Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems
Abstract
:1. Introduction
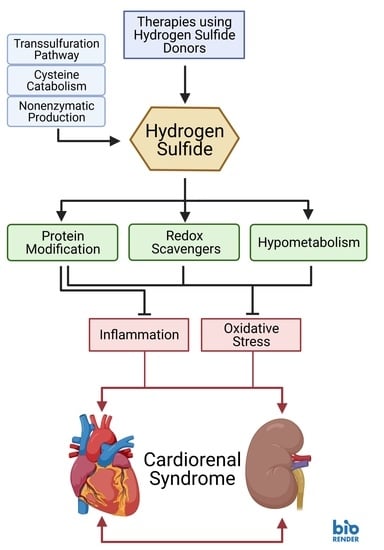
2. Endogenous Production of Hydrogen Sulfide
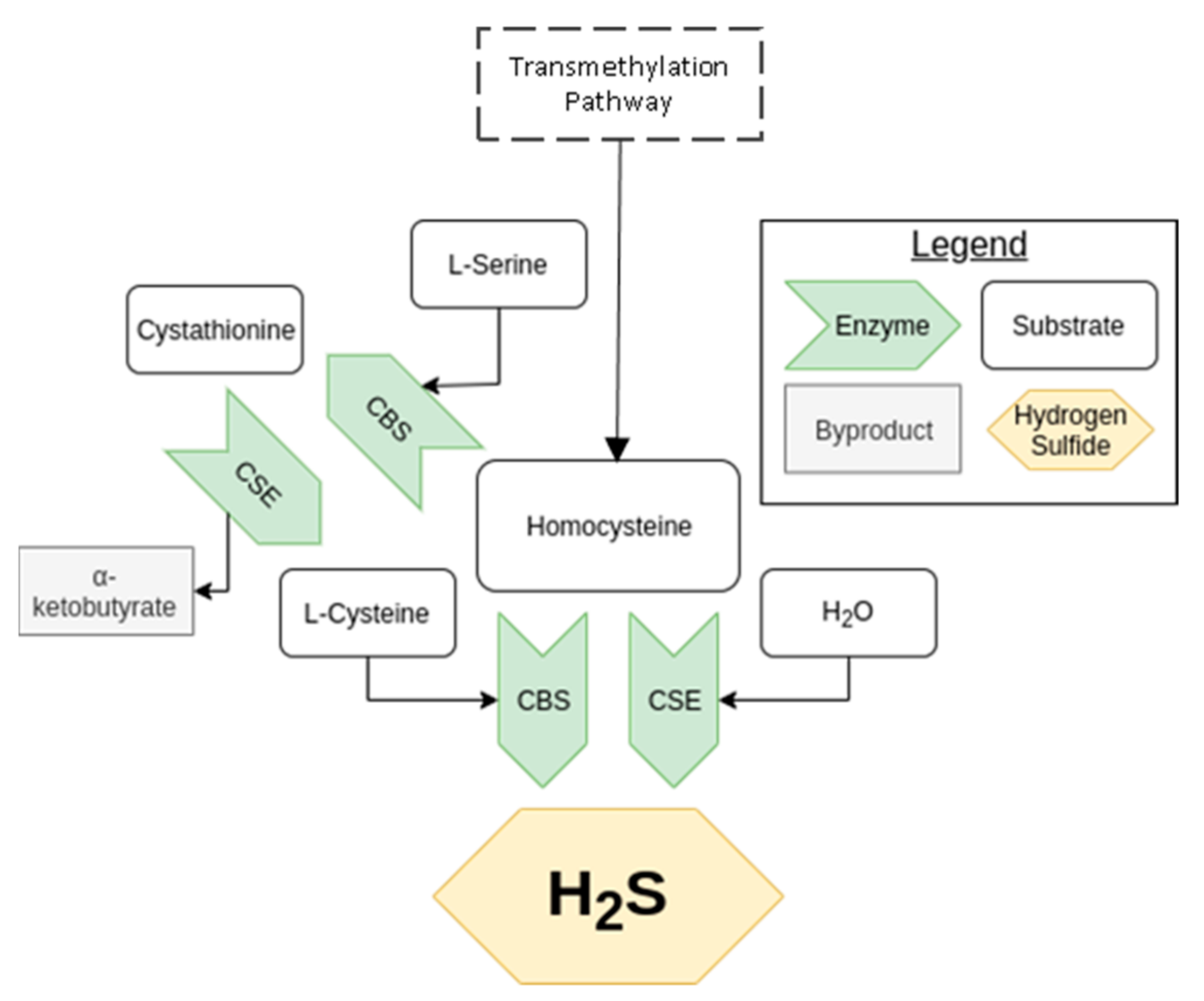
2.1. Transsulfuration Pathway
2.2. Cysteine Catabolism Pathway
2.3. Nonenzymatic Pathways
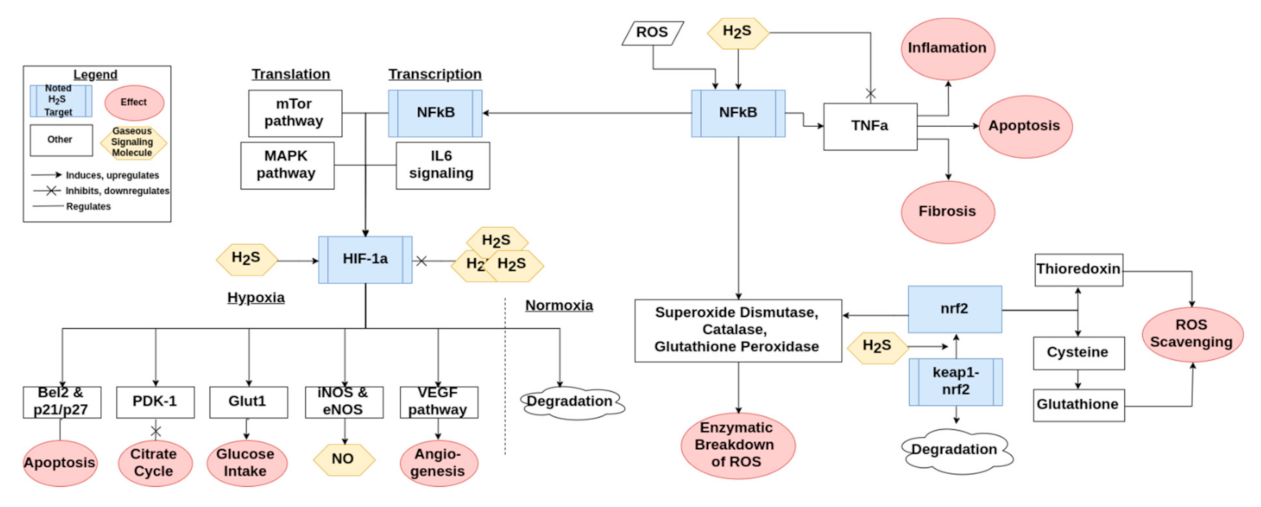
3. Antioxidant Mechanisms of Hydrogen Sulfide
3.1. Reactive Oxygen Species Scavenging
3.2. Protein Modification
3.3. Mitochondria and Respiratory Oxidation
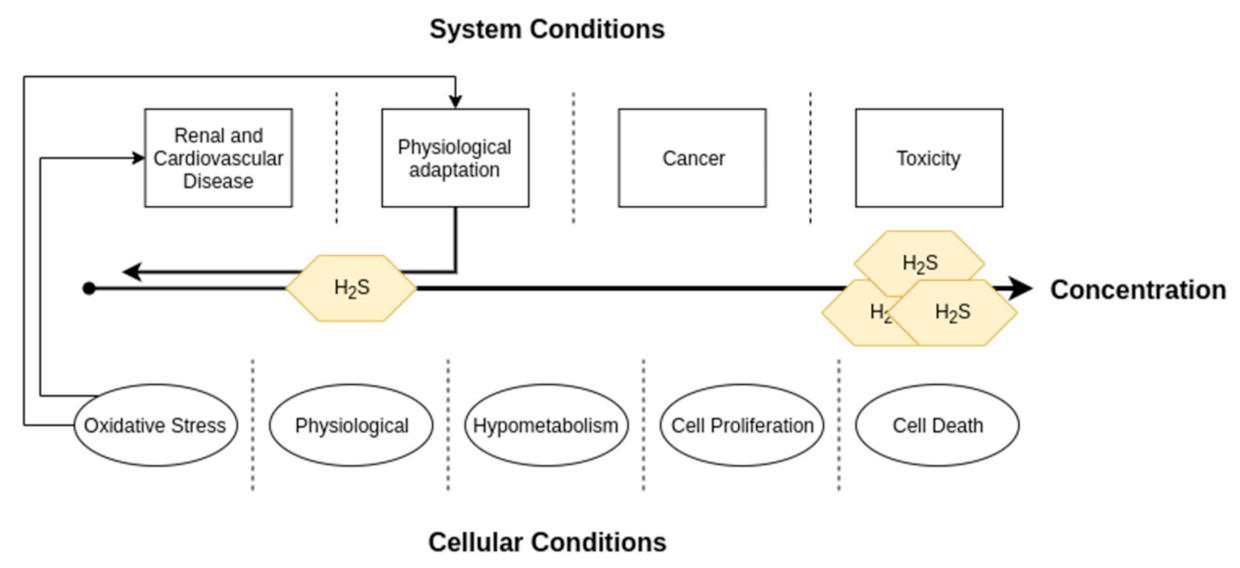
4. Hydrogen Sulfide in Cardiovascular and Renal Physiology
4.1. Cardiovascular
4.2. Renal
5. Role in Cardiorenal Syndrome Pathologies
5.1. Cardiac Cardiorenal Syndrome (CRS) Pathologies
5.2. Renal CRS Pathologies
5.3. Systemic CRS Pathologies
6. Therapeutic Potential in Cardiovascular and Renal Disease
6.1. Treatments Using H2S Donors
6.2. Improving Transplantation Success
6.3. Diet and Exercise
6.4. Regenerative Medicine
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szabo, C. A timeline of hydrogen sulfide (H(2)S) research: From environmental toxin to biological mediator. Biochem. Pharm. 2018, 149, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, T.L. Hydrogen sulphide. Occup. Med. 1996, 46, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Dorman, D.C.; Moulin, F.J.; McManus, B.E.; Mahle, K.C.; James, R.A.; Struve, M.F. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: Correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol. Sci. 2002, 65, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Warenycia, M.W.; Goodwin, L.R.; Benishin, C.G.; Reiffenstein, R.J.; Francom, D.M.; Taylor, J.D.; Dieken, F.P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharm. 1989, 38, 973–981. [Google Scholar] [CrossRef]
- Goodwin, L.R.; Francom, D.; Dieken, F.P.; Taylor, J.D.; Warenycia, M.W.; Reiffenstein, R.J.; Dowling, G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: Postmortem studies and two case reports. J. Anal. Toxicol. 1989, 13, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.C.; Gould, D.H. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J. Chromatogr. 1990, 526, 540–545. [Google Scholar] [CrossRef]
- Binkley, F.; Du Vigneaud, V. The Formation of Cysteine from Homocysteine and Serine by Liver Tissue of Rats. J. Biol. Chem. 1942, 144, 507–511. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Bian, J.S. The Role of Hydrogen Sulfide in Renal System. Front. Pharm. 2016, 7, 385. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Hu, Q.; Zhu, D. An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System. Oxidative Med. Cell. Longev. 2018, 2018, 4579140. [Google Scholar] [CrossRef] [Green Version]
- Koning, A.M.; Frenay, A.R.; Leuvenink, H.G.; Van Goor, H. Hydrogen sulfide in renal physiology, disease and transplantation--the smell of renal protection. Nitric Oxide 2015, 46, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Perridon, B.W.; Leuvenink, H.G.; Hillebrands, J.L.; Van Goor, H.; Bos, E.M. The role of hydrogen sulfide in aging and age-related pathologies. Aging (Albany Ny.) 2016, 8, 2264–2289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 2015, 46, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef] [Green Version]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; Van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Born, J.C.; Frenay, A.S.; Koning, A.M.; Bachtler, M.; Riphagen, I.J.; Minovíc, I.; Feelisch, M.; Dekker, M.M.; Bulthuis, M.L.C.; Gansevoort, R.T.; et al. Urinary Excretion of Sulfur Metabolites and Risk of Cardiovascular Events and All-Cause Mortality in the General Population. Antioxid Redox Signal. 