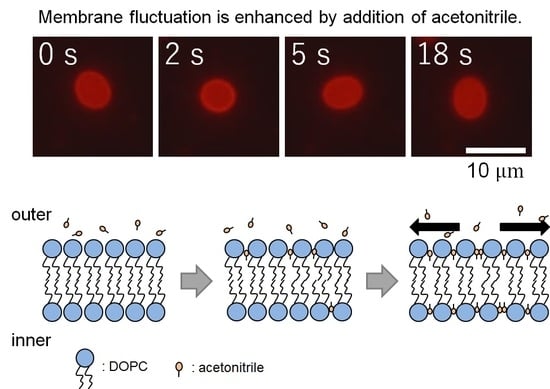
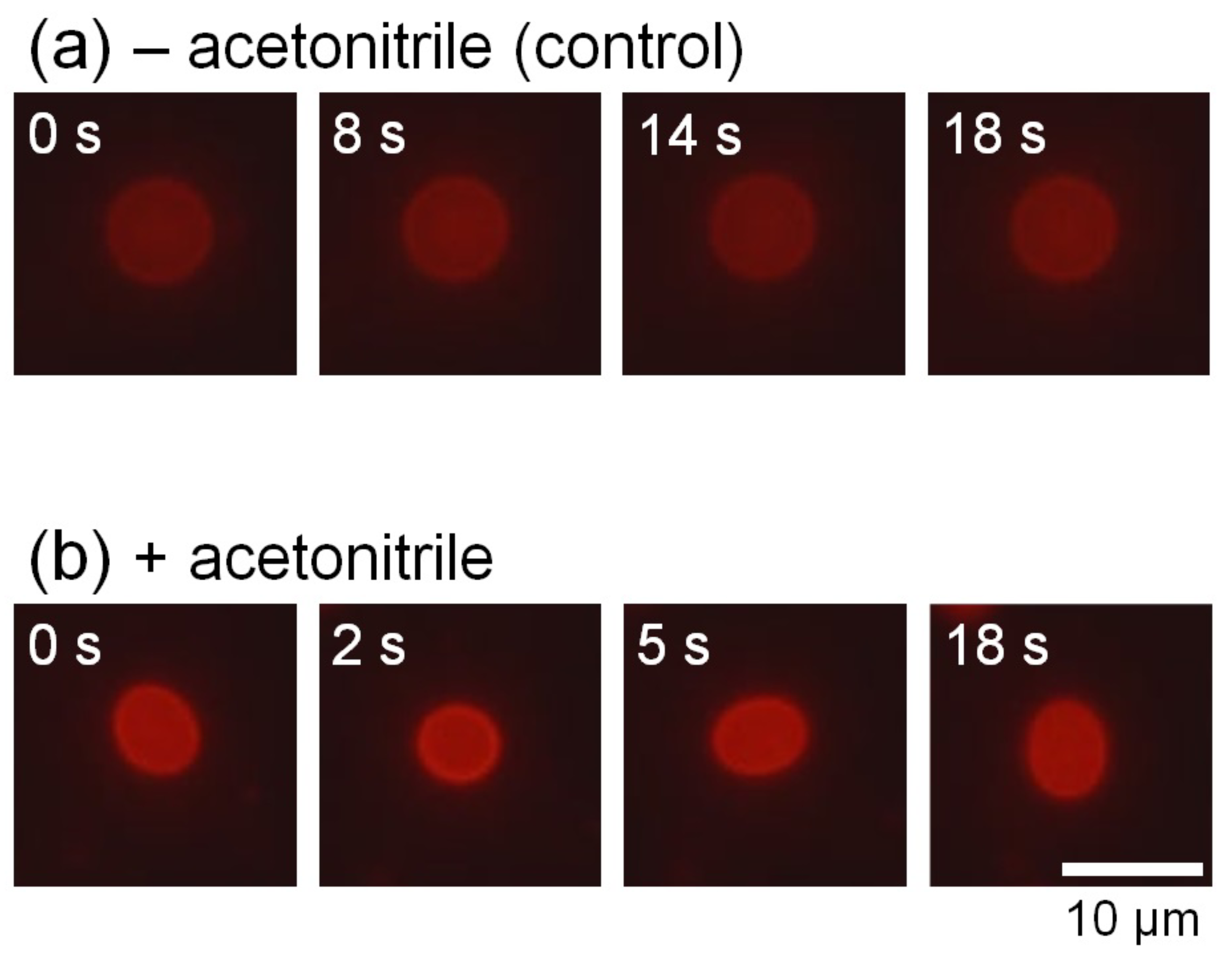
Acetonitrile-Induced Destabilization in Liposomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Giant Lipid Vesicles
2.3. Microscopic Observation
2.4. Extraction of Positions of Membrane Surface
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FFT | Fourier transformation |
| W/O droplet | water-in-oil droplet |
| DOPC | dioleoylphosphatidylcholine |
| rhodamine DHPE | rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt |
References
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Gudipaty, S.; Lindblom, J.; Loftus, P.; Redd, M.; Edes, K.; Davey, C.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A. Cell Division: Breaking Up Is Easy to Do. Curr. Biol. 2009, 19, R327–R328. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Fujii, Y.; Nishio, I. Deformation of Lipid Membranes Containing Photoresponsive Molecules in Response to Ultraviolet Light. J. Phys. Chem. B 2014, 118, 4115–4121. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Takashima, A.; Nishio, I. Effect of Dibucaine Hydrochloride on Raft-Like Lipid Domains in Model Membrane Systems. MedChemComm 2015, 6, 1444–1451. [Google Scholar] [CrossRef]
- Yoshida, K.; Horii, K.; Saito, A.; Takashima, A.; Nishio, I. Confinement Effects on Polymer Dynamics: Thermo-Responsive Behaviours of Hydroxypropyl Cellulose Polymers in Phospholipid-Coated Droplets (Water-in-Oil Emulsion). Polymers 2017, 9, 680. [Google Scholar] [CrossRef]
- Hamada, T.; Hagihara, H.; Morita, M.; Vestergaard, M.C.; Tsujino, Y.; Takagi, M. Physicochemical Profiling of Surfactant-Induced Membrane Dynamics in a Cell-Sized Liposome. J. Phys. Chem. Lett. 2012, 3, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Harris, J.J.; Shelton, R.M.; Perrie, Y. 3D Culture of Bone-Derived Cells Immobilised in Alginate Following Light-Triggered Gelation. J. Control. Release 2007, 119, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lim, J.; Yun, M.; Kim, C. Photoinduced Release of Guest Molecules by Supramolecular Transformation of Self-Assembled Aggregates Derived from Dendrons. Angew. Chem. Int. Ed. 2008, 47, 2959–2963. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-Sensitive Intelligent Drug Delivery Systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, S.; Hosoda, M.; Satake, T.; Okada, S.; Hori, Y.; Furuta, T.; Kikuchi, K. Photocontrolled Compound Release System Using Caged Antimicrobial Peptide. J. Am. Chem. Soc. 2010, 132, 9524–9525. [Google Scholar] [CrossRef] [PubMed]
