Eosinophil Lineage-Committed Progenitors as a Therapeutic Target for Asthma
Abstract
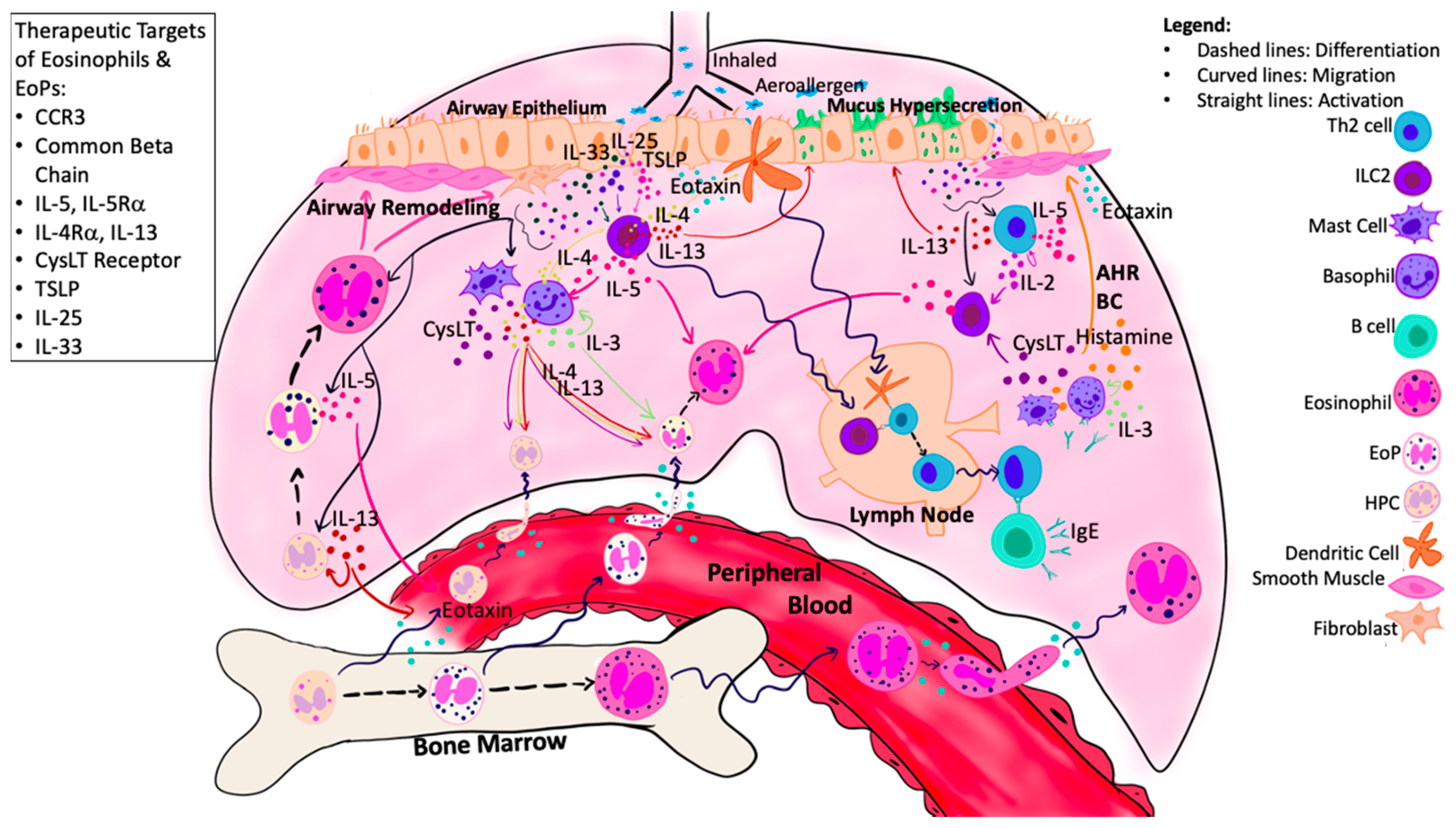
:1. Introduction
2. Identification and Enumeration of Hematopoietic Progenitor Cells
3. Regulation of Eosinophilopoiesis
3.1. Transcription Factors and Eosinophil Lineage Commitment
3.2. Eosinophilopoietic Factors
4. Hematopoietic Processes in Eosinophilic Asthma
5. Targets of Airway Eosinophilopoietic Processes in Asthma
5.1. Anti-Common Beta Chain Therapy
5.2. Anti-Migrational Responsiveness Therapy
5.3. Anti-IL-5 Therapy
5.4. Anti-IL-4/IL-13 Therapy
5.5. Anti-CysLT and PPAR Agonist Therapy
5.6. Anti-Alarmin Therapy
6. Conclusions
Funding
Conflicts of Interest
References
- Leuppi, J.D.; Salome, C.M.; Jenkins, C.R.; Anderson, S.D.; Xuan, W.; Marks, G.B.; Koskela, H.; Brannan, J.D.; Freed, R.; Andersson, M.; et al. Predictive Markers of Asthma Exacerbation during Stepwise Dose Reduction of Inhaled Corticosteroids. Am. J. Respir. Crit. Care Med. 2001, 163, 406–412. [Google Scholar] [CrossRef]
- Green, R.H.; Brightling, C.E.; McKenna, S.; Hargardon, B.; Parker, D.; Bradding, P.; Wardlaw, A.J.P.I. Asthma Exacerbations and Sputum Eosinophil Counts: A Randomised Controlled Trial. Lancet 2002, 9347, 1715–1721. [Google Scholar] [CrossRef]
- Lemière, C.; Ernst, P.; Olivenstein, R.; Yamauchi, Y.; Govindaraju, K.; Ludwig, M.S.; Martin, J.G.; Hamid, Q. Airway Inflammation Assessed by Invasive and Noninvasive Means in Severe Asthma: Eosinophilic and Noneosinophilic Phenotypes. J. Allergy Clin. Immunol. 2006, 118, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid Resistance in Inflammatory Diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Civin, C.I.; Strauss, L.C.; Brovall, C.; Fackler, M.J.O.; Schwartz, J.F.; Shaper, J.H. Antigenic Analysis of Hematopoiesis. III. A Hematopoietic Progenitor Cell Surface Antigen Defined by a Monoclonal Antibody Raised against KG-1a Cells. Information about Subscribing to The Journal of Immunology Is Online at: A Hematopoietic Progenito. J. Immunol. 1984, 133, 157–165. [Google Scholar] [PubMed]
- Krause, D.S.; Fackler, M.J.; Civin, C.I.M.W. CD34: Structure, Biology, and Clinical Utility. Blood 1996, 87, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sergejeva, S.; Johansson, A.K.; Malmhäll, C.; Lötvall, J. Allergen Exposure-Induced Differences in CD34+ Cell Phenotype: Relationship to Eosinophilopoietic Responses in Different Compartments. Blood 2004, 103, 1270–1277. [Google Scholar] [CrossRef]
- Sehmi, R.; Wood, L.J.; Watson, R.; Foley, R.; Hamid, Q.; O’Byrne, P.M.; Denburg, J.A. Allergen-Induced Increases in IL-5 Receptor α-Subunit Expression on Bone Marrow-Derived CD34+ Cells from Asthmatic Subjects: A Novel Marker of Progenitor Cell Commitment towards Eosinophilic Differentiation. J. Clin. Investig. 1997, 100, 2466–2475. [Google Scholar] [CrossRef]
- Iwasaki, H.; Mizuno, S.; Mayfield, R.; Shigematsu, H.; Arinobu, Y.; Seed, B.; Gurish, M.F.; Takatsu, K.; Akashi, K. Identification of Eosinophil Lineage–Committed Progenitors in the Murine Bone Marrow. J. Exp. Med. 2005, 201, 1891–1897. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Iwasaki, H.; Kohno, K.; Yoshimoto, G.; Kikushige, Y.; Okeda, A.; Uike, N.; Niiro, H.; Takenaka, K.; Nagafuji, K.; et al. Identification of the Human Eosinophil Lineage-Committed Progenitor: Revision of Phenotypic Definition of the Human Common Myeloid Progenitor. J. Exp. Med. 2009, 206, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sehmi, R.D.J. Differentiation of Human Eosinophils. Marone G Hum. Eosinophils. Biol. Clin. Asp. Immunol. Basel, Karger 2000, 76, 29–44. [Google Scholar]
- Hassani, M.; van Staveren, S.; van Grinsven, E.; Bartels, M.; Tesselaar, K.; Leijte, G.; Kox, M.; Pickkers, P.; Vrisekoop, Y.; Koenderman, L. Characterization of the Phenotype of Human Eosinophils and Their Progenitors in the Bone Marrow of Healthy Individuals. Haematologica 2020, 105, e52–e56. [Google Scholar] [CrossRef] [PubMed]
- Zon, Y.L.I.; Yamaguchi, K.; Yee, E.A.; Albee, A.; Kimura, J.C.; Bennett, S.H.; Orkin, S.J.A. Expression of MRNA for the GATA-Binding Proteins in Human Eosinophils and Basophils: Potential Role in Gene Transcription. Blood 1993, 81, 3234–3241. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, R.; Shimizu, R.; Takahashi, S.; Osawa, M.; Takayanagi, S.; Kato, Y.; Onodera, M.; Minegishi, N.; Yamamoto, M.; Fukao, K.; et al. Essential and Instructive Roles of GATA Factors in Eosinophil Development. J. Exp. Med. 2002, 195, 1379–1386. [Google Scholar] [CrossRef]