2019, 30, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Van den Born, J.C.; Frenay, A.R.; Bakker, S.J.; Pasch, A.; Hillebrands, J.L.; Lambers Heerspink, H.J.; van Goor, H. High urinary sulfate concentration is associated with reduced risk of renal disease progression in type 2 diabetes. Nitric Oxide 2016, 55–56, 18–24. [Google Scholar] [CrossRef]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- House, A.A.; Anand, I.; Bellomo, R.; Cruz, D.; Bobek, I.; Anker, S.D.; Aspromonte, N.; Bagshaw, S.; Berl, T.; Daliento, L.; et al. Definition and classification of Cardio-Renal Syndromes: Workgroup statements from the 7th ADQI Consensus Conference. Nephrol. Dial. Transpl. 2010, 25, 1416–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braam, B.; Joles, J.A.; Danishwar, A.H.; Gaillard, C.A. Cardiorenal syndrome--current understanding and future perspectives. Nat. Rev. Nephrol. 2014, 10, 48–55. [Google Scholar] [CrossRef]
- Uduman, J. Epidemiology of Cardiorenal Syndrome. Adv. Chronic Kidney Dis. 2018, 25, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellasi, A.; Di Lullo, L. Cardiorenal Syndrome: An Overview. Adv. Chronic Kidney Dis. 2018, 25, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Snijder, P.M.; Frenay, A.R.; Koning, A.M.; Bachtler, M.; Pasch, A.; Kwakernaak, A.J.; Van den Berg, E.; Bos, E.M.; Hillebrands, J.L.; Navis, G.; et al. Sodium thiosulfate attenuates angiotensin II-induced hypertension, proteinuria and renal damage. Nitric Oxide 2014, 42, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, H.; Van den Born, J.C.; Hillebrands, J.L.; Joles, J.A. Hydrogen sulfide in hypertension. Curr. Opin. Nephrol. Hypertens. 2016, 25, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Koning, A.M.; Meijers, W.C.; Minović, I.; Post, A.; Feelisch, M.; Pasch, A.; Leuvenink, H.G.; De Boer, R.A.; Bakker, S.J.; Van Goor, H. The fate of sulfate in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H415–H421. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, I.T.N.; Klooster, A.; Minnion, M.; Feelisch, M.; Verhaar, M.C.; Van Goor, H.; Joles, J.A. Sodium thiosulfate improves renal function and oxygenation in L-NNA-induced hypertension in rats. Kidney Int. 2020, 98, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxidative Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Vlcek, C.; Paces, V.; Kraus, J.P. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch. Biochem. Biophys. 1998, 350, 95–103. [Google Scholar] [CrossRef]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Hine, C.; Kim, H.J.; Zhu, Y.; Harputlugil, E.; Longchamp, A.; Matos, M.S.; Ramadoss, P.; Bauerle, K.; Brace, L.; Asara, J.M.; et al. Hypothalamic-Pituitary Axis Regulates Hydrogen Sulfide Production. Cell Metab. 2017, 25, 1320–1333.e1325. [Google Scholar] [CrossRef] [Green Version]
- Hine, C.; Zhu, Y.; Hollenberg, A.N.; Mitchell, J.R. Dietary and Endocrine Regulation of Endogenous Hydrogen Sulfide Production: Implications for Longevity. Antioxid Redox Signal. 2018, 28, 1483–1502. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharm. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- Bos, E.M.; Wang, R.; Snijder, P.M.; Boersema, M.; Damman, J.; Fu, M.; Moser, J.; Hillebrands, J.L.; Ploeg, R.J.; Yang, G.; et al. Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 2013, 24, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B(6). Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Liu, Z.; Liu, P. Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis. Int. J. Cardiol. 2014, 172, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Koike, S.; Shibuya, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H(2)S(2), H(2)S(3) and H(2)S. Sci. Rep. 2017, 7, 10459. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. Iubmb Life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- Wedmann, R.; Onderka, C.; Wei, S.; Szijártó, I.A.; Miljkovic, J.L.; Mitrovic, A.; Lange, M.; Savitsky, S.; Yadav, P.K.; Torregrossa, R.; et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem. Sci. 2016, 7, 3414–3426. [Google Scholar] [CrossRef] [Green Version]