- Tanii, H.; Hashimoto, K. Studies on the Mechanism of Acute Toxicity of Nitriles in Mice. Arch. Toxicol. 1984, 55, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Boggild, M.; Peck, R.; Tomson, C. Acetonitrile Ingestion: Delayed Onset of Cyanide Poisoning Due to Concurrent Ingestion of Acetone. Postgrad. Med. J. 1990, 66, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Yamazaki, M. Organic Solvents Induce Interdigitated Gel Structures in Multilamellar Vesicles of Dipalmitoylphosphatidylcholine. Biochim. Biophys. Acta (BBA)-Biomembr. 1996, 1284, 233–239. [Google Scholar] [CrossRef]
- Kinoshita, K.; Yamazaki, M. Phase Transition between Hexagonal II(HII) and Liquid-Crystalline Phase Induced by Interaction between Solvents and Segments of the Membrane Surface of Dioleoylphosphatidylethanolamine. Biochim. Biophys. Acta (BBA)-Biomembr. 1997, 1330, 199–206. [Google Scholar] [CrossRef]
- Wu, F.G.; Wang, N.N.; Tao, L.F.; Yu, Z.W. Acetonitrile Induces Nonsynchronous Interdigitation and Dehydration of Dipalmitoylphosphatidylcholine Bilayers. J. Phys. Chem. B 2010, 114, 12685–12691. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Horii, K.; Fujii, Y.; Nishio, I. Real-Time Observation of Liposome Bursting Induced by Acetonitrile. ChemPhysChem 2014, 15, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.i.; Hamada, T.; Hatakeyama, M.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Reversible Control of Exo-and Endo-Budding Transitions in a Photosensitive Lipid Membrane. ChemBioChem 2009, 10, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Salonen, E.; Terama, E.; Vattulainen, I.; Faller, R.; Lee, B.W.; Holopainen, J.; Karttunen, M. Under the Influence of Alcohol: The Effect of Ethanol and Methanol on Lipid Bilayers. Biophys. J. 2006, 90, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.N.; Faller, R. How Alcohol Chain-Length and Concentration Modulate Hydrogen Bond Formation in a Lipid Bilayer. Biophys. J. 2007, 92, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Terama, E.; Ollila, O.H.S.; Salonen, E.; Rowat, A.C.; Trandum, C.; Westh, P.; Patra, M.; Karttunen, M.; Vattulainen, I. Influence of Ethanol on Lipid Membranes: From Lateral Pressure Profiles to Dynamics and Partitioning. J. Phys. Chem. B 2008, 112, 4131–4139. [Google Scholar] [CrossRef] [PubMed]
- Dickey, A.N.; Yim, W.S.; Faller, R. Using Ergosterol To Mitigate the Deleterious Effects of Ethanol on Bilayer Structure. J. Phys. Chem. B 2009, 113, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.J.; Nagy, P.I.; Collantes, E.R. A computer-assisted method for estimation of the partition coefficient. Monte Carlo simulations of the chloroform/water log P for methylamine, methanol, and acetonitrile. J. Am. Chem. Soc. 1991, 113, 7898–7902. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell—A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Seifert, U.; Berndl, K.; Lipowsky, R. Shape Transformations of Vesicles: Phase Diagram for Spontaneous- Curvature and Bilayer-Coupling Models. Phys. Rev. A 1991, 44, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Duwe, H.P.; Sackmann, E. Bending Elasticity and Thermal Excitations of Lipid Bilayer Vesicles: Modulation by Solutes. Phys. A Stat. Mech. Appl. 1990, 163, 410–428. [Google Scholar] [CrossRef]
