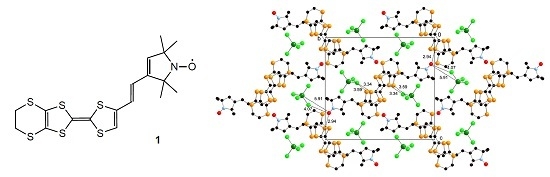
New Ethylenedithio-TTF Containing a 2,2,5,5-Tetramethylpyrrolin-1-yloxyl Radical through a Vinylene Spacer and Its FeCl4− Salt—Synthesis, Physical Properties and Crystal Structure Analyses
Abstract
:1. Introduction
2. Results and Discussion
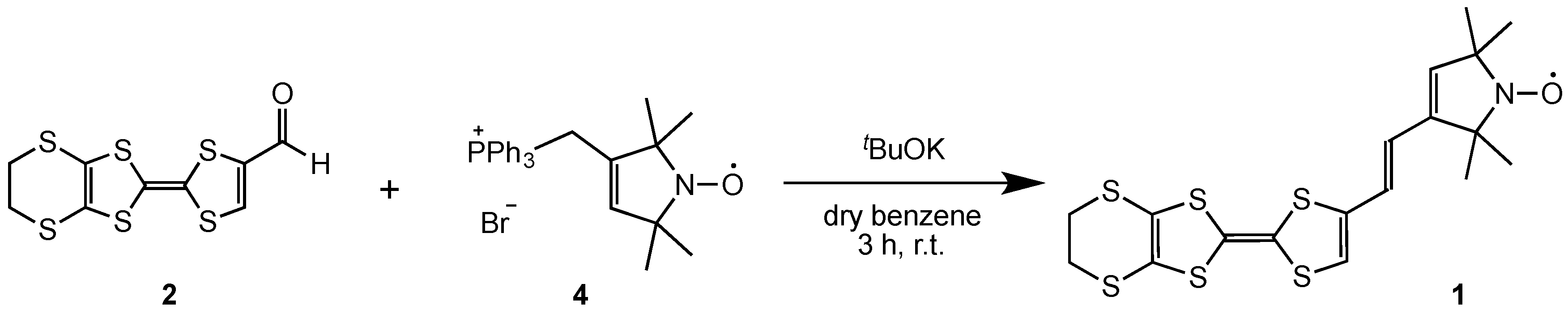
2.1. Synthesis of Donor 1
2.2. Electrochemical Properties and DFT Calculation of Donor 1
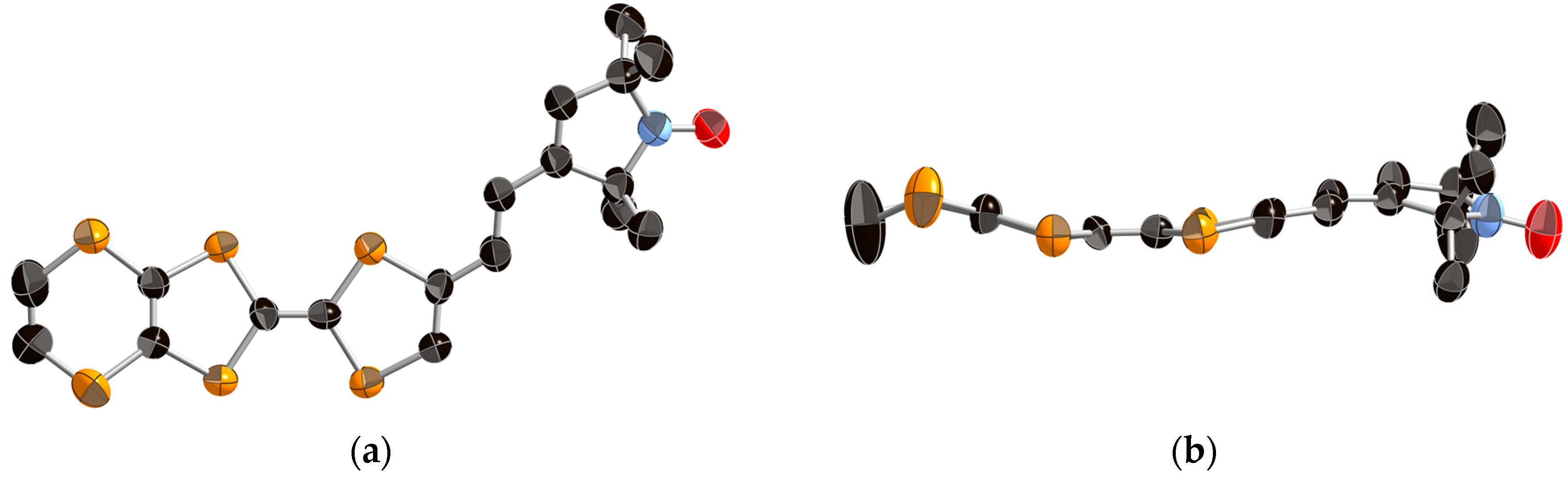
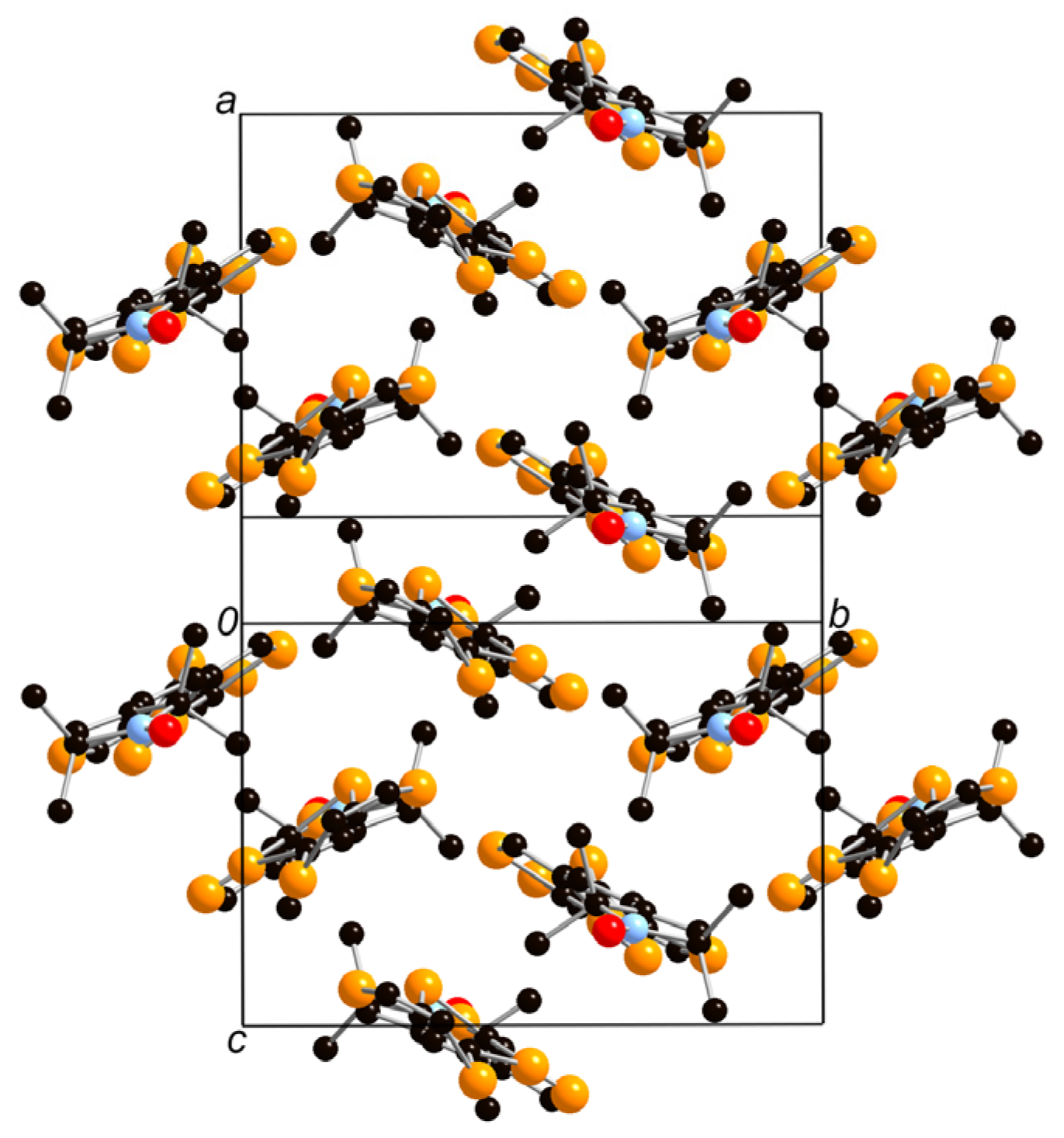
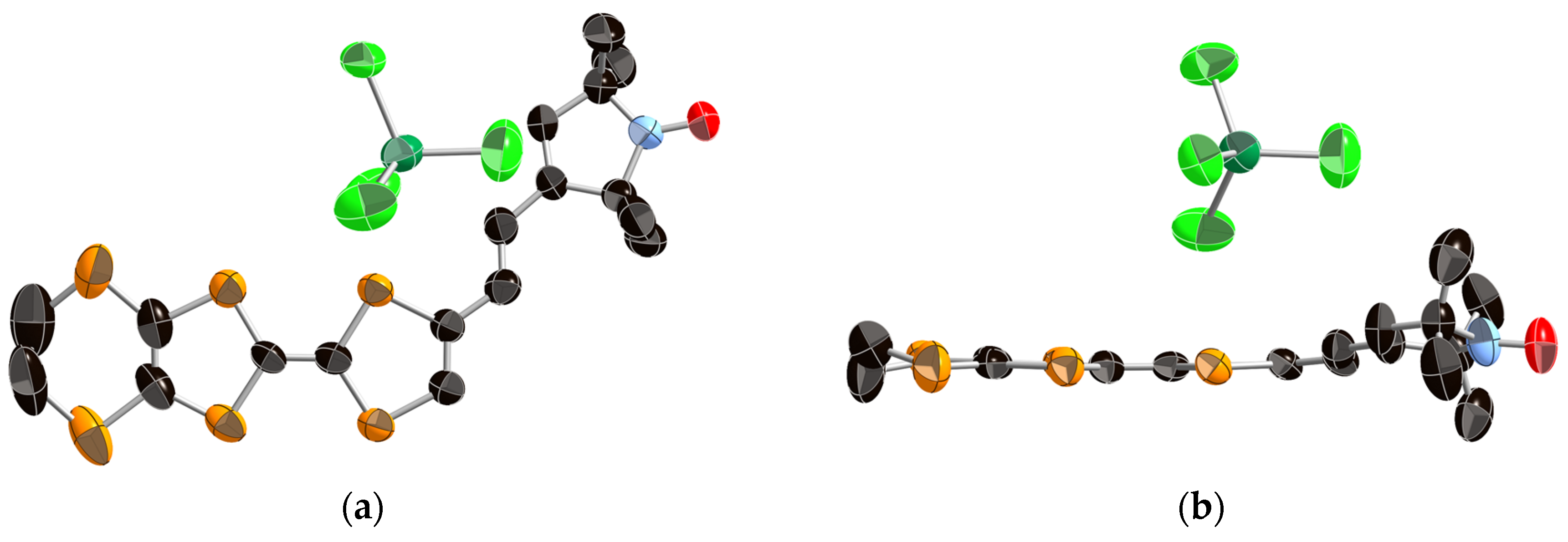
2.3. Crystal Structure Analysis and Physical Properties of Donor 1
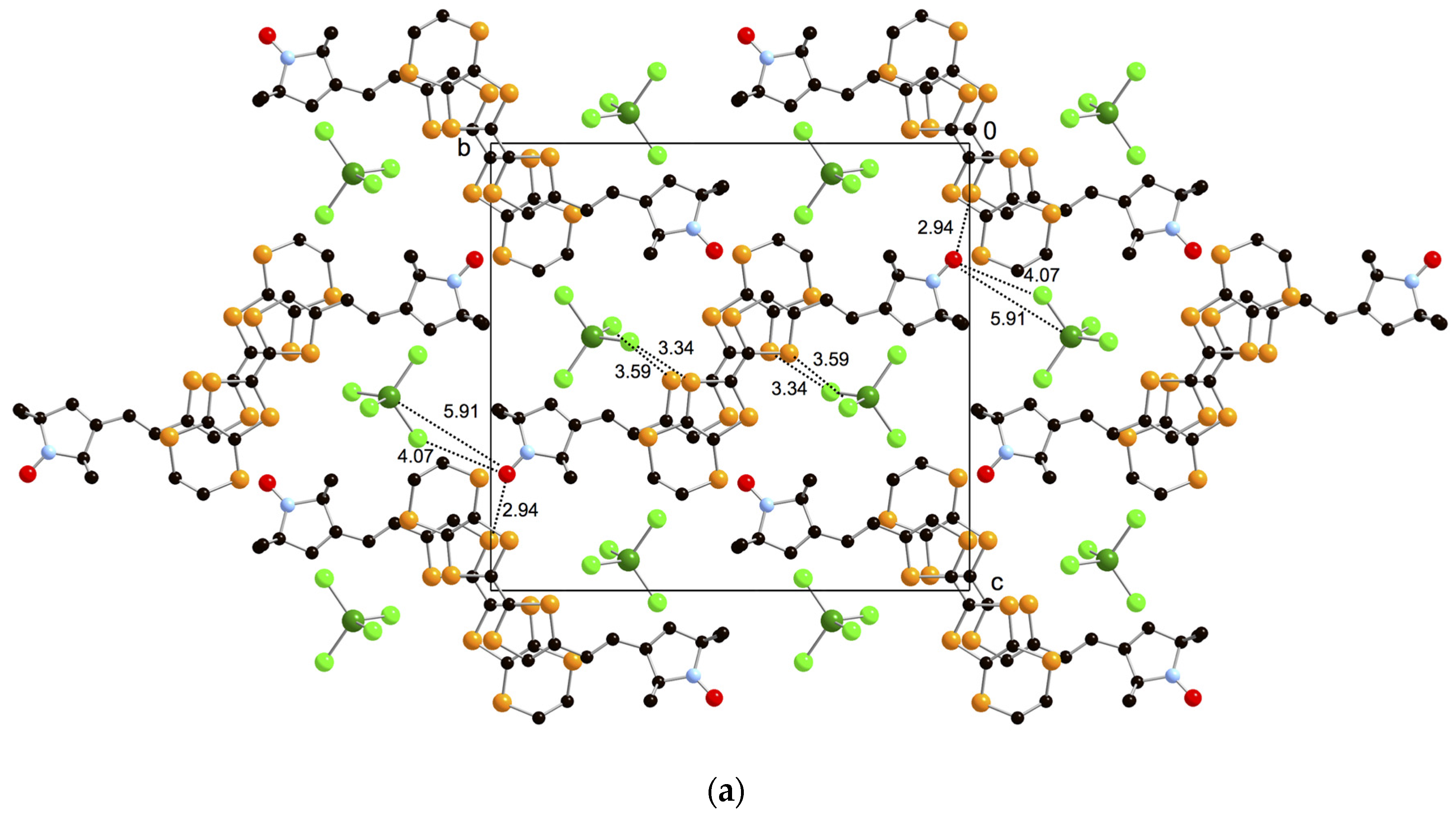
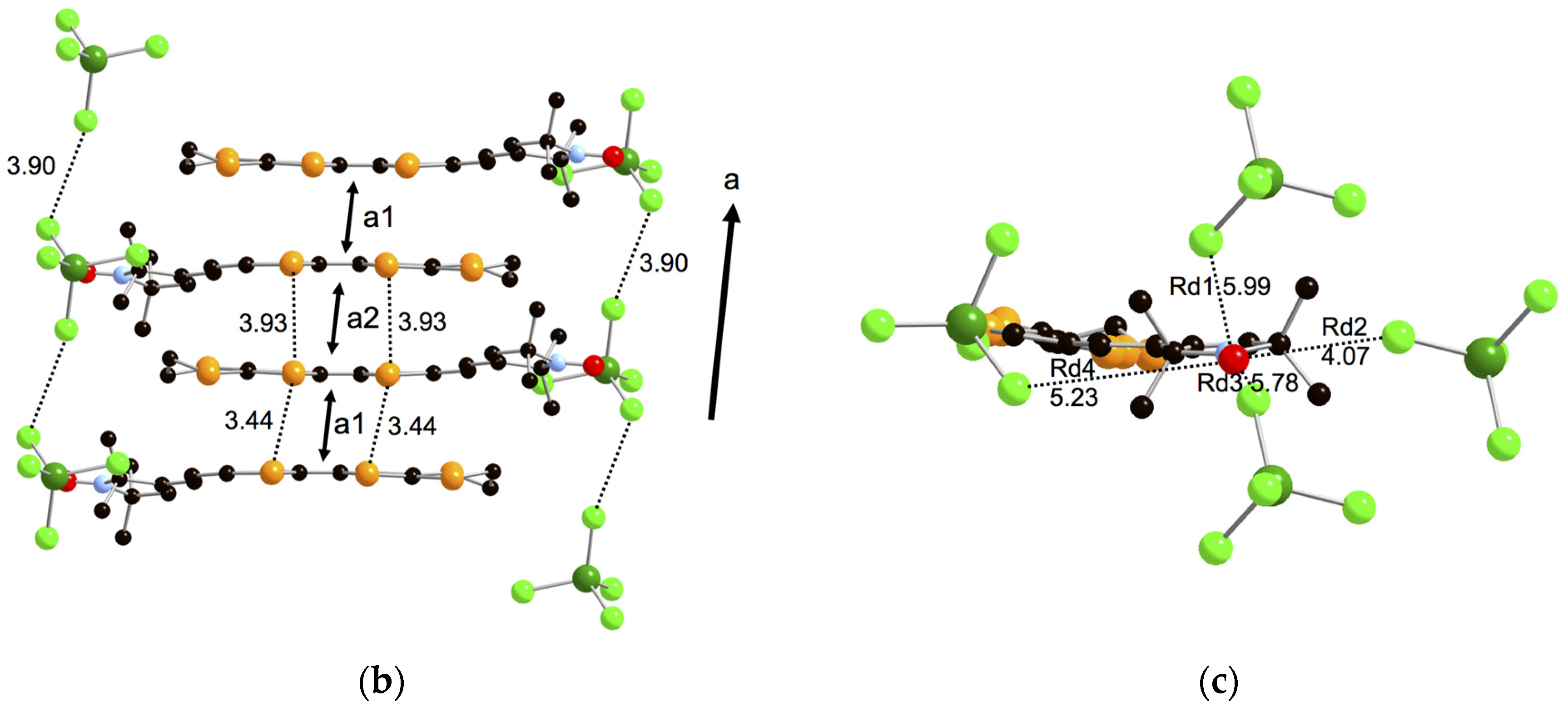
2.4. Crystal Structure Analyses and Magnetic Properties of the Cation Rarical Salts of Donor 1
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Uji, S.; Shinagawa, H.; Terashima, T.; Yakabe, T.; Terai, Y.