- Querfurth, E.; Schuster, M.; Kulessa, H.; Crispino, J.D.; Döderlein, G.; Orkin, S.H.; Graf, T.; Nerlov, C. Antagonism between C/EBPβ and FOG in Eosinophil Lineage Commitment of Multipotent Hematopoietic Progenitors. Genes Dev. 2000, 14, 2515–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Roure, C.; Versavel, A.; Doll, T.; Cao, C.; Pillonel, V.; Matthias, G.; Kaller, M.; Spetz, J.F.; Kopp, P.; Kohler, H.; et al. Hematopoietic Overexpression of FOG1 Does Not Affect B-Cells but Reduces the Number of Circulating Eosinophils. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Mancini, E.; Sanjuan-Pla, A.; Luciani, L.; Moore, S.; Grover, A.; Zay, A.; Rasmussen, K.D.; Luc, S.; Bilbao, D.; O’Carroll, D.; et al. FOG-1 and GATA-1 Act Sequentially to Specify Definitive Megakaryocytic and Erythroid Progenitors. EMBO J. 2012, 31, 351–365. [Google Scholar] [CrossRef]
- Milanovic, M.; Terszowski, G.; Struck, D.; Liesenfeld, O.; Carstanjen, D. IFN Consensus Sequence Binding Protein (Icsbp) Is Critical for Eosinophil Development. J. Immunol. 2014, 181, 5045–5053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Nishio, H.; Kishi, K.; Ackerman, S.J.S.T. C/EBPbeta and GATA-1 Synergistically Regulate Activity of the Eosinophil Granule Major Basic Protein Promoter: Implication for C/EBPbeta Activity in Eosinophil Gene Expression. Blood 1999, 94, 1429–1439. [Google Scholar] [CrossRef]
- McNagny, K.M.; Sieweke, M.H.; Döderlein, G.; Graf, T.; Nerlov, C. Regulation of Eosinophil-Specific Gene Expression by a C/EBP-Ets Complex and GATA-1. EMBO J. 1998, 17, 3669–3680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwama, A.; Pan, J.; Zhang, P.; Reith, W.; Mach, B.; Tenen, D.G.; Sun, Z. Dimeric RFX Proteins Contribute to the Activity and Lineage Specificity of the Interleukin-5 Receptor α Promoter through Activation and Repression Domains. Mol. Cell. Biol. 1999, 19, 3940–3950. [Google Scholar] [CrossRef] [Green Version]
- Iwama, A.; Osawa, M.; Hirasawa, R.; Uchiyama, N.; Kaneko, S.; Onodera, M.; Shibuya, K.; Shibuya, A.; Vinson, C.; Tenen, D.G.; et al. Reciprocal Roles for CCAAT/Enhancer Binding Protein (C/EBP) and PU.1 Transcription Factors in Langerhans Cell Commitment. J. Exp. Med. 2002, 195, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.E.; Zhang, P.; Wang, N.D.; Hetherington, C.J.; Darlington, G.J.; Tenen, D.G. Absence of Granulocyte Colony-Stimulating Factor Signaling and Neutrophil Development in CCAAT Enhancer Binding Protein Alpha-Deficient Mice. Proc. Natl. Acad. Sci. USA 1997, 94, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Satoh, T.; Kidoya, H.; Naito, H.; Yamamoto, M.; Takemura, N.; Nakagawa, K.; Yoshioka, Y.; Morii, E.; Takakura, N.; Takeuchi, O.; et al. Critical Role of Trib1 in Differentiation of Tissue-Resident M2-like Macrophages. Nature 2013, 495, 524–528. [Google Scholar] [CrossRef]
- Iwasaki, H.; Mizuno, S.I.; Arinobu, Y.; Ozawa, H.; Mori, Y.; Shigematsu, H.; Takatsu, K.; Tenen, D.G.; Akashi, K. The Order of Expression of Transcription Factors Directs Hierarchical Specification of Hematopoietic Lineages. Genes Dev. 2006, 20, 3010–3021. [Google Scholar] [CrossRef] [Green Version]
- Bedi, R.; Du, J.; Sharma, A.K.; Gomes, I.; Ackerman, S.J. Human C/EBP-ε Activator and Repressor Isoforms Differentially Reprogram Myeloid Lineage Commitment and Differentiation. Blood 2009, 113, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, R.; Barlow, C.; Lekstrom-Himes, J.; Castilla, L.H.; Liu, P.P.; Eckhaus, M.; Decker, T.; Wynshaw-Boris, A.; Xanthopoulos, K.G. Impaired Granulopoiesis, Myelodysplasia, and Early Lethality in CCAAT/Enhancer Binding Protein ε-Deficient Mice. Proc. Natl. Acad. Sci. USA 1997, 94, 13187–13192. [Google Scholar] [CrossRef] [Green Version]
- Bettigole, S.E.; Lis, R.; Adoro, S.; Lee, A.H.; Spencer, L.A.; Weller, P.F.; Glimcher, L.H. The Transcription Factor XBP1 Is Selectively Required for Eosinophil Differentiation. Nat. Immunol. 2015, 16, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.W.; Simon, J.M.C.; Anastasi, H.S. Requirement of Transcription Factor PU.1 in the Development of Multiple Hematopoietic Lineages. Science 1994, 265, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.J.; Du, J.; Xin, F.; Dekoter, R.; Mckercher, S.; Maki, R.; Singh, H.; Yamaguchi, Y. Eosinophilopoiesis. Respir. Med. 2000, 94, 1135–1138. [Google Scholar] [CrossRef] [Green Version]
