Recent Advances of Taxol-Loaded Biocompatible Nanocarriers Embedded in Natural Polymer-Based Hydrogels
Abstract
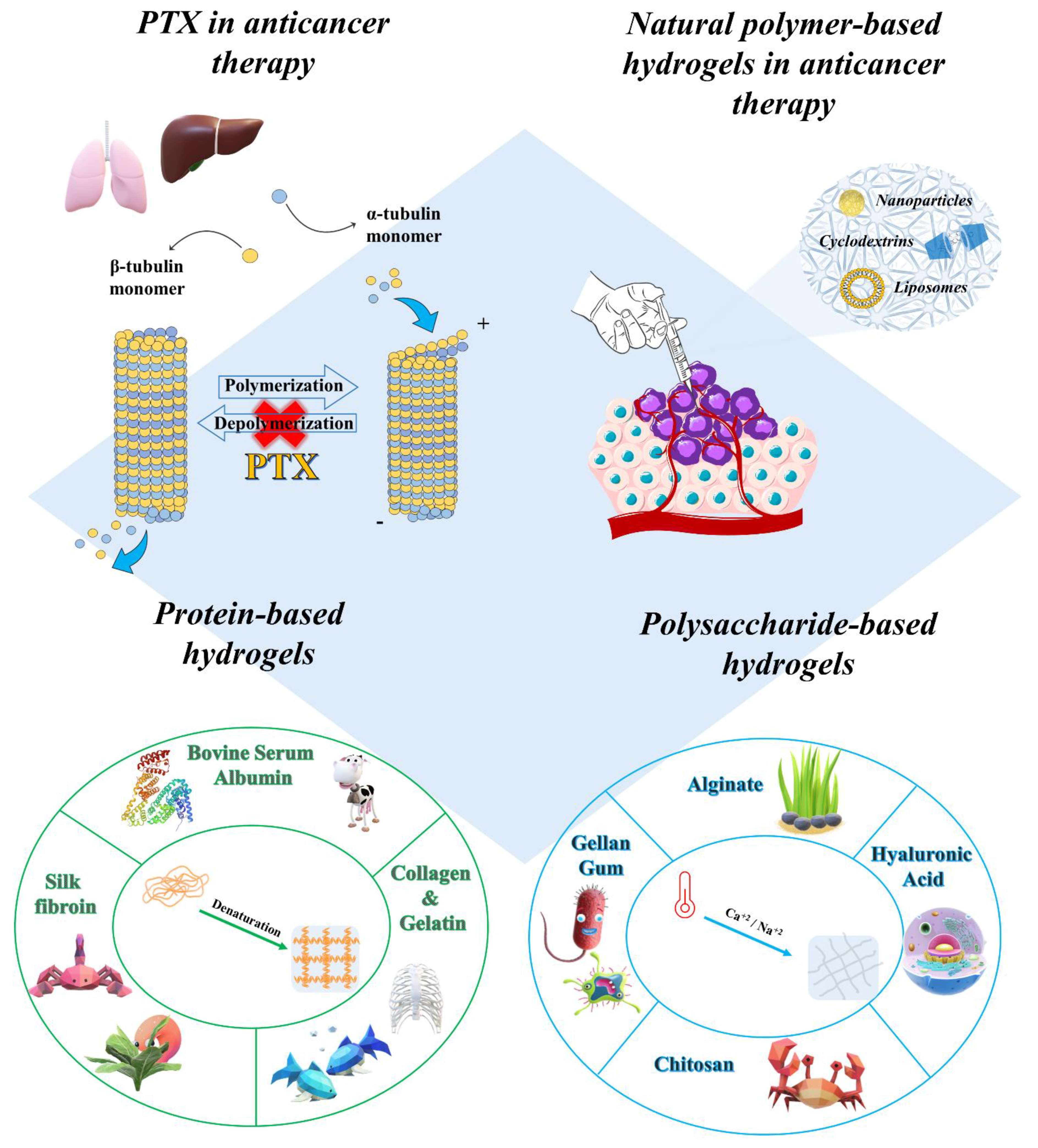
:1. Introduction
1.1. Paclitaxel in Anti-Cancer Therapy
1.2. Overview on Marketed PTX-Loaded Nanosystems
2. Natural Polymer-Based Hydrogels in Anti-Cancer Therapy
3. Polysaccharide-Based Hydrogels
3.1. Hyaluronic Acid-Based Hydrogels
3.2. Gellan Gum-Based Hydrogels
3.3. Alginate-Based Hydrogels
3.4. Chitosan-Based Hydrogels
4. Protein-Based Hydrogels
4.1. Bovine Serum Albumin-(BSA) Based Hydrogels
4.2. Collagen-Based Hydrogels
Gelatin-Based Hydrogels
4.3. Silk Fibroin (SF)-Based Hydrogels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Dou, Q.P. Are we seeing a resurgence in the use of natural products for new drug discovery? Expert Opin. Drug Deliv. 2019, 14, 417. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.; Khan, S.; Hafeez, B.B.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Development of polyvinylpyrrolidone/paclitaxel self-assemblies for breast cancer. Acta Pharm. Sin. B. 2018, 8, 602–614. [Google Scholar] [CrossRef]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, e02422. [Google Scholar] [CrossRef] [Green Version]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Russo, D.; Fresta, M. Liposomes as multicompartmental carriers for multidrug delivery in anticancer chemotherapy. Drug Deliv. Transl. Res. 2011, 1, 66–75. [Google Scholar] [CrossRef]
- Tiainen, L.; Tanner, M.; Lahdenperä, O.; Vihinen, P.; Jukkola, A.; Karihtala, P.; Paunu, N.; Huttunen, T.; Kellokumpu-Lehtinen, P.L. Bevacizumab Combined with Docetaxel or Paclitaxel as First-line Treatment of HER2-negative Metastatic Breast Cancer. Anticancer Res. 2016, 36, 6431–6438. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Beaudoin, J.J.; Vinod, N.; Min, Y.; Makita, N.; Bludau, H.; Kabanov, A.V. Co-delivery of paclitaxel and cisplatin in poly (2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 2019, 192, 1–14. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, H.; Chen, J.; Wang, S.; Xin, Y.; Qu, Y.; Xu, X. Ratiometric co-encapsulation and co-delivery of doxorubicin and paclitaxel by tumor-targeted lipodisks for combination therapy of breast cancer. Int. J. Pharm. 2019, 560, 191–204. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- Holton, R.A.; Somoza, C.; Kim, H.B.; Liang, F.; Biediger, R.J.; Boatman, P.D.; Kim, S. First total synthesis of taxol. 1. Functionalization of the B ring. J. Am. Chem. Soc. 1994, 116, 1597–1598. [Google Scholar] [CrossRef]
- Holton, R.A.; Kim, H.B.; Somoza, C.; Liang, F.; Biediger, R.J.; Boatman, P.D.; Kim, S. First total synthesis of taxol. 2. Completion of the C and D rings. J. Am. Chem. Soc. 1994, 116, 1599–1600. [Google Scholar] [CrossRef]
- Ezrahi, S.; Aserin, A.; Garti, N. Basic principles of drug delivery systems–the case of paclitaxel. Adv. Colloid Interface Sci. 2019, 263, 95–130. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Salehi, M.; Moieni, A.; Safaie, N.; Farhadi, S. Whole fungal elicitors boost paclitaxel biosynthesis induction in Corylus avellana cell culture. PLoS ONE 2020, 15, e0236191. [Google Scholar] [CrossRef]
- Fang, W.S.; Liang, X.T. Recent progress in structure activity relationship and mechanistic studies of taxol analogues. Mini Rev. Med. Chem. 2005, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Qu, X.X.; Wang, D.; Chen, X.X.; Tian, X.C.; Gao, F.; Zhou, X.L. Recent advances in design, synthesis and bioactivity of paclitaxel-mimics. Fitoterapia 2016, 110, 26–37. [Google Scholar] [CrossRef]
- Yang, C.P.H.; Horwitz, S.B. Taxol®: The first microtubule stabilizing agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.N.; Zheng, L.L.; Wang, D.; Liang, X.X.; Gao, F.; Zhou, X.L. Recent advances in microtubule-stabilizing agents. Eur. J. Med. Chem. 2018, 143, 806–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.; Khanam, J.; Sarkar, P.; Pal, T.K. A chemotherapeutic approach targeting the acidic tumor microenvironment: Combination of a proton pump inhibitor and paclitaxel for statistically optimized nanotherapeutics. RSC Adv. 2019, 9, 240–254. [Google Scholar] [CrossRef] [Green Version]
- Bonacci, S.; Paonessa, R.; Costanzo, P.; Salerno, R.; Maiuolo, J.; Nardi, M.; Procopio, A.; Manuela, O. Peracetylation as a strategy to improve oleuropein stability and its affinity to fatty foods. Food Funct. 2018, 9, 5759–5767. [Google Scholar] [CrossRef]