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-field-induced superconductivity in a two-dimensional organic conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Fujiwara, E.; Nakazawa, Y.; Narymbetov, B.Z.; Kato, K.; Kobayashi, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. A novel antiferromagnetic organic superconductor κ-(BETS)2FeBr4 [where BETS = bis(ethylenedithio)tetraselenafulvalene]. J. Am. Chem. Soc. 2001, 123, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Cui, H.B.; Kobayashi, A. Organic metals and superconductors based on BETS (BETS = bis(ethylenedithio)tetraselenafulvalene). Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Xiao, X.; Fujiwara, H.; Sugimoto, T.; Nakazumi, H.; Noguchi, S.; Fujimoto, T.; Yasuzuka, S.; Yoshino, H.; Murata, K.; et al. A metallic (EDT-DSDTFVSDS)2FeBr4 salt: Antiferromagnetic ordering of d spins of FeBr4– ions and anomalous magnetoresistance due to preferential π-d interaction. J. Am. Chem. Soc. 2006, 128, 11746–11747. [Google Scholar] [CrossRef] [PubMed]
- Maesato, M.; Kawashima, T.; Furushima, Y.; Saito, G.; Kitagawa, H.; Shirahata, T.; Kibune, M.; Imakubo, T. Spin-Flop Switching and Memory in a Molecular Conductor. J. Am. Chem. Soc. 2012, 134, 17452–17455. [Google Scholar]