- Mack, E.A.; Stein, S.J.; Rome, K.S.; Xu, L.; Wertheim, G.B.; Melo, R.C.N.; Pear, W.S. Trib1 Regulates Eosinophil Lineage Commitment and Identity by Restraining the Neutrophil Program. Blood 2019, 133, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.A. SHP2 Tyrosine Phosphatase Stimulates CEBPA Gene Expression to Mediate Cytokine-Dependent Granulopoiesis. Blood 2011, 118, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.X.; Hua, W.; Jin, Y.; Tian, B.P.; Qiu, Z.W.; Zhang, C.; Che, L.Q.; Zhou, H.B.; Wu, Y.F.; Huang, H.Q.; et al. Eosinophil Differentiation in the Bone Marrow Is Promoted by Protein Tyrosine Phosphatase SHP2. Cell Death Dis. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Buitenhuis, M.; Van Deutekom, H.W.M.; Verhagen, L.P.; Castor, A.; Jacobsen, S.E.W.; Lammers, J.W.J.; Koenderman, L.; Coffer, P.J. Differential Regulation of Granulopoiesis by the Basic Helix-Loop-Helix Transcriptional Inhibitors Id1 and Id2. Blood 2005, 105, 4272–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.S.; Damia, R.; Zeibecoglou, K.; Molet, S.; North, J.; Yamada, T.; Kay, A.B.; Hamid, Q. CD34+/Interleukin-5Rα Messenger RNA+ Cells in the Bronchial Mucosa in Asthma: Potential Airway Eosinophil Progenitors. Am. J. Respir. Cell Mol. Biol. 1999, 20, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Folkesson, H.G.; Warnock, M.L.; Erle, D.J.; Matthay, M.A.; Wiener-Kronish, J.P.; Locksley, R.M. Interleukin 4, but Not Interleukin 5 or Eosinophils, Is Required in a Murine Model of Acute Airway Hyperreactivity. J. Exp. Med. 1996, 183, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Kopf, M.; Brombacher, F.; Hodgkin, P.D.; Ramsay, A.J.; Milbourne, E.A.; Dai, W.J.; Ovington, K.S.; Behm, C.A.; Köhler, G.; Young, I.G.; et al. IL-5-Deficient Mice Have a Developmental Defect in CD5+ B-1 Cells and Lack Eosinophilia but Have Normal Antibody and Cytotoxic T Cell Responses. Immunity 1996, 4, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Hui, C.C.K.; Rusta-Sallehy, S.; Asher, I.; Heroux, D.; Denburg, J.A. The Effects of Thymic Stromal Lymphopoietin and Il-3 on Human Eosinophil–Basophil Lineage Commitment: Relevance to Atopic Sensitization. Immun. Inflamm. Dis. 2014, 2, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Salter, B.M.A.; Smith, S.G.; Mukherjee, M.; Plante, S.; Krisna, S.; Nusca, G.; Oliveria, J.P.; Irshad, A.; Gauvreau, G.M.; Chakir, J.; et al. Human Bronchial Epithelial Cell–Derived Factors from Severe Asthmatic Subjects Stimulate Eosinophil Differentiation. Am. J. Respir. Cell Mol. Biol. 2018, 58. [Google Scholar] [CrossRef]
- Johnston, L.K.; Hsu, C.-L.; Krier-Burris, R.A.; Chhiba, K.D.; Chien, K.B.; McKenzie, A.; Berdnikovs, S.; Bryce, P.J. IL-33 Precedes IL-5 in Regulating Eosinophil Commitment and Is Required for Eosinophil Homeostasis. J. Immunol. 2016, 197, 3445–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boberg, E.; Johansson, K.; Malmhäll, C.; Weidner, J.; Rådinger, M. House Dust Mite Induces Bone Marrow Il-33responsive ILC2S and TH Cells. Int. J. Mol. Sci. 2020, 21, 3751. [Google Scholar] [CrossRef] [PubMed]
- Stolarski, B.; Kurowska-Stolarska, M.; Kewin, P.; Xu, D.; Liew, F.Y. IL-33 Exacerbates Eosinophil-Mediated Airway Inflammation. J. Immunol. 2010, 185, 3472–3480. [Google Scholar] [CrossRef] [Green Version]
- Allakhverdi, Z.; Comeau, M.R.; Smith, D.E.; Toy, D.; Endam, L.M.; Desrosiers, M.; Liu, Y.J.; Howie, K.J.; Denburg, J.A.; Gauvreau, G.M.; et al. CD34+ Hemopoietic Progenitor Cells Are Potent Effectors of Allergic Inflammation. J. Allergy Clin. Immunol. 2009, 123, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Maeda, K.; Yashiro, T.; Inage, E.; Kasakura, K.; Suzuki, R.; Niyonsaba, F.; Hara, M.; Tanabe, A.; Ogawa, H.; et al. GATA2 Is a Critical Transactivator for the Human IL1RL1/ST2 Promoter in Mast Cells/Basophils: Opposing Roles for GATA2 and GATA1 in Human IL1RL1/ST2 Gene Expression. J. Biol. Chem. 2012, 287, 32689–32696. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.G.; Gugilla, A.; Mukherjee, M.; Merim, K.; Irshad, A.; Tang, W.; Kinoshita, T.; Watson, B.; Oliveria, J.P.; Comeau, M.; et al. Thymic Stromal Lymphopoietin and IL-33 Modulate Migration of Hematopoietic Progenitor Cells in Patients with Allergic Asthma. J. Allergy Clin. Immunol. 2015, 135, 1594–1602. [Google Scholar] [CrossRef]