- Palde, P.B.; Carroll, K.S. A universal entropy-driven mechanism for thioredoxin-target recognition. Proc. Natl. Acad. Sci. USA 2015, 112, 7960–7965. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, A Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress in Vitro and in Acute Renal Injury in Vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef] [Green Version]
- Strutyns’ka, N.A.; Semenykhina, O.M.; Chorna, S.V.; Vavilova, H.L.; Sahach, V.F. Hydrogen sulfide inhibits Ca(2+)-induced mitochondrial permeability transition pore opening in adult and old rat heart. Fiziol. Zh. 2011, 57, 3–14. [Google Scholar] [CrossRef]
- Bruce King, S. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radic. Biol. Med. 2013, 55, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shi, C.; Wang, H.; Gao, C.; Chang, P.; Chen, X.; Shan, H.; Zhang, M.; Tao, L. Hydrogen sulfide protects against cell damage through modulation of PI3K/Akt/Nrf2 signaling. Int. J. Biochem. Cell Biol. 2019, 117, 105636. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.H.; Rizzi, E.S.; Alves-Lopes, R.; Pinheiro, L.C.; Tostes, R.C.; Tanus-Santos, J.E. Antioxidant and antihypertensive responses to oral nitrite involves activation of the Nrf2 pathway. Free Radic. Biol. Med. 2019, 141, 261–268. [Google Scholar] [CrossRef]
- Kai, S.; Tanaka, T.; Daijo, H.; Harada, H.; Kishimoto, S.; Suzuki, K.; Takabuchi, S.; Takenaga, K.; Fukuda, K.; Hirota, K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid Redox Signal. 2012, 16, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yan, J.; Cao, X.; Hua, P.; Li, Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1α activation. Biochem. Pharm. 2020, 172, 113775. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharm. Rep. 2018, 70, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Zhu, H.; Blake, S.; Chan, K.T.; Pearson, R.B.; Kang, J. Cystathionine β-Synthase in Physiology and Cancer. BioMed Res. Int. 2018, 2018, 3205125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maassen, H.; Hendriks, K.D.W.; Venema, L.H.; Henning, R.H.; Hofker, S.H.; Van Goor, H.; Leuvenink, H.G.D.; Coester, A.M. Hydrogen sulphide-induced hypometabolism in human-sized porcine kidneys. PLoS ONE 2019, 14, e0225152. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; et al. Cytochrome c Reduction by H(2)S Potentiates Sulfide Signaling. ACS Chem. Biol. 2018, 13, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Radic. Biol. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.B.; Van Eyk, J.E.; Marbán, E.; O’Rourke, B. Redox signaling and protein phosphorylation in mitochondria: Progress and prospects. J. Bioenerg. Biomembr. 2009, 41, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Flannigan, K.L.; Agbor, T.A.; Motta, J.P.; Ferraz, J.G.; Wang, R.; Buret, A.G.; Wallace, J.L. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. Faseb. J. 2015, 29, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Drachuk, K.O.; Dorofeyeva, N.A.; Sagach, V.F. The role of hydrogen sulfide in diastolic function restoration during aging. Fiziol. Zh. 2016, 62, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Pu, S.X.; Hou, C.L.; Ma, F.F.; Li, N.; Li, X.H.; Tan, B.; Tao, B.B.; Wang, M.J.; Zhu, Y.C. Cardiac H2S Generation Is Reduced in Ageing Diabetic Mice. Oxidative Med. Cell Longev. 2015, 2015, 758358. [Google Scholar] [CrossRef] [Green Version]