- Alyane, M.; Barratt, G.; Lahouel, M. Remote loading of doxorubicin into liposomes by transmembrane pH gradient to reduce toxicity toward H9c2 cells. SPJ 2016, 24, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, A.; Cosco, D.; Udongo, B.P.; Dini, L.; Viglietto, G.; Paolino, D. Design and Characterization of Glyceryl Monooleate-Nanostructures Containing Doxorubicin Hydrochloride. Pharmaceutics 2020, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Zhuang, J.; Wei, X.; Zhang, X.; Zhang, Y.; Jiang, Y.; Zhang, L. Remote-Loaded Platelet Vesicles for Disease-Targeted Delivery of Therapeutics. Adv. Funct. Mater. 2018, 28, 1801032. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, S.; Li, J.; Wang, Y.; Su, Y.; Chi, D.; Wang, Y. Simple weak-acid derivatives of paclitaxel for remote loading into liposomes and improved therapeutic effects. RSC Adv. 2020, 10, 27676–27687. [Google Scholar] [CrossRef]
- Ojima, I.; Lichtenthal, B.; Lee, S.; Wang, C.; Wang, X. Taxane anticancer agents: A patent perspective. Expert Opin. Ther. Pat. 2016, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Zhang, H. Improved antitumor efficacy of paclitaxel with nano-formulation in breast cancer. Nanotechnol. Rev. 2017, 6, 291–299. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef]
- Kakde, D.; Jain, D.; Shrivastava, V.; Kakde, R.; Patil, A.T. Cancer therapeutics-opportunities, challenges and advances in drug delivery. J. Appl. Pharm. Sci. 2011, 1, 1–10. [Google Scholar]
- Sohail, M.F.; Rehman, M.; Sarwar, H.S.; Naveed, S.; Salman, O.; Bukhari, N.I.; Shahnaz, G. Advancements in the oral delivery of Docetaxel: Challenges, current state-of-the-art and future trends. Int. J. Nanomedicine 2018, 13, 3145. [Google Scholar] [CrossRef] [Green Version]
- Abu Samaan, T.M.; Samec, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules 2019, 9, 789. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Khan, A.R.; Fu, M.; Ji, J.; Yu, A.; Zhai, G. Current development in the formulations of non-injection administration of paclitaxel. Int. J. Pharm. 2018, 542, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, L.; Xu, H.Q.; Huang, X.E.; Qian, Y.D.; Xiang, J. Clinical comparison between paclitaxel liposome (Lipusu®) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2591–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koudelka, Š.; Turánek, J. Liposomal paclitaxel formulations. JCR 2012, 163, 322–334. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein-vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2021, 118, 111538. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Palma, E.; Pasqua, A.; Gagliardi, A.; Britti, D.; Fresta, M.; Cosco, D. Antileishmanial activity of amphotericin B-loaded-PLGA nanoparticles: An overview. Materials 2018, 11, 1167. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Shin, H.S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 1–29. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardi, A.; Giuliano, E.; Eeda, V.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomedicine 2009, 4, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.; Trieu, V.; Damascelli, B.; Soon-Shiong, P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl. Oncol. 2009, 2, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parodi, A.; Miao, J.; Soond, S.M.; Rudzińska, M.; Zamyatnin, A.A. Albumin nanovectors in cancer therapy and imaging. Biomolecules 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 31, 7794–7803. [Google Scholar] [CrossRef]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef]
- Zang, X.; Lee, J.B.; Deshpande, K.; Garbuzenko, O.B.; Minko, T.; Kagan, L. Prevention of paclitaxel-induced neuropathy by formulation approach. JCR 2019, 303, 109–116. [Google Scholar] [CrossRef]
- Ye, L.; He, J.; Hu, Z.; Dong, Q.; Wang, H.; Fu, F.; Tian, J. Antitumor effect and toxicity of Lipusu in rat ovarian cancer xenografts. FCT 2013, 52, 200–206. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, G.; Du, Y.; Ye, L.; Chen, W.; Zhang, L.; Fu, F. Hypersensitivity reaction studies of a polyethoxylated castor oil-free, liposome-based alternative paclitaxel formulation. Mol. Med. Rep. 2013, 7, 947–952. [Google Scholar] [CrossRef] [Green Version]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Harshita, B.M.A.; Beg, S.; Pottoo, F.H.; Ahmad, F.J. Nanopaclitaxel therapy: An evidence based review on the battle for next-generation formulation challenges. Nanomedicine 2019, 14, 1323–1341. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. JCR 2011, 72, 191–202. [Google Scholar] [CrossRef]