- Tang, W.; Smith, S.G.; Du, W.; Gugilla, A.; Du, J.; Oliveria, J.P.; Howie, K.; Salter, B.M.; Gauvreau, G.M.; O’Byrne, P.M.; et al. Interleukin-25 and Eosinophils Progenitor Cell Mobilization in Allergic Asthma. Clin. Transl. Allergy 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenz, S.A.; Siracusa, M.C.; Perrigoue, J.G.; Spencer, S.P.; Urban, J.F.; Tocker, J.E.; Budelsky, A.L.; Kleinschek, M.A.; Kastelein, R.A.; Kambayashi, T.; et al. IL25 Elicits a Multipotent Progenitor Cell Population That Promotes T H 2 Cytokine Responses. Nature 2010, 464, 1362–1366. [Google Scholar] [CrossRef]
- Saenz, S.A.; Siracusa, M.C.; Monticelli, L.A.; Ziegler, C.G.K.; Kim, B.S.; Brestoff, J.R.; Peterson, L.W.; John Wherry, E.; Goldrath, A.W.; Bhandoola, A.; et al. IL-25 Simultaneously Elicits Distinct Populations of Innate Lymphoid Cells and Multipotent Progenitor Type 2 (MPPtype2) Cells. J. Exp. Med. 2013, 210, 1823–1837. [Google Scholar] [CrossRef] [Green Version]
- Braccioni, F.; Dorman, S.C.; O’Byrne, P.M.; Inman, M.D.; Denburg, J.A.; Parameswaran, K.; Baatjes, A.J.; Foley, R. The Effect of Cysteinyl Leukotrienes on Growth of Eosinophil Progenitors From Peripheral Blood and Bone Marrow of Atopic Subjects. J. Allergy Clin. Immunol. 2002, 110, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Lamkhioued, B.; Abdelilah, S.G.; Hamid, Q.; Mansour, N.; Delespesse, G.; Renzi, P.M. The CCR3 Receptor Is Involved in Eosinophil Differentiation and Is Up-Regulated by Th2 Cytokines in CD34 + Progenitor Cells. J. Immunol. 2003, 170, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Sehmi, R.; Howie, K.; Sutherland, D.R.; Schragge, W.; O’Byrne, P.M.; Denburg, J.A. Increased Levels of CD34+ Hemopoietic Progenitor Cells in Atopic Subjects. Am. J. Respir. Cell Mol. Biol. 1996, 15, 645–654. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsieh, W.Y.; Shih, L.Y.; Lin, H.C.; Liu, C.Y.; Chung, K.F.; Kuo, H.P. Increased Progenitor Cell Proliferation in the Peripheral Blood of Patients with Bronchial Asthma:The Role of Nitric Oxide. J. Allergy Clin. Immunol. 1999, 104, 803–810. [Google Scholar] [CrossRef]
- Denburg, J.A.; Telizyn, S.; Belda, A.; Dolovich, J.; Bienenstock, J. Increased Numbers of Circulating Basophil Progenitors in Atopic Patients. J. Allergy Clin. Immunol. 1985, 76, 466–472. [Google Scholar] [CrossRef]
- Gibson, P.G.; Dolovich, J.; Girgis-Gabardo, A.; Morris, M.M.; Anderson, M.; Hargreave, F.E.; Denburg, J.A. The Inflammatory Response in Asthma Exacerbation: Changes in Circulating Eosinophils, Basophils and Their Progenitors. Clin. Exp. Allergy 1990, 20, 661–668. [Google Scholar] [CrossRef]
- Gibson, P.G.; Manning, P.J.; Byrne, P.M.O.; Girgis-gabardo, A.; Dolovich, J.; Denburg, J.A.; Hargreave, F.E. Allergen-Induced Asthmatic Responses. Am. Rev. Respir. Dis. 1991, 143, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; O’Byrne, P.M.; Moqbel, R.; Velazquez, J.; Watson, R.M.; Howie, K.J.; Denburg, J.A. Enhanced Expression of GM-CSF in Differentiating Eosinophils of Atopic and Atopic Asthmatic Subjects. Am. J. Respir. Cell Mol. Biol. 1998, 19, 55–62. [Google Scholar] [CrossRef]
- Kuo, H.P.; Wang, C.H.; Lin, H.C.; Hwang, K.S.; Liu, S.L.; Chung, K.F. Interleukin-5 in Growth and Differentiation of Blood Eosinophil Progenitors in Asthma: Effect of Glucocorticoids. Br. J. Pharmacol. 2001, 134, 1539–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.G.; Chen, R.; Kjarsgaard, M.; Huang, C.; Oliveria, J.-P.; O’Byrne, P.M.; Gauvreau, G.M.; Boulet, L.-P.; Lemiere, C.; Martin, J.; et al. Increased Numbers of Activated Group 2 Innate Lymphoid Cells in the Airways of Patients with Severe Asthma and Persistent Airway Eosinophilia. J. Allergy Clin. Immunol. 2016, 137, 75–86.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehmi, R.; Smith, S.G.; Kjarsgaard, M.; Radford, K.; Boulet, L.P.; Lemiere, C.; Prazma, C.M.; Ortega, H.; Martin, J.G.; Nair, P. Role of Local Eosinophilopoietic Processes in the Development of Airway Eosinophilia in Prednisone-Dependent Severe Asthma. Clin. Exp. Allergy 2016, 46, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.; Christodoulopoulos, P.; Lavigne, F.; Nakamura, Y.; Eidelman, D.; McEuen, A.; Walls, A.; Tavernier, J.; Minshall, E.; Moqbel, R.; et al. Evidence for Local Eosinophil Differentiation Within Allergic Nasal Mucosa: Inhibition with Soluble IL-5 Receptor. J. Immunol. 2000, 164, 1538–1545. [Google Scholar] [CrossRef]
- Menzies-Gow, A.N.; Flood-Page, P.T.; Robinson, D.S.; Kay, A.B. Effect of Inhaled Interleukin-5 on Eosinophil Progenitors in the Bronchi and Bone Marrow of Asthmatic and Non-Asthmatic Volunteers. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2007, 37, 1023–1032. [Google Scholar] [CrossRef]