- Mys, L.A.; Budko, A.Y.; Strutynska, N.A.; Sagach, V.F. Pyridoxal-5-phosphate restores hydrogen sulfide synthes and redox state of heart and blood vessels tissue in old animals. Fiziol. Zh. 2017, 63, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majzunova, M.; Dovinova, I.; Barancik, M.; Chan, J.Y. Redox signaling in pathophysiology of hypertension. J. BioMed. Sci. 2013, 20, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenyiova, A.; Drobna, M.; Cebova, M.; Kristek, F.; Cacanyiova, S. Changes in the vasoactive effects of nitric oxide, hydrogen sulfide and the structure of the rat thoracic aorta: The role of age and essential hypertension. J. Physiol. Pharm. 2018, 69. [Google Scholar] [CrossRef]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pr. 2019, 38, 414–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Feliers, D.; Barnes, J.L.; Oh, S.; Choudhury, G.G.; Diaz, V.; Galvan, V.; Strong, R.; Nelson, J.; Salmon, A.; et al. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience 2018, 40, 163–176. [Google Scholar] [CrossRef]
- Kurtz, A. Control of renin synthesis and secretion. Am. J. Hypertens. 2012, 25, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Wu, Z.; Xiong, S.; Cao, L.; Sethi, G.; Bian, J.S. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharm. 2018, 149, 20–28. [Google Scholar] [CrossRef]
- Luo, R.; Hu, S.; Liu, Q.; Han, M.; Wang, F.; Qiu, M.; Li, S.; Li, X.; Yang, T.; Fu, X.; et al. Hydrogen sulfide upregulates renal AQP-2 protein expression and promotes urine concentration. Faseb. J. 2019, 33, 469–483. [Google Scholar] [CrossRef]
- Olson, K.R. Hydrogen sulfide as an oxygen sensor. Clin. Chem. Lab. Med. 2013, 51, 623–632. [Google Scholar] [CrossRef]
- Leigh, J.; Juriasingani, S.; Akbari, M.; Shao, P.; Saha, M.N.; Lobb, I.; Bachtler, M.; Fernandez, B.; Qian, Z.; Van Goor, H.; et al. Endogenous H(2)S production deficiencies lead to impaired renal erythropoietin production. Can. Urol. Assoc. J. 2018, 13, E210–E219. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Maksuti, E.; Westerhof, N.; Westerhof, B.E.; Broomé, M.; Stergiopulos, N. Contribution of the Arterial System and the Heart to Blood Pressure during Normal Aging—A Simulation Study. PLoS ONE 2016, 11, e0157493. [Google Scholar] [CrossRef] [Green Version]
- Strutynska, N.A.; Kotsiuruba, A.V.; Budko, A.Y.; Mys, L.A.; Sagach, V.F. Mitochondrial dysfunction in the aging heart is accompanied by constitutive no-synthases uncoupling on the background of oxidative and nitrosative stress. Fiziol. Zh. 2016, 62, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Zhao, Y.; Wang, L.; Peng, X.; Li, H.; Zhao, Y.; He, Y.; Shao, H.; Zhong, X.; Li, H.; et al. H2 S restores the cardioprotection from ischemic post-conditioning in isolated aged rat hearts. Cell Biol. Int. 2015, 39, 1173–1176. [Google Scholar] [CrossRef]
- Liang, M.; Jin, S.; Wu, D.D.; Wang, M.J.; Zhu, Y.C. Hydrogen sulfide improves glucose metabolism and prevents hypertrophy in cardiomyocytes. Nitric Oxide 2015, 46, 114–122. [Google Scholar] [CrossRef]
- Ma, N.; Liu, H.M.; Xia, T.; Liu, J.D.; Wang, X.Z. Chronic aerobic exercise training alleviates myocardial fibrosis in aged rats through restoring bioavailability of hydrogen sulfide. Can. J. Physiol. Pharm. 2018, 96, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Jin, H.F.; Chen, S.; Chen, Q.H.; Tang, C.S.; Du, J.B.; Huang, Y.Q. Hydrogen Sulfide Regulating Myocardial Structure and Function by Targeting Cardiomyocyte Autophagy. Chin. Med. J. 2018, 131, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, S.E.; Calvani, R.; Marzetti, E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J. Mol. Cell. Cardiol. 