- He, Z.; Wan, X.; Schulz, A.; Bludau, H.; Dobrovolskaia, M.A.; Stern, S.T.; Kabanov, A.V. A high capacity polymeric micelle of paclitaxel: Implication of high dose drug therapy to safety and in vivo anti-cancer activity. Biomaterials 2016, 101, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, I.; Ichimura, E.; Goda, R.; Hayashi, H.; Mashiba, H.; Nagai, D.; Masuda, A. An in vivo mechanism for the reduced peripheral neurotoxicity of NK105: A paclitaxel-incorporating polymeric micellar nanoparticle formulation. Int. J. Nanomedicine 2017, 12, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madaan, A.; Singh, P.; Awasthi, A.; Verma, R.; Singh, A.T.; Jaggi, M.; Kulkarni, H. Efficiency and mechanism of intracellular paclitaxel delivery by novel nanopolymer-based tumor-targeted delivery system, Nanoxel TM. Clin. Transl. Oncol. 2013, 15, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Giodini, L.; Re, F.L.; Campagnol, D.; Marangon, E.; Posocco, B.; Dreussi, E.; Toffoli, G. Nanocarriers in cancer clinical practice: A pharmacokinetic issue. NBM 2017, 13, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Fernández-Carballido, A.; Torres-Suárez, A.I. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother. Pharmacol. 2019, 1–18. [Google Scholar] [CrossRef]
- Vergote, I.; Brize, A.; Lisyanskaya, A.S.; Lichinitser, M. Randomized phase III study comparing paclical-carboplatin with paclitaxel-carboplatin in patients with recurrent platinum-sensitive epithelial ovarian cancer. J. Clin. Oncol. 2015, 33, 5517. [Google Scholar] [CrossRef]
- Khanna, C.; Rosenberg, M.; Vail, D.M. A review of paclitaxel and novel formulations including those suitable for use in dogs. J. Vet. Intern. Med. 2015, 29, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, C.R.; Lejeune, A.; Thompson, P.M.; Shiomitsu, K. In vitro effects of the chemotherapy agent water-soluble micellar paclitaxel (Paccal Vet) on canine hemangiosarcoma cell lines. Vet. Comp. Oncol. 2019, 17, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Drug-Loaded Biocompatible Nanocarriers Embedded in Poloxamer 407 Hydrogels as Therapeutic Formulations. Medicines 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. JCR 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, E.; Paolino, D.; Cristiano, M.C.; Fresta, M.; Cosco, D. Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials 2020, 10, 1069. [Google Scholar] [CrossRef]
- Bonacucina, G.; Martelli, S.; Palmieri, G.F. Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int. J. Pharm. 2004, 282, 115–130. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan/β-glycerophosphate in situ gelling mucoadhesive systems for intravesical delivery of mitomycin-C. Int. J. Pharm. X 2019, 1, 100007. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Spizzirri, U.G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics 2019, 11, 486. [Google Scholar] [CrossRef] [Green Version]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Pol. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Parsons, D.L.; Navarre, C.; Kompella, U.B. Development and in-vitro evaluation of sustained release poloxamer 407 (P407) gel formulations of ceftiofur. JCR 2002, 85, 73–81. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Cafaggi, S.; Russo, E.; Caviglioli, G.; Parodi, B.; Stefani, R.; Sillo, G.; Bignardi, G. Poloxamer 407 as a solubilising agent for tolfenamic acid and as a base for a gel formulation. Eur. J. Pharm. Sci. 2008, 35, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ci, L.; Huang, Z.; Liu, Y.; Liu, Z.; Wei, G.; Lu, W. Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: Preparation, characterization and application in a vaginal drug delivery system. Acta Pharm. Sin. B 2017, 7, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Gagliardi, A.; Froiio, F.; Salvatici, M.C.; Paolino, D.; Fresta, M.; Cosco, D. Characterization and refinement of zein-based gels. Food Hydrocoll. 2020, 101, 105555. [Google Scholar] [CrossRef]