- Sehmi, R.; Dorman, S.; Baatjes, A.; Watson, R.; Foley, R.; Ying, S.U.N.; Robinson, S.; Kay, A.B.; Byrne, P.M.O.; Denburg, J.A. Allergen-Induced Fluctuation in CC Chemokine Receptor 3 Expression on Bone Marrow CD34+ Cells from Asthmatic Subjects: Significance for Mobilization of Haemopoietic Progenitor Cells in Allergic Inflammation. Immunology 2003, 109, 536–546. [Google Scholar] [CrossRef]
- Dorman, S.C.; Babirad, I.; Post, J.; Watson, R.M.; Foley, R.; Jones, G.L.; O’Byrne, P.M.; Sehmi, R. Progenitor Egress from the Bone Marrow after Allergen Challenge: Role of Stromal Cell-Derived Factor 1α and Eotaxin. J. Allergy Clin. Immunol. 2005, 115, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Catalli, A.E.; Thomson, J.V.; Babirad, I.M.; Duong, M.; Doyle, T.M.; Howie, K.J.; Newbold, P.; Craggs, R.I.; Foster, M.; Gauvreau, G.M.; et al. Modulation of Beta1-Integrins on Hemopoietic Progenitor Cells after Allergen Challenge in Asthmatic Subjects. J. Allergy Clin. Immunol. 2008, 122, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.T.; Magier, A.Z.; Marshall, S.A.F.P. Eosinophil Progenitor Cell Blood Levels Inversely Correlate with Disease Control in Pediatric Patients with Asthma. J. Allergy Clin. Immunol. 2019, 143, AB5. [Google Scholar] [CrossRef] [Green Version]
- Pageau, R.; Gauvreau, G.; Seguin, R.; Carballo, D.; Anjou, H.D.; Campbell, H.; Watson, R.; Parry-Billings, M.; Killian, K.R.P. TPI ASM8 Decreases Inflammatory Markers and Improves Airway Responsiveness in Asthmatics. J. Allergy Clin. Immunol. 2011, 127, AB82. [Google Scholar] [CrossRef]
- Imaoka, H.; Campbell, H.; Babirad, I.; Watson, R.M.; Mistry, M.; Sehmi, R.; Gauvreau, G.M. TPI ASM8 Reduces Eosinophil Progenitors in Sputum after Allergen Challenge. Clin. Exp. Allergy 2011, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Panousis, C.; Dhagat, U.; Edwards, K.M.; Rayzman, V.; Hardy, M.P.; Braley, H.; Gauvreau, G.M.; Hercus, T.R.; Smith, S.; Sehmi, R.; et al. CSL311, a Novel, Potent, Therapeutic Monoclonal Antibody for the Treatment of Diseases Mediated by the Common β Chain of the IL-3, GM-CSF and IL-5 Receptors. MAbs 2016, 8, 436–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neighbour, H.; Boulet, L.; Lemiere, C.; Sehmi, R.; Leigh, R.; Sousa, A.R.; Martin, J.; Dallow, N.; Gilbert, J.; Allen, A. Safety and Efficacy of an Oral CCR3 Antagonist in Patients with Asthma and Eosinophilic Bronchitis: A Randomized. Placebo-Controll. Clin. Trial Exp. Allergy 2013, 2, 508–516. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Flood-Page, P.; Sehmi, R.; Burman, J.; Hamid, Q.; Robinson, D.S.; Kay, A.B.; Denburg, J. Anti-IL-5 (Mepolizumab) Therapy Induces Bone Marrow Eosinophil Maturational Arrest and Decreases Eosinophil Progenitors in the Bronchial Mucosa of Atopic Asthmatics. J. Allergy Clin. Immunol. 2003, 111, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Paramo, F.A.; Kjarsgaard, M.; Salter, B.; Nair, G.; LaVigne, N.; Radford, K.; Sehmi, R.; Nair, P. Weight-Adjusted Intravenous Reslizumab in Severe Asthma with Inadequate Response to Fixed-Dose Subcutaneous Mepolizumab. Am. J. Respir. Crit. Care Med. 2018, 197. [Google Scholar] [CrossRef] [PubMed]
- Sehmi, R.; Lim, H.F.; Mukherjee, M.; Huang, C.; Radford, K.; Newbold, P.; Boulet, L.P.; Dorscheid, D.; Martin, J.G.; Nair, P. Benralizumab Attenuates Airway Eosinophilia in Prednisone-Dependent Asthma. J. Allergy Clin. Immunol. 2018, 141, 1529–1532.e8. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, K.; Watson, R.; Gauvreau, G.M.; Sehmi, R.; O’Byrne, P.M. The Effect of Pranlukast on Allergen-Induced Bone Marrow Eosinophilopoiesis in Subjects with Asthma. Am. J. Respir. Crit. Care Med. 2004, 169, 915–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauvreau, G.M.; Pageau, R.; Séguin, R.; Carballo, D.; Gauthier, J.; D’Anjou, H.; Campbell, H.; Watson, R.; Mistry, M.; Parry-Billings, M.; et al. Dose-Response Effects of TPI ASM8 in Asthmatics after Allergen. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Komai, M.; Tanaka, H.; Nagao, K.; Ishizaki, M.; Kajiwara, D.; Miura, T.; Ohashi, H.; Haba, T.; Kawakami, K.; Sawa, E.; et al. A Novel CC-Chemokine Receptor 3 Antagonist, Ki19003, Inhibits Airway Eosinophilia and Subepithelial/Peribronchial Fibrosis Induced by Repeated Antigen Challenge in Mice. J. Pharmacol Sci. 2010, 112, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauvreau, G.M.; FitzGerald, J.M.; Boulet, L.P.; Watson, R.M.; Hui, L.; Villineuve, H.; Scime, T.X.; Schlatman, A.R.; Obminski, C.; Kum, J.; et al. The Effects of a CCR3 Inhibitor, AXP1275, on Allergen-Induced Airway Responses in Adults with Mild-to-Moderate Atopic Asthma. Clin. Exp. Allergy 2018, 48, 445–451. [Google Scholar] [CrossRef]