2014, 71, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Bilska-Wilkosz, A.; Górny, M. Sulfane sulfur—New findings on an old topic. Acta Biochim. Pol. 2019, 66, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Dudoignon, E.; Dépret, F.; Legrand, M. Is the Renin-Angiotensin-Aldosterone System Good for the Kidney in Acute Settings? Nephron 2019, 143, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Zarbock, A. Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesth Analg. 2017, 125, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Thiele, R.H.; Isbell, J.M.; Rosner, M.H. AKI associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2015, 10, 500–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, F.; Seifi, B.; Kadkhodaee, M.; Ahghari, P. Administration of hydrogen sulfide protects ischemia reperfusion-induced acute kidney injury by reducing the oxidative stress. Ir. J. Med. Sci. 2016, 185, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.M.; Leuvenink, H.G.; Snijder, P.M.; Kloosterhuis, N.J.; Hillebrands, J.L.; Leemans, J.C.; Florquin, S.; Van Goor, H. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2009, 20, 1901–1905. [Google Scholar] [CrossRef] [Green Version]
- Caravaca-Fontán, F.; Fernández-Juárez, G.; Praga, M. Acute kidney injury in interstitial nephritis. Curr. Opin. Crit. Care 2019, 25, 558–564. [Google Scholar] [CrossRef]
- Clark, M.R.; Trotter, K.; Chang, A. The Pathogenesis and Therapeutic Implications of Tubulointerstitial Inflammation in Human Lupus Nephritis. Semin. Nephrol. 2015, 35, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen Sulfide Attenuates LPS-Induced Acute Kidney Injury by Inhibiting Inflammation and Oxidative Stress. Oxid Med. Cell Longev. 2018, 2018, 6717212. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.L.; Vaughan, D.; Dicay, M.; MacNaughton, W.K.; De Nucci, G. Hydrogen Sulfide-Releasing Therapeutics: Translation to the Clinic. Antioxid Redox Signal. 2018, 28, 1533–1540. [Google Scholar] [CrossRef]
- Van Dingenen, J.; Pieters, L.; Vral, A.; Lefebvre, R.A. The H2S-Releasing Naproxen Derivative ATB-346 and the Slow-Release H2S Donor GYY4137 Reduce Intestinal Inflammation and Restore Transit in Postoperative Ileus. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Donnan, P.T.; Bell, S.; Guthrie, B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: Systematic review and meta-analysis. Bmc Nephrol. 2017, 18, 256. [Google Scholar] [CrossRef] [Green Version]
- Ozkaya, O.; Genc, G.; Bek, K.; Sullu, Y. A case of acetaminophen (paracetamol) causing renal failure without liver damage in a child and review of literature. Ren. Fail. 2010, 32, 1125–1127. [Google Scholar] [CrossRef]
- Ozatik, F.Y.; Teksen, Y.; Kadioglu, E.; Ozatik, O.; Bayat, Z. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int. Urol. Nephrol. 2019, 51, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, W.; Moore, P.K.; Bian, J. Protective Smell of Hydrogen Sulfide and Polysulfide in Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 313. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhu, L.; Li, L.; Liu, J.; Chen, Y.; Cheng, J.; Peng, T.; Lu, Y. S-Sulfhydration of SIRT3 by Hydrogen Sulfide Attenuates Mitochondrial Dysfunction in Cisplatin-Induced Acute Kidney Injury. Antioxid Redox Signal. 2019, 31, 1302–1319. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Sun, Y.; Zhang, A.; Yang, T. A H 2 S Donor GYY4137 Exacerbates Cisplatin-Induced Nephrotoxicity in Mice. Mediat. Inflamm. 