- Pécréaux, J.; Döbereiner, H.G.; Prost, J.; Joanny, J.F.; Bassereau, P. Refined Contour Analysis of Giant Unilamellar Vesicles. Euro. Phys. J. E 2004, 13, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Gracia, R.S.; Bezlyepkina, N.; Knorr, R.L.; Lipowsky, R.; Dimova, R. Effect of Cholesterol on the Rigidity of Saturated and Unsaturated Membranes: Fluctuation and Electrodeformation Analysis of Giant Vesicles. Soft Matter 2010, 6, 1472–1482. [Google Scholar] [CrossRef]
- Shi, Z.; Baumgart, T. Dynamics and Instabilities of Lipid Bilayer Membrane Shapes. Adv. Colloid Interface Sci. 2014, 208, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Loftus, A.F.; Noreng, S.; Hsieh, V.L.; Parthasarathy, R. Robust Measurement of Membrane Bending Moduli Using Light Sheet Fluorescence Imaging of Vesicle Fluctuations. Langmuir 2013, 29, 14588–14594. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Negishi, L.; Kitoh, T.; Sonoyama, M.; Asami, Y.; Mitaku, S. Effect of Lipid Phase Transition on Molecular Assembly and Structural Stability of Bacteriorhodopsin Reconstituted into Phosphatidylcholine Liposomes with Different Acyl-Chain Lengths. J. Phys. Chem. B 2010, 114, 15706–15711. [Google Scholar] [CrossRef] [PubMed]
- Negishi, L.; Mitaku, S. Electrostatic Effects Influence the Formation of Two-Dimensional Crystals of Bacteriorhodopsin Reconstituted into Dimyristoylphosphatidylcholine Membranes. J. Biochem. 2011, 150, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rieger, S.; Grill, D.; Gerke, V.; Fallnich, C. Quantitative spontaneous Raman scattering spectroscopy in artificial binary lipid membranes. J. Raman Spectrosc. 2017, 48, 1264–1269. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Mitsumori, R.; Horii, K.; Takashima, A.; Nishio, I. Acetonitrile-Induced Destabilization in Liposomes. Colloids Interfaces 2018, 2, 6. https://doi.org/10.3390/colloids2010006
Yoshida K, Mitsumori R, Horii K, Takashima A, Nishio I. Acetonitrile-Induced Destabilization in Liposomes. Colloids and Interfaces. 2018; 2(1):6. https://doi.org/10.3390/colloids2010006
Chicago/Turabian StyleYoshida, Kazunari, Ryohei Mitsumori, Keitaro Horii, Akito Takashima, and Izumi Nishio. 2018. "Acetonitrile-Induced Destabilization in Liposomes" Colloids and Interfaces 2, no. 1: 6. https://doi.org/10.3390/colloids2010006