- Grozdanovic, M.; Laffey, K.G.; Abdelkarim, H.; Hitchinson, B.; Harijith, A.; Moon, H.G.; Park, G.Y.; Rousslang, L.K.; Masterson, J.C.; Furuta, G.T.; et al. Novel Peptide Nanoparticle–Biased Antagonist of CCR3 Blocks Eosinophil Recruitment and Airway Hyperresponsiveness. J. Allergy Clin. Immunol. 2019, 143, 669–680.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N. Mepolizumab for Severe Eosinophilic Asthma (DREAM): A Multicentre, Double-Blind, Placebo-Controlled Trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W. Oral Glucocorticoid-Sparing Effect of Mepolizumab in Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Leckie, M.J.; Brinke, A.T.; Khan, J.; Diamant, Z.; O’Connor, B.J.; Walls, C.M.M.A.; Cowley, H.C.; Chung, K.F.; Djukanovic, R.; Hansel, T.T.; et al. Effects of an Interleukin-5 Blocking Monoclonal Antibody on Eosinophils, Airway Hyper-Responsìveness, and the Late Asthmatic Response. Lancet 2000, 356, 2144–2148. [Google Scholar] [CrossRef]
- Büttner, C.; Lun, A.; Splettstoesser, T.; Kunkel, G.R.H. Monoclonal Antiinterleukin-5 Treatment Suppresses Eosinophil but Not T-cell Functions. Eur. Respir. J. 2003, 21, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Flood-Page, P.T.; Menzies-Gow, A.N.; Kay, A.B. Eosinophil’s Role Remains Uncertain as Anti–Interleukin-5 Only Partially Depletes Numbers in Asthmatic Airway. Am. J. Respir. Crit. Care Med. 2003, 167, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Flood-Page, P.; Swenson, C.; Faiferman, I.; Matthews, J.; Williams, M.B.L.; Robinson, D.; Wenzel, S.; Busse, W.; Hansel, T.T.B.N. A Study to Evaluate Safety and Efficacy of Mepolizumab in Patients with Moderate Persistent Asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 1062–1071. [Google Scholar] [CrossRef]
- Nair, P.; Pizzichini, M.M.M.; Kjarsgaard, M.; Inman, M.D.; Efthimiadis, A.P.E.; Hargreave, F.E.O.P. Mepolizumab for Prednisone-Dependent Asthma with Sputum Eosinophilia. N. Engl. J. Med. 2009, 360, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.M.; Bradding, P.; Green, R.H.; Wardlaw, A.J. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Kips, J.C.; O’Connor, B.J.; Langley, S.J.; Woodcock, A.; Kerstjens, H.A.M.; Danzig, M.; Cuss, F. Effect of SCH55700, a Humanized Anti-Human Interleukin-5 Antibody, in Severe Persistent Asthma. Am. J. Respir. Crit. Care Med. 2003, 167, 1655–1659. [Google Scholar] [CrossRef]
- Castro, M.; Mathur, S.; Hargreave, F.; Boulet, L.-P.; Xie, F.; Young, J.; Henkel, T. Reslizumab for Poorly Controlled, Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 1125–1132. [Google Scholar] [PubMed]
- Castro, M.; Zangrilli, J.; Wechsler, M.E.; Bateman, E.D.; Brusselle, G.G.; Bardin, P.; Murphy, K.; Maspero, J.F.; O’Brien, C.K.S. Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Counts: Results From Two Multicentre, Parallel, Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trials. Lancet Respir. Med. 2015, 3, 355–366. [Google Scholar] [CrossRef]
- Corren, J.; Weinstein, S.; Janka, L.; Zangrilli, J.; Garin, M. Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. Chest 2016, 150, 799–810. [Google Scholar] [CrossRef] [Green Version]
- Bjermer, L.; Lemiere, C.; Maspero, J.; Weiss, S.; Zangrilli, J.; Germinaro, M. Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase 3 Study. Chest 2016, 150, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, K.; Jacobs, J.; Bjermer, L.; Fahrenholz, J.M.; Shalit, Y.; Garin, M.; Zangrilli, J.; Castro, M. Long-Term Safety and Efficacy of Reslizumab in Patients with Eosinophilic Asthma. J. Allergy Clin. Immunol. Pract. 2017, 5, 1572–1581.e3. [Google Scholar] [CrossRef]
- Ibrahim, H.; O’Sullivan, R.; Casey, D.; Murphy, J.; MacSharry, J.; Plant, B.J.; Murphy, D.M. The Effectiveness of Reslizumab in Severe Asthma Treatment: A Real-World Experience. Respir. Res. 2019, 20, 1–5. [Google Scholar] [CrossRef]
- Chanez, P.; McDonald, M. Early Decreases in Blood Eosinophil Levels with Reslizumab. J. Allergy Clin. Immunol. 2019, 143, 1653–1655. [Google Scholar] [CrossRef] [Green Version]