2016, 2016, 8145785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrar, A. Acute Kidney Injury. Nurs. Clin. North Am. 2018, 53, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lian, D.; Liu, W.; Haig, A.; Lobb, I.; Hijazi, A.; Razvi, H.; Burton, J.; Whiteman, M.; Sener, A. Daily therapy with a slow-releasing H(2)S donor GYY4137 enables early functional recovery and ameliorates renal injury associated with urinary obstruction. Nitric Oxide 2018, 76, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Visram, F.; Liu, W.; Haig, A.; Jiang, J.; Mok, A.; Lian, D.; Wood, M.E.; Torregrossa, R.; Whiteman, M.; et al. GYY4137, a Slow-Releasing Hydrogen Sulfide Donor, Ameliorates Renal Damage Associated with Chronic Obstructive Uropathy. J. Urol. 2016, 196, 1778–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminzadeh, M.A.; Vaziri, N.D. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transpl. 2012, 27, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmin, O.; Siow, Y.L. Metabolic Imbalance of Homocysteine and Hydrogen Sulfide in Kidney Disease. Curr. Med. Chem. 2018, 25, 367–377. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Isoni, C.A.; Borges, E.A.; Veloso, C.A.; Mattos, R.T.; Chaves, M.M.; Nogueira-Machado, J.A. cAMP activates the generation of reactive oxygen species and inhibits the secretion of IL-6 in peripheral blood mononuclear cells from type 2 diabetic patients. Oxidative Med. Cell Longev. 2009, 2, 317–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhao, H.; Qiang, Y.; Qian, G.; Lu, S.; Chen, J.; Wang, X.; Guan, Q.; Liu, Y.; Fu, Y. Effects of hydrogen sulfide on high glucose-induced glomerular podocyte injury in mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6814–6820. [Google Scholar] [PubMed]
- Xue, R.; Hao, D.D.; Sun, J.P.; Li, W.W.; Zhao, M.M.; Li, X.H.; Chen, Y.; Zhu, J.H.; Ding, Y.J.; Liu, J.; et al. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal. 2013, 19, 5–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Shi, X.; Wang, H.; Fan, J.; Feng, Y.; Lin, X.; Yang, J.; Cui, Q.; Tang, C.; Xu, G.; et al. Cystathionine γ lyase-hydrogen sulfide increases peroxisome proliferator-activated receptor γ activity by sulfhydration at C139 site thereby promoting glucose uptake and lipid storage in adipocytes. Biochim. Biophys. Acta 2016, 1861, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.P.; Thambar, S.V. Resistant hypertension: An approach to management in primary care. J. Fam. Med. Prim. Care 2015, 4, 193–199. [Google Scholar] [CrossRef]
- Van Der Sande, N.G.C.; Blankestijn, P.J.; Visseren, F.L.J.; Beeftink, M.M.; Voskuil, M.; Westerink, J.; Bots, M.L.; Spiering, W. Prevalence of potential modifiable factors of hypertension in patients with difficult-to-control hypertension. J. Hypertens. 2019, 37, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Doroszko, A.; Janus, A.; Szahidewicz-Krupska, E.; Mazur, G.; Derkacz, A. Resistant Hypertension. Adv. Clin. Exp. Med. 2016, 25, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Magableh, M.R.; Kemp-Harper, B.K.; Hart, J.L. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens. Res. 2015, 38, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Snijder, P.M.; Frenay, A.R.; De Boer, R.A.; Pasch, A.; Hillebrands, J.L.; Leuvenink, H.G.; Van Goor, H. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease in rats. Br. J. Pharm. 2015, 172, 1494–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucci, M.; Vellecco, V.; Cantalupo, A.; Brancaleone, V.; Zhou, Z.; Evangelista, S.; Calderone, V.; Papapetropoulos, A.; Cirino, G. Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ACE inhibition. Cardiovasc. Res. 2014, 102, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Born, J.C.; Mencke, R.; Conroy, S.; Zeebregts, C.J.; Van Goor, H.; Hillebrands, J.L. Cystathionine γ-lyase is expressed in human atherosclerotic plaque microvessels and is involved in micro-angiogenesis. Sci. Rep. 2016, 6, 34608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.J.; Wu, J.; Guo, W.; Zhu, Y.Z. Atherosclerosis and the Hydrogen Sulfide Signaling Pathway—Therapeutic Approaches to Disease Prevention. Cell Physiol. Biochem. 2017, 42, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.D.; Wang, H.; Zhu, Y.Z. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxidative Med. Cell Longev. 2018, 2018, 4010395. [Google Scholar] [CrossRef] [Green Version]