- Voci, S.; Fresta, M.; Cosco, D. Gliadins as versatile biomaterials for drug delivery applications. J. Contr. Release 2021, 329, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bajaj, A. Advances in self-assembled injectable hydrogels for cancer therapy. Biomater. Sci. 2020, 8, 2055–2073. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Y.; Gou, Y.; Wang, X.; Zhao, X.; Tao, L. Improving tumor chemotherapy effect using an injectable self-healing hydrogel as drug carrier. Polym. Chem. 2017, 8, 5071–5076. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Zhao, L. Injectable and self-healing thermosensitive magnetic hydrogel for asynchronous control release of doxorubicin and docetaxel to treat triple-negative breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 33660–33673. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, T.L.; Hillaireau, H.; Noiray, M.; Bourgaux, C.; Arpicco, S.; Pehau-Arnaudet, G.; Taverna, M.; Cosco, D.; Tsapis, N.; Fattal, E. Supramolecular organization and siRNA binding of hyaluronic acid-coated lipoplexes for targeted delivery to the CD44 receptor. Langmuir 2015, 31, 11186–11194. [Google Scholar] [CrossRef]
- Cosco, D.; Tsapis, N.; Nascimento, T.L.; Fresta, M.; Chapron, D.; Taverna, M.; Fattal, E. Polysaccharide-coated liposomes by post-insertion of a hyaluronan-lipid conjugate. Colloids Surf. B 2017, 158, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic acid: The influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers 2018, 10, 1133. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liu, M.; Mo, R. Polysaccharide-Based Biomaterials for Protein Delivery. Med. Drug Dev. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Ueda, K.; Akiba, J.; Ogasawara, S.; Todoroki, K.; Nakayama, M.; Sumi, A.; Kusano, H.; Sanada, S.; Suekane, S.; Xu, K.; et al. Growth inhibitory effect of an injectable hyaluronic acid-tyramine hydrogels incorporating human natural interferon and sorafenib on renal cell carcinoma cells. Acta. Biomater. 2016, 29, 103–111. [Google Scholar] [CrossRef]
- Ohta, S.; Hiramoto, S.; Amano, Y.; Emoto, S.; Yamaguchi, H.; Ishigami, H.; Kitayama, J.; Ito, T. Intraperitoneal Delivery of Cisplatin via a Hyaluronan-Based Nanogel/in Situ Cross-Linkable Hydrogel Hybrid System for Peritoneal Dissemination of Gastric Cancer. Mol. Pharm. 2017, 14, 3105–3113. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Akbari, V.; Amuaghae, E.; Emami, J. Preparation and characterization of an injectable thermosensitive hydrogel for simultaneous delivery of paclitaxel and doxorubicin. Res. Pharm. Sci. 2018, 13, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Paolino, D.; Cilurzo, F.; Casale, F.; Fresta, M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Int. J. Pharm. 2012, 422, 229–237. [Google Scholar] [CrossRef]
- He, M.; Sui, J.; Chen, Y.; Bian, S.; Cui, Y.; Zhou, C.; Zhang, X. Localized multidrug co-delivery by injectable self-crosslinking hydrogel for synergistic combinational chemotherapy. J. Mater. Chem. B 2017, 5, 4852–4862. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V.P. Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs’ Dosage Ratio Effect. Molecules 2019, 24, 1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, G.; Consumi, M.; Pepi, S.; Pardini, A.; Bonechi, C.; Tamasi, G.; Magnani, A. Enriched Gellan Gum hydrogel as visco-supplement. Carbohydr. Polym. 2020, 227, 115347. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [Green Version]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef] [Green Version]
- Tako, M. The principle of polysaccharide gels. Adv. Biosci. Biotechnol. 2015, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guhasarkar, S.; More, P.; Banerjee, R. Urothelium-adherent, ion-triggered liposome-in-gel system as a platform for intravesical drug delivery. J. Contr. Release 2017, 245, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 1–22. [Google Scholar] [CrossRef]
- Otterlei, M.; Østgaard, K.; Skjåk-Bræk, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Gurikov, P.; Smirnova, I. Non-conventional methods for gelation of alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Ikoma, T.; Monkawa, A.; Yunoki, S.; Abe, T.; Sakane, M.; Tanaka, M. Preparation of hydroxyapatite-alginate gels as a carrier for controlled release of paclitaxel. Key Eng. Mater. 2007, 330, 1053–1056. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Q.; Shen, M.; Sun, Y.; Zhang, X.; Huang, C.; Duan, Y. Thermoresponsive nanocomposite gel for local drug delivery to suppress the growth of glioma by inducing autophagy. Autophagy 2017, 13, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Hu, X.; Wu, B.; Jiang, H.; Young, C.Y.; Pang, Y.; Yuan, H. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011, 307, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, Y.; Zhang, X.; Yan, Z.; Wang, M.; Xu, F. Preparation of covalently crosslinked sodium alginate/hydroxypropyl methylcellulose pH-sensitive microspheres for controlled drug release. BioResources 2018, 13, 8614–8628. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Iannone, M.; Mare, R.; Paolino, D.; Gagliardi, A.; Froiio, F.; Cosco, D.; Fresta, M. Characterization and in vitro anticancer properties of chitosan-microencapsulated flavan-3-ols-rich grape seed extracts. Int. J. Biol. Macromol. 2017, 104, 1039–1045. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 1–18. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydr. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of partially N-acetylated chitosans with hen egg white and human lysozyme. Carbohydr. Polym. 1996, 29, 163–167. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef]
- Kong, X.; Xu, W.; Zhang, C.; Kong, W. Chitosan temperature-sensitive gel loaded with drug microspheres has excellent effectiveness, biocompatibility and safety as an ophthalmic drug delivery system. Exp. Ther. Med. 2018, 15, 1442–1448. [Google Scholar] [CrossRef]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Paolino, D. Rutin-loaded chitosan microspheres: Characterization and evaluation of the anti-inflammatory activity. Carbohydr. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Özdoğan, A.I.; Akca, G.; Şenel, S. Development and in vitro evaluation of chitosan based system for local delivery of atorvastatin for treatment of periodontitis. Eur. J. Pharm. Sci. 2018, 124, 208–216. [Google Scholar] [CrossRef]
- Palma, E.; Costa, N.; Molinaro, R.; Francardi, M.; Paolino, D.; Cosco, D.; Fresta, M. Improvement of the therapeutic treatment of inflammatory bowel diseases following rectal administration of mesalazine-loaded chitosan microparticles vs. Asamax®. Carbohydr. Polym. 2019, 212, 430–438. [Google Scholar] [CrossRef]
- Cosco, D.; Federico, C.; Maiuolo, J.; Bulotta, S.; Molinaro, R.; Paolino, D.; Fresta, M. Physicochemical features and transfection properties of chitosan/poloxamer 188/poly (D, L-lactide-co-glycolide) nanoplexes. Int. J. Nanomed. 2014, 9, 2359. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Crake, C.; Teo, B.; Carugo, D.; de Saint Victor, M.; Seth, A.; Stride, E. Ultrasound-Enhanced siRNA Delivery Using Magnetic Nanoparticle-Loaded Chitosan-Deoxycholic Acid Nanodroplets. Adv. Healthc. Mater. 2017, 6, 1601246. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, G.E.; Maggisano, V.; Celano, M.; Cosco, D.; Mignogna, C.; Baldan, F.; Puxeddu, E. Anti-hTERT siRNA-loaded nanoparticles block the growth of anaplastic thyroid cancer xenograft. Mol. Cancer Ther. 2018, 17, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, G.; Murray, P.; Zhang, H. Porous chitosan by crosslinking with tricarboxylic acid and tuneable release. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Han, Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Shive, M.; Bichara, A.; Berrada, M.; Le Garrec, D.; Chenite, A.; Leroux, J.C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004, 57, 53–63. [Google Scholar] [CrossRef]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.A. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Choromanska, A.; Kulbacka, J.; Harasym, J.; Oledzki, R.; Szewczyk, A.; Saczko, J. High-and low-molecular weight oat beta-glucan reveals antitumor activity in human epithelial lung cancer. Pathol. Oncol. Res. 2018, 24, 583–592. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wang, J.; Ge, L.; Zhu, J. Development of a thermally responsive nanogel based on chitosan–poly (N-isopropylacrylamide-co-acrylamide) for paclitaxel delivery. J. Pharm. Sci. 2014, 103, 2012–2021. [Google Scholar] [CrossRef]
- Castro, J.S.; Tapia, L.V.; Silveyra, R.A.; Martinez, C.A.; Deymier, P.A. Negative impact of paclitaxel crystallization on hydrogels and novel approaches for anticancer drug delivery systems. In Current Cancer Treatment—Novel Beyond Conventional Approaches; Ozdemir, O., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 767–782. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1043.7594&rep=rep1&type=pdf (accessed on 16 December 2020).