- Laviolette, M.; Gossage, D.L.; Gauvreau, G.; Leigh, R.; Olivenstein, R.; Katial, R.; Busse, W.W.; Wenzel, S.; Wu, Y.; Datta, V.; et al. Effects of Benralizumab on Airway Eosinophils in Asthmatic Patients with Sputum Eosinophilia. J. Allergy Clin. Immunol. 2013, 132, 1086–1096.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.H.; Damera, G.; Newbold, P.; Ranade, K. Reductions in Eosinophil Biomarkers by Benralizumab in Patients with Asthma. Respir. Med. 2016, 111, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Busse, W.W.; Katial, R.; Gossage, D.; Sari, S.; Wang, B.; Kolbeck, R.; Coyle, A.J.K.M.; Spitalny, G.L.; Kiener, P.A.; Geba, G.P.M.N. Safety Profile, Pharmacokinetics, and Biologic Activity of MEDI-563, an Anti–IL-5 Receptor α Antibody, in a Phase I Study of Subjects with Mild Asthma. J. Allergy Clin. Immunol. 2010, 125, 1244. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in Persistent Asthma with Elevated Eosinophil Levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.; De Feyter, S.; Maes, T.; Joos, G.; Lahousse, L. Monoclonal Antibodies in Type 2 Asthma: A Systematic Review and Network Meta-Analysis. Respir. Res. 2019, 20, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.J.; et al. Efficacy and Safety of Lebrikizumab in Patients with Uncontrolled Asthma (LAVOLTA I and LAVOLTA II): Replicate, Phase 3, Randomised, Double-Blind, Placebo-Controlled Trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Trifilieff, A.; Bench, A.; Hanley, M.; Bayley, D.; Campbell, E.; Whittaker, P. PPAR-α and -γ but Not -δ Agonists Inhibit Airway Inflammation in a Murine Model of Asthma: In Vitro Evidence for an NF-ΚB-Independent Effect. Br. J. Pharmacol. 2003, 139, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.G.; Hill, M.; Oliveria, J.P.; Watson, B.M.; Baatjes, A.J.; Dua, B.; Howie, K.; Campbell, H.; Watson, R.M.; Sehmi, R.; et al. Evaluation of Peroxisome Proliferator-Activated Receptor Agonists on Interleukin-5-Induced Eosinophil Differentiation. Immunology 2014, 142, 484–491. [Google Scholar] [CrossRef]
- Kaler, M.; Barcochia, A.V.; Weir, N.A.; Cuento, R.A.; Stylianou, M.; Roth, M.J.; Filie, A.C.; Vaughey, E.C.; Nathan, S.D. A Randomized, Placebo-Controlled Double-Blinded, Crossover Trial of Pioglitazone for Severe Asthma. J. Allergy Clin. Immunol. 2017, 140, 1716–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.G.; Zhang, T.T.; Li, H.T.; Chen, F.H.; Zou, X.L.; Ji, J.Z.; Chen, H. Neutralization of TSLP Inhibits Airway Remodeling in a Murine Model of Allergic Asthma Induced by Chronic Exposure to House Dust Mite. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Cheng, D.T.; Ma, C.; Niewoehner, J.; Dahl, M.; Tsai, A.; Zhang, J.; Gonsiorek, W.; Apparsundaram, S.; Pashine, A.; Ravindran, P.; et al. Thymic Stromal Lymphopoietin Receptor Blockade Reduces Allergic Inflammation in a Cynomolgus Monkey Model of Asthma. J. Allergy Clin. Immunol. 2013, 132, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Leu, S.W.; Xu, F.; Zhou, X.; Yin, H.; Cai, L.; Zhang, L. Local Blockade of TSLP Receptor Alleviated Allergic Disease by Regulating Airway Dendritic Cells. Clin. Immunol. 2008, 129, 202–210. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Pham, T.-H.; Ren, P.; Parnes, J.R.; Griffiths, J.M. Tezepelumab Reduces Multiple Key Inflammatory Biomarkers in Patients with Severe, Uncontrolled Asthma in the Phase 2b PATHWAY Study. Am. J. Respir. Crit. Care Med. 2019, 224, A2677. [Google Scholar] [CrossRef]
- Lee, H.Y.; Rhee, C.K.; Kang, J.Y.; Byun, J.H.; Choi, J.Y.; Kim, S.J.; Kim, Y.K.; Kwon, S.S.; Lee, S.Y. Blockade of IL-33/ST2 Ameliorates Airway Inflammation in a Murine Model of Allergic Asthma. Exp. Lung Res. 2014, 40, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Allinne, J.; Scott, G.; Lim, W.K.; Birchard, D.; Erjefält, J.S.; Sandén, C.; Ben, L.H.; Agrawal, A.; Kaur, N.; Kim, J.H.; et al. IL-33 Blockade Affects Mediators of Persistence and Exacerbation in a Model of Chronic Airway Inflammation. J. Allergy Clin. Immunol. 2019, 144, 1624–1637.e10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-Concept Clinical Trial of Etokimab Shows a Key Role for IL-33 in Atopic Dermatitis Pathogenesis. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Chinthrajah, S.; Cao, S.; Liu, C.; Lyu, S.C.; Sindher, S.B.; Long, A.; Sampath, V.; Petroni, D.; Londei, M.; Nadeau, K.C. Phase 2a Randomized, Placebo-Controlled Study of Anti–IL-33 in Peanut Allergy. JCI Insight 2019, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AnaptysBio. Proof of concept study to investigate ANB020 activity in adult patients with severe eosinophilic asthma.