- Sugano, T.; Fukasawa, T.; Kinoshita, M. Magnetic interactions among unpaired electrons in charge-transfer complexes of organic donors having a neutral radical. Synth. Met. 1991, 43, 3281–3284. [Google Scholar] [CrossRef]
- Kumai, R.; Matsushita, M.M.; Izuoka, A.; Sugawara, T. Intramolecular Exchange Interaction in a Novel Cross-Conjugated Spin System Composed of π-Ion Radical and Nitronyl Nitroxide. J. Am. Chem. Soc. 1994, 116, 4523–4524. [Google Scholar] [CrossRef]
- Komatsu, H.; Matsushita, M.M.; Yamamura, S.; Sugawara, Y.; Suzuki, K.; Sugawara, T. Influence of Magnetic Field upon the Conductance of a Unicomponent Crystal of a Tetrathiafulvalene-Based Nitronyl Nitroxide. J. Am. Chem. Soc. 2010, 132, 4528–4529. [Google Scholar] [CrossRef] [PubMed]
- Souto, M.; Solano, M.V.; Jensen, M.; Bendixen, D.; Delchiaro, F.; Girlando, A.; Painelli, A.; Jeppesen, J.O.; Rovira, C.; Ratera, I.; et al. Self-Assembled Architectures with Segregated Donor and Acceptor Units of a Dyad Based on a Monopyrrolo-Annulated TTF-PTM Radical. Chem. Eur. J. 2015, 21, 8816–8825. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.M.; Kawakami, H.; Sugawara, T.; Ogata, M. Molecule-based system with coexisting conductivity and magnetism and without magnetic inorganic ions. Phys. Rev. B 2008, 77, 195208. [Google Scholar] [CrossRef]
- Fujiwara, H.; Kobayashi, H. New pi-extended organic donor containing a stable TEMPO radical as a candidate for conducting magnetic multifunctional materials. Chem. Commun. 1999, 2417–2418. [Google Scholar] [CrossRef]
- Fujiwara, H.; Fujiwara, E.; Kobayashi, H. Novel π-electron donors for magnetic conductors containing a PROXYL radical. Chem. Lett. 2002, 1048–1049. [Google Scholar] [CrossRef]
- Fujiwara, H.; Fujiwara, E.; Kobayashi, H. Synthesis, structure and physical properties of donors containing a PROXYL radical. Synth. Met. 2003, 135, 533–534. [Google Scholar] [CrossRef]
- Fujiwara, H.; Lee, H.J.; Kobayashi, H.; Fujiwara, E.; Kobayashi, A. A novel TTP donor containing a PROXYL radical for magnetic molecular conductors. Chem. Lett. 2003, 32, 482–483. [Google Scholar] [CrossRef]
- Fujiwara, H.; Lee, H.J.; Cui, H.B.; Kobayashi, H.; Fujiwara, E.; Kobayashi, A. Synthesis, structure, and physical properties of a new organic conductor based on a π-extended donor containing a stable 2,2,5,5-tetramethyl-1-pyrrolidinyloxy radical. Adv. Mater. 2004, 16, 1765–1769. [Google Scholar] [CrossRef]
- Otsubo, S.; Cui, H.B.; Lee, H.J.; Fujiwara, H.; Takahashi, K.; Okano, Y.; Kobayashi, H. A magnetic organic conductor based on a π donor with a stable radical and a magnetic anion—A step to magnetic organic metals with two kinds of localized spin systems. Chem. Lett. 2006, 35, 130–131. [Google Scholar] [CrossRef]
- Fujiwara, E.; Aonuma, S.; Fujiwara, H.; Sugimoto, T.; Misaki, Y. New π-electron donors with a 2,2,5,5-tetramethylpyrrolin-1-yloxyl radical designed for magnetic molecular conductors. Chem. Lett. 2008, 37, 84–85. [Google Scholar] [CrossRef]