- Snijder, P.M.; De Boer, R.A.; Bos, E.M.; Van den Born, J.C.; Ruifrok, W.P.; Vreeswijk-Baudoin, I.; van Dijk, M.C.; Hillebrands, J.L.; Leuvenink, H.G.; Van Goor, H. Gaseous hydrogen sulfide protects against myocardial ischemia-reperfusion injury in mice partially independent from hypometabolism. PLoS ONE 2013, 8, e63291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, J.L.; Nagy, P.; Feener, T.; Allain, T.; Ditrói, T.; Vaughan, D.; Muscara, M.; De Nucci, G.; Buret, A. A3 A proof-of-concept, phase 2 clinical trial of the gi safety of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346). J. Can. AsSoc. Gastroenterol. 2019, 2, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Capasso, A. Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules 2013, 18, 690–700. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.H.; et al. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat. Prod. Res. 2018, 32, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Parker-Cote, J.L.; Rizer, J.; Vakkalanka, J.P.; Rege, S.V.; Holstege, C.P. Challenges in the diagnosis of acute cyanide poisoning. Clin. Toxicol. 2018, 56, 609–617. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Brunelli, S.M.; Meade, D.; Wang, W.; Hymes, J.; Lacson, E., Jr. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin. J. Am. Soc. Nephrol. 2013, 8, 1162–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.J.; Wu, Z.Y.; Cao, L.; Zhu, M.Y.; Liu, T.T.; Guo, L.; Lin, Y.; Nie, X.W.; Bian, J.S. Hydrogen Sulfide: Recent Progression and Perspectives for the Treatment of Diabetic Nephropathy. Molecules 2019, 24, 2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosterhuis, N.R.; Frenay, A.R.; Wesseling, S.; Snijder, P.M.; Slaats, G.G.; Yazdani, S.; Fernandez, B.O.; Feelisch, M.; Giles, R.H.; Verhaar, M.C.; et al. DL-propargylglycine reduces blood pressure and renal injury but increases kidney weight in angiotensin-II infused rats. Nitric Oxide 2015, 49, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannan, S.; Boovarahan, S.R.; Rengaraju, J.; Prem, P.; Kurian, G.A. Attenuation of cardiac ischemia-reperfusion injury by sodium thiosulfate is partially dependent on the effect of cystathione beta synthase in the myocardium. Cell Biochem. Biophys. 2019, 77, 261–272. [Google Scholar] [CrossRef]
- Ravindran, S.; Jahir Hussain, S.; Boovarahan, S.R.; Kurian, G.A. Sodium thiosulfate post-conditioning protects rat hearts against ischemia reperfusion injury via reduction of apoptosis and oxidative stress. Chem. Biol. Interact. 2017, 274, 24–34. [Google Scholar] [CrossRef]
- Terstappen, F.; Clarke, S.M.; Joles, J.A.; Ross, C.A.; Garrett, M.R.; Minnion, M.; Feelisch, M.; Goor, H.V.; Sasser, J.M.; Lely, A.T. Sodium Thiosulfate in the Pregnant Dahl Salt-Sensitive Rat, a Model of Preeclampsia. Biomolecules 2020, 10, 302. [Google Scholar] [CrossRef] [Green Version]
- Mizuta, Y.; Tokuda, K.; Guo, J.; Zhang, S.; Narahara, S.; Kawano, T.; Murata, M.; Yamaura, K.; Hoka, S.; Hashizume, M.; et al. Sodium thiosulfate prevents doxorubicin-induced DNA damage and apoptosis in cardiomyocytes in mice. Life Sci. 2020, 257, 118074. [Google Scholar] [CrossRef]