- Sharkhuu, T.; Matthaei, K.I.; Forbes, E.; Mahalingam, S.; Hogan, S.P.; Hansbro, P.M.; Foster, P.S. Mechanism of Interleukin-25 (IL-17E)-Induced Pulmonary Inflammation and Airways Hyper-Reactivity. Clin. Exp. Allergy 2006, 36, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Angkasekwinai, P.; Park, H.; Wang, Y.H.; Wang, Y.H.; Seon, H.C.; Corry, D.B.; Liu, Y.J.; Zhu, Z.; Dong, C. Interleukin 25 Promotes the Initiation of Proallergic Type 2 Responses. J. Exp. Med. 2007, 204, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Hurst, S.D.; Muchamuel, T.; Gorman, D.M.; Gilbert, J.M.; Clifford, T.; Kwan, S.; Menon, S.; Seymour, B.; Jackson, C.; Kung, T.T.; et al. New IL-17 Family Members Promote Th1 or Th2 Responses in the Lung: In Vivo Function of the Novel Cytokine IL-25. J. Immunol. 2002, 169, 443–453. [Google Scholar] [CrossRef]
- Ballantyne, S.J.; Barlow, J.L.; Jolin, H.E.; Nath, P.; Williams, A.S.; Chung, K.F.; Sturton, G.; Wong, S.H.; McKenzie, A.N.J. Blocking IL-25 Prevents Airway Hyperresponsiveness in Allergic Asthma. J. Allergy Clin. Immunol. 2007, 120, 1324–1331. [Google Scholar] [CrossRef]
- Gregory, L.G.; Jones, C.P.; Walker, S.A.; Sawant, D.; Gowers, K.H.C.; Campbell, G.A.; McKenzie, A.N.J.; Lloyd, C.M. IL-25 Drives Remodelling in Allergic Airways Disease Induced by House Dust Mite. Thorax 2013, 68, 82–90. [Google Scholar] [CrossRef] [Green Version]

| Study | Therapeutic Agent | Subjects | Effects on Mature Eosinophils | Effects on Eosinophil Progenitors | Other Inflammatory Outcomes |
|---|---|---|---|---|---|
| Pageau et al. 2011 [66] | TPI ASM8 (4 mg bid, 8 mg o.d. inhaled for 4d) Anti-sense oligonucleotide against common β chain and CCR3 | MA | Reduced SP Eos | Reduced SP CCR3+HPC and EoP | Reduced SP ECP levels |
| Imaoka et al. 2011 [67] | TPI ASM8 (4 mg bid or 8 mg o.d. inhaled, for 4d) Anti-sense oligonucleotide against common β chain and CCR3 | MA | Reduced SP Eos at 7 h and 24 h post-Ag | Reduced SP CCR3+HPC at pre- and 24 h post-Ag No effect on total HPC | Not reported |
| Panousis et al. 2016 [68] | CSL 311 (100 µg/mL) Common β chain mAb | HC and MA | Reduced PB Eos survival | Decreased BM and PB Eo/B-CFU pre- and 24 h post-Ag | Not reported |
| Neighbour et al. 2013 [69] | GW766944 (300 mg PO bid for 10d) CCR3 Antagonist | MA | No effect on PB or SP Eos | No effect on PB or SP EoP | Not reported |
| Menzies-Gow et al. 2003 [70] | Mepolizumab (750 mg IV at 1, 4, 8 weeks) Anti-IL-5 mAb | MA | Reduced BM and PB Eos | Reduced EoP in bronchial mucosa No effect on BM or PB EoP or Eo/B-CFU | Not reported |
| Sehmi et al. 2016 [59] | Mepolizumab (100 mg q4 weeks SC for 10 weeks) Anti-IL-5 mAb | SA (>3% SP Eos, >300 cells/µL) | Reduced PB Eos No effect on SP Eos | Increased PB EoP No effect on SP EoP | -Not reported |
| Mukherjee et al. 2018 [71] | Reslizumab (3.0 mg/kg IV q4 weeks, total of 16 weeks) Anti-IL-5 mAb | SA (>3% SP Eos, >300 cells/µL) | Reduced PB and SP Eos | Reduced PB and SP HPC, PB EoP No effect on SP EoP | Not reported |
| Sehmi et al. 2018 [72] | Benralizumab (30 mg SC q4 weeks for 28 weeks) Anti-IL-5Rα mAb | SA (>3% SP Eos, >300 cells/µL) | Reduced PB and SP Eos | Reduced PB EoP Trend toward decrease in SP EoP | Not reported |
| Parameswaran N et al. 2004 [73] | Pranlukast CysLT Receptor Antagonist (300 mg PO bid for 2 weeks) | MA | Reduced SP Eos pre- and 24 h post-Ag Reduced SP EG2+ cells pre- and 24 h post-Ag | Reduced BM Eo/B-CFU and CCR3+HPC | Trends for reduced SP cells + for IL-5 Eotaxin and RANTES 24 h post-Ag |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salter, B.M.; Ju, X.; Sehmi, R. Eosinophil Lineage-Committed Progenitors as a Therapeutic Target for Asthma. Cells 2021, 10, 412. https://doi.org/10.3390/cells10020412
Salter BM, Ju X, Sehmi R. Eosinophil Lineage-Committed Progenitors as a Therapeutic Target for Asthma. Cells. 2021; 10(2):412. https://doi.org/10.3390/cells10020412
Chicago/Turabian StyleSalter, Brittany M., Xiaotian Ju, and Roma Sehmi. 2021. "Eosinophil Lineage-Committed Progenitors as a Therapeutic Target for Asthma" Cells 10, no. 2: 412. https://doi.org/10.3390/cells10020412
APA StyleSalter, B. M., Ju, X., & Sehmi, R. (2021). Eosinophil Lineage-Committed Progenitors as a Therapeutic Target for Asthma. Cells, 10(2), 412. https://doi.org/10.3390/cells10020412