- Bajaj, G.; Kim, M.R.; Mohammed, S.I.; Yeo, Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. JCR 2012, 158, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, M.; Utreja, P.; Kumar Jain, S. Paclitaxel loaded nanoliposomes in thermosensitive hydrogel: A dual approach for sustained and localized delivery. Curr. Med. Chem. Anticancer Agents 2016, 16, 365–376. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, X.; Wu, Z.; Qi, X. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohydr. Polym. 2016, 144, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Yoon, S.J.; Kim, S.Y.; Lee, D.W.; Um, S.; Hyun, H.; Yang, D.H. A local drug delivery system based on visible light-cured glycol chitosan and doxorubicin⋅ hydrochloride for thyroid cancer treatment in vitro and in vivo. Drug Deliv. 2018, 25, 1664–1671. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.J.; Hyun, H.; Lee, D.-W.; Yang, D.H. Visible Light-Cured Glycol Chitosan Hydrogel Containing a Beta-Cyclodextrin-Curcumin Inclusion Complex Improves Wound Healing In Vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.J.; Yoo, Y.; Nam, S.E.; Hyun, H.; Lee, D.-W.; Um, S.; Kim, S.Y.; Hong, S.O.; Yang, D.H.; Chun, H.J. The Cocktail Effect of BMP-2 and TGF-β1 Loaded in Visible Light-Cured Glycol Chitosan Hydrogels for the Enhancement of Bone Formation in a Rat Tibial Defect Model. Mar. Drugs 2018, 16, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-cured glycol chitosan hydrogel for ovarian cancer drug delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Pesoa, J.I.; Rico, M.J.; Rozados, V.R.; Scharovsky, O.G.; Luna, J.A.; Mengatto, L.N. Paclitaxel delivery system based on poly (lactide-co-glycolide) microparticles and chitosan thermo-sensitive gel for mammary adenocarcinoma treatment. J. Pharm. Pharmacol. 2018, 70, 1494–1502. [Google Scholar] [CrossRef]
- Totosaus, A.; Montejano, J.G.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food gels: Gelling process and new applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Paolino, D.; Fresta, M.; Cosco, D. Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels. Molecules 2020, 25, 3174. [Google Scholar] [CrossRef] [PubMed]
- Munialo, C.D.; Euston, S.R.; de Jongh, H.H.J. Protein gels. In Proteins in Food Processing; Woodhead Publishing: Duxford, UK, 2018; pp. 501–521. ISBN 9780081007228. [Google Scholar]
- Katzav, H.; Chirug, L.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. Comparison of Thermal and High-Pressure Gelation of Potato Protein Isolates. Foods 2020, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Singh, H.; Castro-Aceituno, V.; Ahn, S.; Kim, Y.J.; Yang, D.C. Bovine serum albumin as a nanocarrier for the efficient delivery of ginsenoside compound K: Preparation, physicochemical characterizations and in vitro biological studies. RSC Adv. 2017, 7, 15397–15407. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, B. Bovine Serum Albumin as a Versatile Platform for Cancer Imaging and Therapy. Curr. Med. Chem. 2018, 25, 2938–2953. [Google Scholar] [CrossRef]
- Arabi, S.H.; Aghelnejad, B.; Schwieger, C.; Meister, A.; Kerth, A.; Hinderberger, D. Serum albumin hydrogels in broad pH and temperature ranges: Characterization of their self-assembled structures and nanoscopic and macroscopic properties. Biomater. Sci. 2018, 6, 478–492. [Google Scholar] [CrossRef] [Green Version]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1278–1289. [Google Scholar] [CrossRef]
- Wani, T.A.; Bakheit, A.H.; Abounassif, M.A.; Zargar, S. Study of interactions of an anticancer drug neratinib with bovine serum albumin: Spectroscopic and molecular docking approach. Front. Chem. 2018, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Wani, T.A.; Bakheit, A.H.; Zargar, S.; Hamidaddin, M.A.; Darwish, I.A. Spectrophotometric and molecular modelling studies on in vitro interaction of tyrosine kinase inhibitor linifanib with bovine serum albumin. PLoS ONE 2017, 12, e0176015. [Google Scholar] [CrossRef]
- Tayyab, S.; Min, L.H.; Kabir, M.Z.; Kandandapani, S.; Ridzwan, N.F.W.; Mohamad, S.B. Exploring the interaction mechanism of a dicarboxamide fungicide, iprodione with bovine serum albumin. Chem. Papers 2019, 1–14. [Google Scholar] [CrossRef]
- Boye, J.I.; Alli, I.; Ismail, A.A. Interactions involved in the gelation of bovine serum albumin. J. Agric. Food Chem. 1996, 44, 996–1004. [Google Scholar] [CrossRef]
- Qian, H.; Qian, K.; Cai, J.; Yang, Y.; Zhu, L.; Liu, B. Therapy for Gastric Cancer with Peritoneal Metastasis Using Injectable Albumin Hydrogel Hybridized with Paclitaxel-Loaded Red Blood Cell Membrane Nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 1100–1112. [Google Scholar] [CrossRef]
- Friess, W. Collagen–biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-based drug-delivery materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Alpaslan, D.; Bitlisli, B.O. Collagen-based hydrogel films as drug-delivery devices with antimicrobial properties. Polym. Bull. 2014, 71, 3017–3033. [Google Scholar] [CrossRef]