- Krishna, M.C.; DeGraff, W.; Hankovszky, O.H.; Sár, C.P.; Kálai, T.; Jekő, J.; Russo, A.; Mitchell, J.B.; Hideg, K. Studies of Structure−Activity Relationship of Nitroxide Free Radicals and Their Precursors as Modifiers Against Oxidative Damage. J. Med. Chem. 1998, 41, 3477–3492. [Google Scholar] [CrossRef] [PubMed]
- Garin, J.; Orduna, J.; Uriel, S.; Moore, A.J.; Bryce, M.R.; Wegener, S.; Yufit, D.S.; Howard, J.A.K. Improved Syntheses of Carboxytetrathiafulvalene, Formyltetrathiafulvalene and (Hydroxymethyl)Tetrathiafulvalene —Versatile Building-Blocks for New Functionalized Tetrathiafulvalene Derivatives. Synthesis 1994, 1994, 489–493. [Google Scholar] [CrossRef]
- Kálai, T.; Balog, M.; Jekö, J.; Hideg, K. Synthesis and Reactions of a Symmetric Paramagnetic Pyrrolidine Diene. Synthesis 1999, 1999, 973–980. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The Intermolecular Interaction of Tetrathiafulvalene and Bis(ethylenedithio)tetrathiafulvalene in Organic Metals. Calculation of Orbital Overlaps and Models of Energy-band Structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Mori, T.; Katsuhara, M. Estimation of πd-Interactions in Organic Conductors Including Magnetic Anions. J. Phys. Soc. Jpn. 2002, 71, 826–844. [Google Scholar] [CrossRef]
- Mori, T.; Katsuhara, M.; Akutsu, H.; Kikuchi, K.; Yamada, J.; Fujiwara, H.; Matsumoto, T.; Sugimoto, T. Estimation of π d-interactions in magnetic molecular conductors. Polyhedron 2005, 24, 2315–2320. [Google Scholar] [CrossRef]
- Batail, P.; Livage, C.; Parkin, S.S.P.; Coulon, C.; Martin, J.D.; Canadell, E. Antiperovskite Structure with Ternary Tetrathiafulvalenium Salts: Construction, Distortion, and Antiferromagnetic Ordering. Angew. Chem. Int. Ed. Engl. 1991, 30, 1498–1500. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Crystallogr. 1994, 27, 435. [Google Scholar] [CrossRef]
- Beurskens, P.T.A.; Beurskens, G.; Bosman, W.P.; de Gelder, D.; Israel, R.; Smith, J.M.M. Technical Report of the Crystallography Laboratory; University of Nijmegen: Nijmegen, The Netherlands, 1994. [Google Scholar]
- CrystalStructure, 4.0; Crystal Structure Analysis Package; Rigaku Corp.: Akishima, Tokyo, Japan, 2000–2010.