- De Koning, M.L.Y.; Assa, S.; Maagdenberg, C.G.; Van Veldhuisen, D.J.; Pasch, A.; Van Goor, H.; Lipsic, E.; Van der Harst, P. Safety and Tolerability of Sodium Thiosulfate in Patients with an Acute Coronary Syndrome Undergoing Coronary Angiography: A Dose-Escalation Safety Pilot Study (SAFE-ACS). J. Interv. Cardiol. 2020, 2020, 6014915. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wei, C.; Bai, S.; Zhao, Y.; Li, H.; Wu, B.; Wang, R.; Wu, L.; Xu, C. Mediation of exogenous hydrogen sulfide in recovery of ischemic post-conditioning-induced cardioprotection via down-regulating oxidative stress and up-regulating PI3K/Akt/GSK-3β pathway in isolated aging rat hearts. Cell Biosci. 2015, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Snijder, P.M.; Van den Berg, E.; Whiteman, M.; Bakker, S.J.; Leuvenink, H.G.; Van Goor, H. Emerging role of gasotransmitters in renal transplantation. Am. J. Transpl. 2013, 13, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Antioxidant supplements and mortality. Curr. Opin. Clin. Nutr Metab. Care 2014, 17, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Tehzeeb, J.; Manzoor, A.; Ahmed, M.M. Is Stem Cell Therapy an Answer to Heart Failure: A Literature Search. Cureus 2019, 11, e5959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.Q.; Suda, T. Reactive Oxygen Species and Mitochondrial Homeostasis as Regulators of Stem Cell Fate and Function. Antioxid. Redox Signal. 2018, 29, 149–168. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, R.; Liu, X.; Zhou, Y.; Qu, C.; Kikuiri, T.; Wang, S.; Zandi, E.; Du, J.; Ambudkar, I.S.; et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 2014, 15, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Abdelmonem, M.; Shahin, N.N.; Rashed, L.A.; Amin, H.A.A.; Shamaa, A.A.; Shaheen, A.A. Hydrogen sulfide enhances the effectiveness of mesenchymal stem cell therapy in rats with heart failure: In vitro preconditioning versus in vivo co-delivery. BioMed. Pharm. 2019, 112, 108584. [Google Scholar] [CrossRef] [PubMed]



| Concentration | 20–50 ppm | 100–200 ppm | 250–500 ppm | 500+ ppm | 1000+ ppm |
|---|---|---|---|---|---|
| Effects | Kerato- conjunctivitis, Airway agitation | Olfactory paralysis (smell disappears), Eye and airway agitation becomes severe | Lung edema that worsens with longer exposure time | Serious eye damage within 30 min Unconscious or dead within 8 h Amnesia | Immediate collapse due to respiratory failure |
| Administration Type | Examples | Considerations |
|---|---|---|
| Donors | NaHS, Na2S | Easy to use in vitro and in animal models. Phase 1 clinical trials. |
| Sodium Thiosulfate (Na2S2O3) | Already used in the clinic, increasingly tested in animal models. | |
| Modified Drugs | ACE-inhibitors (Zofenopril, Captopril) | Improved safety and efficacy. |
| NSAIDS (Diclonefac, Ibuprofen, Naproxen) | Improved safety. | |
| Gas | H2S | Non-oral administration, potentially dangerous. |
| Modified Treatments | Transplantation | Improvements over cold preservation with greater success rates. |
| Stem Cell Treatments | Protection from oxidative stress during processing. Better treatment results. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scammahorn, J.J.; Nguyen, I.T.N.; Bos, E.M.; Van Goor, H.; Joles, J.A. Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants 2021, 10, 373. https://doi.org/10.3390/antiox10030373
Scammahorn JJ, Nguyen ITN, Bos EM, Van Goor H, Joles JA. Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants. 2021; 10(3):373. https://doi.org/10.3390/antiox10030373
Chicago/Turabian StyleScammahorn, Joshua J., Isabel T. N. Nguyen, Eelke M. Bos, Harry Van Goor, and Jaap A. Joles. 2021. "Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems" Antioxidants 10, no. 3: 373. https://doi.org/10.3390/antiox10030373
APA StyleScammahorn, J. J., Nguyen, I. T. N., Bos, E. M., Van Goor, H., & Joles, J. A. (2021). Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants, 10(3), 373. https://doi.org/10.3390/antiox10030373