- An, B.; Lin, Y.S.; Brodsky, B. Collagen interactions: Drug design and delivery. Adv. Drug Deliv. Rev. 2016, 97, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.P.; Dutta, D.; Ganguly, S.; Kapat, K.; Dixit, K.; Chowdhury, A.R.; Dhara, S. Isolation and mass spectrometry based hydroxyproline mapping of type II collagen derived from Capra hircus ear cartilage. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Watanabe, K.; Nishio, Y.; Makiura, R.; Nakahira, A.; Kojima, C. Paclitaxel-loaded hydroxyapatite/collagen hybrid gels as drug delivery systems for metastatic cancer cells. Int. J. Pharm. 2013, 446, 81–86. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Li, W.; Zang, F.; Liu, X.; Qin, S. Paclitaxel-nanoparticles-loaded double network hydrogel for local treatment of breast cancer after surgical resection. Mater. Sci. Eng. C 2020, 114, 111046. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. JCR 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan, Y.H.; Nafchi, A.M.; Huda, N.; Ariffin, F.; Karim, A.A. Comparison of physicochemical and functional properties of duck feet and bovine gelatins. J. Sci. Food Agric. 2017, 97, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. JCR 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Zhu, Z.; Xu, H.; Li, X.; Zheng, D.; Sun, W. Efficient antitumor effect of co-drug-loaded nanoparticles with gelatin hydrogel by local implantation. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lu, X.; Xu, H.; Zhu, Z.; Yin, H.; Qian, X.; Liu, B. Paclitaxel/tetrandrine coloaded nanoparticles effectively promote the apoptosis of gastric cancer cells based on “oxidation therapy”. Mol. Pharm. 2012, 9, 222–229. [Google Scholar] [CrossRef]
- Ramanathan, B.; Jan, K.Y.; Chen, C.H.; Hour, T.C.; Yu, H.J.; Pu, Y.S. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005, 65, 8455–8460. [Google Scholar] [CrossRef] [Green Version]
- Fidanboylu, M.; Griffiths, L.A.; Flatters, S.J. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS ONE 2011, 6, e25212. [Google Scholar] [CrossRef]
- Yang, J.C.; Lu, M.C.; Lee, C.L.; Chen, G.Y.; Lin, Y.Y.; Chang, F.R.; Wu, Y.C. Selective targeting of breast cancer cells through ROS-mediated mechanisms potentiates the lethality of paclitaxel by a novel diterpene, gelomulide K. Free Radic. Biol. Med. 2011, 51, 641–657. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Pahoff, S.; Bock, N.; Hutmacher, D.W. Gelatin Methacryloyl Hydrogels Control the Localized Delivery of Albumin-Bound Paclitaxel. Polymers 2020, 12, 501. [Google Scholar] [CrossRef] [Green Version]
- Im, D.S.; Kim, M.H.; Yoon, Y.I.; Park, W.H. Gelation behaviors and mechanism of silk fibroin according to the addition of nitrate salts. Int. J. Mol. Sci. 2016, 17, 1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. JCR 2011, 150, 128–141. [Google Scholar] [CrossRef]
- Pham, D.T.; Tiyaboonchai, W. Fibroin nanoparticles: A promising drug delivery system. Drug Deliv. 2020, 27, 431–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.H.; Le, T.H.; Huynh, V.Q.N.; Vo, D.V.N.; Le, Q.V. Silk fibroin-based biomaterials for biomedical applications: A review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, A.; Chen, J.; Collette, A.L.; Kim, U.J.; Altman, G.H.; Cebe, P.; Kaplan, D.L. Mechanisms of silk fibroin sol− gel transitions. J. Phys. Chem. B 2006, 110, 21630–21638. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, Q.; Wang, Q.; Qian, H.; Yu, L.; Liu, B.; Li, R. Novel silk fibroin nanoparticles incorporated silk fibroin hydrogel for inhibition of cancer stem cells and tumor growth. Int. J. Nanomed. 2018, 13, 5405. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Liu, Q.; Li, R.; Wang, J.; Zhen, X.; Yue, G.; Liu, B. Facile preparation of paclitaxel loaded silk fibroin nanoparticles for enhanced antitumor efficacy by locoregional drug delivery. ACS Appl. Mater. Interfaces 2013, 5, 12638–12645. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Salvatici, M.C.; Fresta, M.; Cosco, D. Brij-stabilized zein-based nanoparticles as potential drug carriers. Colloids Surf. B: Biointerfaces 2021, 111647. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: From bench to bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Antitumor Features of Vegetal Protein-Based Nanotherapeutics. Pharmaceutics 2020, 12, 65. [Google Scholar] [CrossRef] [Green Version]
- Elstad, N.L.; Fowers, K.D. OncoGel (ReGel/paclitaxel)—Clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009, 61, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Vukelja, S.J.; Anthony, S.P.; Arseneau, J.C.; Berman, B.S.; Cunningham, C.C.; Nemunaitis, J.J.; Fowers, K.D. Phase 1 study of escalating-dose OncoGel®(ReGel®/paclitaxel) depot injection, a controlled-release formulation of paclitaxel, for local management of superficial solid tumor lesions. Anti-Cancer Drugs 2007, 18, 283–289. [Google Scholar] [CrossRef]