- König, E. Landolt Bornstein, Group II: Atomic and Molecular Physics, Vol. 2, Magnetic Properties of Coordination and Organometallic Transition Metal Compounds; Spring: Berlin, Germany, 1966. [Google Scholar]

| Compound | E1 | E2 | E3 | E2–E1 |
|---|---|---|---|---|
| 1 | +0.50 | +0.87 | +0.96 | 0.37 |
| EDT-TTF | +0.48 | +0.88 | - | 0.40 |
| 5 | - | - | +0.99 | - |
| Crystal Data | 1 | 1·FeCl4 | 1·GaCl4 |
| Temperature/K | 293.3 | 293.3 | 293.1 |
| Chemical Formula | C18H20NOS6 | C18H20NOS6FeCl4 | C18H20NOS6GaCl4 |
| Formula weight | 458.72 | 656.38 | 670.25 |
| Crystal color, habit | orange, platelet | black, platelet | black, platelet |
| Dimensions, mm | 0.50 × 0.40 × 0.10 | 0.10 × 0.05 × 0.04 | 0.10 × 0.10 × 0.08 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| a/Å | 15.172(9) | 7.209(3) | 7.242(2) |
| b/Å | 12.274(8) | 20.106(7) | 20.166(5) |
| c/Å | 11.458(7) | 18.831(7) | 18.930(5) |
| β/° | 93.166(9) | 95.504(11) | 95.571(6) |
| V/Å3 | 2130(3) | 2717(2) | 2751.7(11) |
| Space group, Z | P21/c, 4 | P21/n, 4 | P21/n, 4 |
| Dcalc./g·cm−3 | 1.430 | 1.605 | 1.618 |
| µ/cm−1 | 6.50 | 14.217 | 18.561 |
| F000 | 956.00 | 1332.00 | 1352.00 |
| 2θmax/° | 61.7 | 61.1 | 60.8 |
| Reflections collected | 18831 | 29638 | 29387 |
| Independent reflections | 5971 (Rint = 0.0749) | 7008 (Rint = 0.0758) | 7104 (Rint = 0.0474) |
| Reflections used | 1879 (I > 2.50σ(I)) | 2308 (I > 2.50σ(I)) | 3520 (I > 3.00σ(I)) |
| Number of variables | 255 | 280 | 280 |
| GOF on F | 1.087 | 1.034 | 1.077 |
| R1 | 0.0688 (I > 2.50σ(I)) | 0.0704 (I > 2.50σ(I)) | 0.0787 (I > 3.00σ(I)) |
| wR | 0.0738 (I > 2.50σ(I)) | 0.0818 (I > 2.50σ(I)) | 0.0733(I > 3.00σ(I)) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikiri, K.; Fujiwara, H. New Ethylenedithio-TTF Containing a 2,2,5,5-Tetramethylpyrrolin-1-yloxyl Radical through a Vinylene Spacer and Its FeCl4− Salt—Synthesis, Physical Properties and Crystal Structure Analyses. Magnetochemistry 2017, 3, 8. https://doi.org/10.3390/magnetochemistry3010008
Horikiri K, Fujiwara H. New Ethylenedithio-TTF Containing a 2,2,5,5-Tetramethylpyrrolin-1-yloxyl Radical through a Vinylene Spacer and Its FeCl4− Salt—Synthesis, Physical Properties and Crystal Structure Analyses. Magnetochemistry. 2017; 3(1):8. https://doi.org/10.3390/magnetochemistry3010008
Chicago/Turabian StyleHorikiri, Kazuki, and Hideki Fujiwara. 2017. "New Ethylenedithio-TTF Containing a 2,2,5,5-Tetramethylpyrrolin-1-yloxyl Radical through a Vinylene Spacer and Its FeCl4− Salt—Synthesis, Physical Properties and Crystal Structure Analyses" Magnetochemistry 3, no. 1: 8. https://doi.org/10.3390/magnetochemistry3010008