| Polymer | Carrier Composition | Physico-chemical Features | Hydrogel Composition | Proposed Application | Main Results | Reference |
|---|---|---|---|---|---|---|
| HA | Micelles P127/TPGS 7:3 w/w mixture; 3 mg of PTX | MS: ~160 ± 20 nm PdI: ~0.30 ± 0.10 ZP: ~−10 ± 1 mV | PF127 20% w/v HA 2% w/v DOX 2 mg/mL | Treatment of solid tumors | Sustained release of PTX from mixed micelles embedded in HA-PF127 hydrogels. | [99] |
| GG | Liposomes PTX:lipid 1:2 w/w | MS: 124 ± 7 nm PdI: 0.22 ± 0.001 ZP: −17 ± 2 mV | 0.1% w/v of gellan gum in saline solution | Intravesical delivery | Cation-triggered gelation. | [109] |
| SA | HAp Microparticles PTX 2.4-7.3 w/v | MS: 4 ± 0.2 µm PdI: / ZP: / | SA 0.5-3% w/t SA:HAp-PTX 10-40% w/t | Treatment of bone cancer | HAp microparticles embedded in alginate matrix can act as both drug carrier and filler for the cancerous, resected bone tissue. | [117] |
| HPMC-SA-PF127 | Nanoparticles mPEG-DPPE-CaP-NP PTX:TMZ 1:100 w/w | MS:38 ± 1 nm PdI: 0.16 ± 0.02 ZP: −40 ± 1 mV | / | Treatment of glioma | Thermo-responsive hydrogel. | [118] |
| CS | Liposomes SP 4.25% w/v PTX 0.6% w/v SD 0.75% w/v S80 0.75% w/v | SD-Liposomes MS: 176 ± 6 nm PdI: 0.3 ZP: −16 ± 1 mV | CS 2% w/v DSP 4-12% w/v | Localized drug delivery system | CS hydrogel containing PTX-liposomes showed improved localization of the drug in the target site and improved safety as compared to Taxol®. | [145] |
| S80-Liposomes MS 136 ± 4 nm PdI: 0.3 ZP: −19 ± 0.5 mV | ||||||
| CS-PVA-GA | Inclusion complex HP- β-CD 0.1% w/v PTX: HP-β-CD 1:2 w/w | / | CS 2% w/v CS:PVA 1:1 w/w GA 220 µM | Intratumoral treatment of solid cancers | Combination of chemical and physical crosslinking resulted in modified CS hydrogels with considerable mechanical strength. The modified porous matrix allowed the sustained release of PTX into the tumor site for 13 days. | [146] |
| CS-Glycerol | β-CD complexes PTX 1 mg 2 mM β-CD 3 mg 2 mM | / | GC 1.5 g GM 7 mg | Treatment of ovarian cancer | Improved PTX solubility after its inclusion in β-CD host cavity. | [150] |
| CS | PLGA Microparticles PTX 1.30 mg PTX/g formulation PVA 2% w/v | MS: ~6 ± 0.13 µm PdI: / ZP: / | / | Treatment of mammary adenocarcinoma | Improved anti-tumor efficacy of chitosan-based hydrogel with respect to PTX solution. | [151] |
| Polymer | Carrier Composition | Physico-chemical Features | Hydrogel Composition | Proposed Application | Main Results | Reference | |
|---|---|---|---|---|---|---|---|
| PEG-BSA | RBC nanoparticles | MS: ~130 ± 2 nm PdI:/ ZP:~ −10 ± 1mV | PEG-BSA 16% w/v | Treatment of gastric cancer | Sustained release of PTX at the tumor site with decrease in intraperitoneal metastasis. | [166] | |
| Collagen | HAp nanoparticles | / | PTX-HAp NPs 2% w/v Collagen: high concentration medium:alkali solution 8:1:1 v/v | Treatment of metastatic cancer | Improved anti-tumor efficacy against MDA-MB-231 cells. | [162] | |
| Collagen-PVA | PLGA nanoparticles PLGA 500 mg PTX 50 mg PVA 0.625% w/v | MS: ~62 nm PdI: 0.22 ± 0.022 ZP:~−30 mV | PVA 5% Collagen 10 mg/mL PLGA-PTX NPs 5% w/v | Breast cancer | Injection of collagen-PVA hydrogel for PTX release in the tumor-resection cavity and as tissue implant to replace the cancerous mammary tissue. | [173] | |
| Gelatin | PEG-b-PCL 20 mg | MS: 70–90 nm PdI:/ ZP: / | Gelatin 500 mg PTX and Tet 0.5 mg/mL | Treatment of gastric cancer | Tet improved the anti-proliferative activity of PTX. | [178] | |
| GelMA | Abraxane® 1.07 µg/µL and 2.65 µg/µL per µl of hydrogel formulation | / | GelMA 5-10-15% w/v | Prevention of breast cancer recurrence after mastectomy and/or lumpectomy | GelMA improved the cytotoxic activity of Abraxane. | [183] | |
| SF | SF-based nanoparticles Sal 6 mg PTX 5 mg | Sal-SF | MS: ~240 nm PdI: 0.15 ZP: ~−15 mV | SF 15 mg/mL SF/PTX 25:5 w/w | Locoregional chemotherapy | Increase in Sal maximum tolerated dose; co-administration of SF-based nanoparticles encapsulating PTX and Sal as single agents in SF-based hydrogels improved their anti-tumor efficacy against CD44+CD133+ cancer stem cells. | [189] |
| PTX-SF | MS: 158 ± 0.4 nm PdI: 0.12 ± 0.02 ZP: ~−3 mV | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voci, S.; Gagliardi, A.; Molinaro, R.; Fresta, M.; Cosco, D. Recent Advances of Taxol-Loaded Biocompatible Nanocarriers Embedded in Natural Polymer-Based Hydrogels. Gels 2021, 7, 33. https://doi.org/10.3390/gels7020033
Voci S, Gagliardi A, Molinaro R, Fresta M, Cosco D. Recent Advances of Taxol-Loaded Biocompatible Nanocarriers Embedded in Natural Polymer-Based Hydrogels. Gels. 2021; 7(2):33. https://doi.org/10.3390/gels7020033
Chicago/Turabian StyleVoci, Silvia, Agnese Gagliardi, Roberto Molinaro, Massimo Fresta, and Donato Cosco. 2021. "Recent Advances of Taxol-Loaded Biocompatible Nanocarriers Embedded in Natural Polymer-Based Hydrogels" Gels 7, no. 2: 33. https://doi.org/10.3390/gels7020033







