A Grape Juice Supplemented with Natural Grape Extracts Is Well Accepted by Consumers and Reduces Brain Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Extracts and Grape Juice
2.2. Preparation of MecobalActive®- and Red Grape Polyphenol-Containing Grape Juice
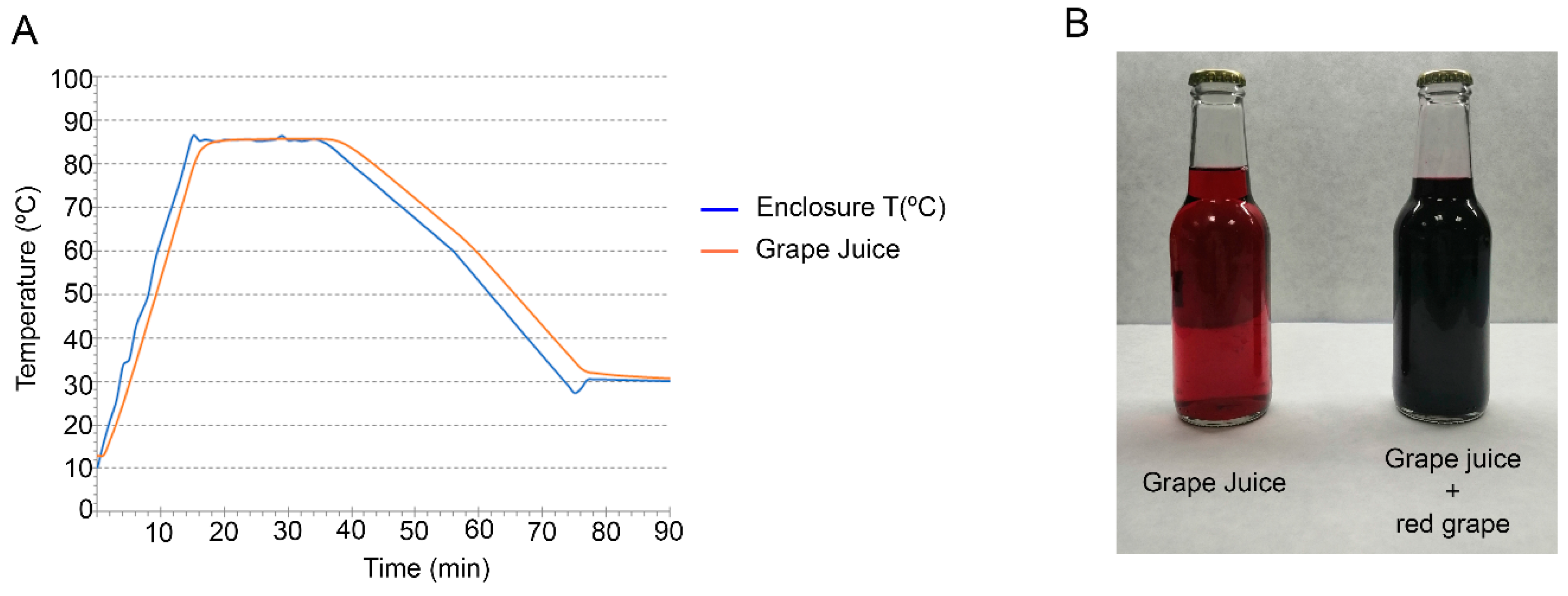
2.3. Pasteurization Process
2.4. Determination of Total Vitamin B12 Content
2.5. Determination of Total Polyphenols Content
2.6. Participants and Testing Location
2.7. Testing Procedure
2.7.1. Descriptive Analysis
2.7.2. Assessment of Acceptability
2.7.3. Testing Preference
2.8. Restrain Stress and In Vivo Treatments
2.9. Quantitative Real-Time PCR
2.10. TBARS, SOD, and Catalase Activity
2.11. Statistical Analysis
3. Results
3.1. Vitamin B12 and Polyphenol Levels Are Not Negatively Affected by Grape Juice Pasteurization
3.2. MecobalActive® Addition to the Grape Juice Does Not Cause Significant Changes in Its Aspect, Texture, and Color and Odor Intensities
3.3. The Addition of Red Grape Polyphenols Does Not Cause Relevant Changes in the Intensity of Flavor and Texture of the Grape Juice
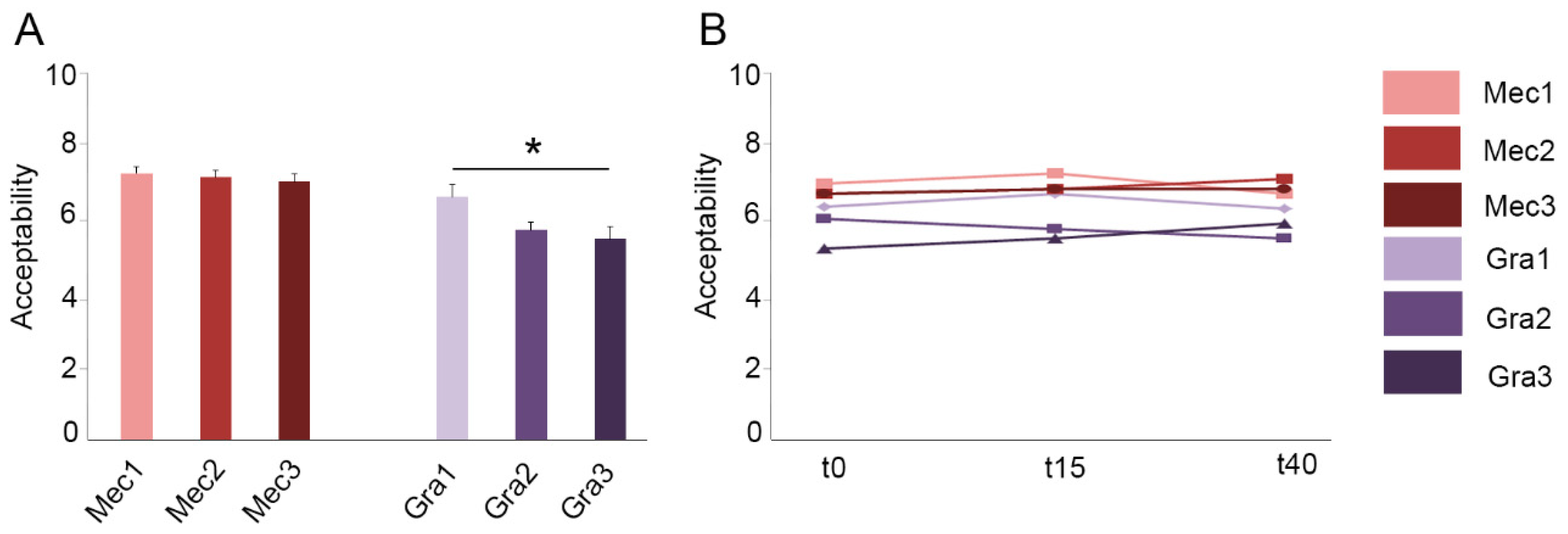
3.4. MecobalActive® and Red Grape Polyphenols Addition to the Grape Juice Does Not Cause Variation in Its Organoleptic Standards
3.5. The Sample with the Lowest Concentration of Red Grape Polyphenols Shows Higher Preference by the Judges
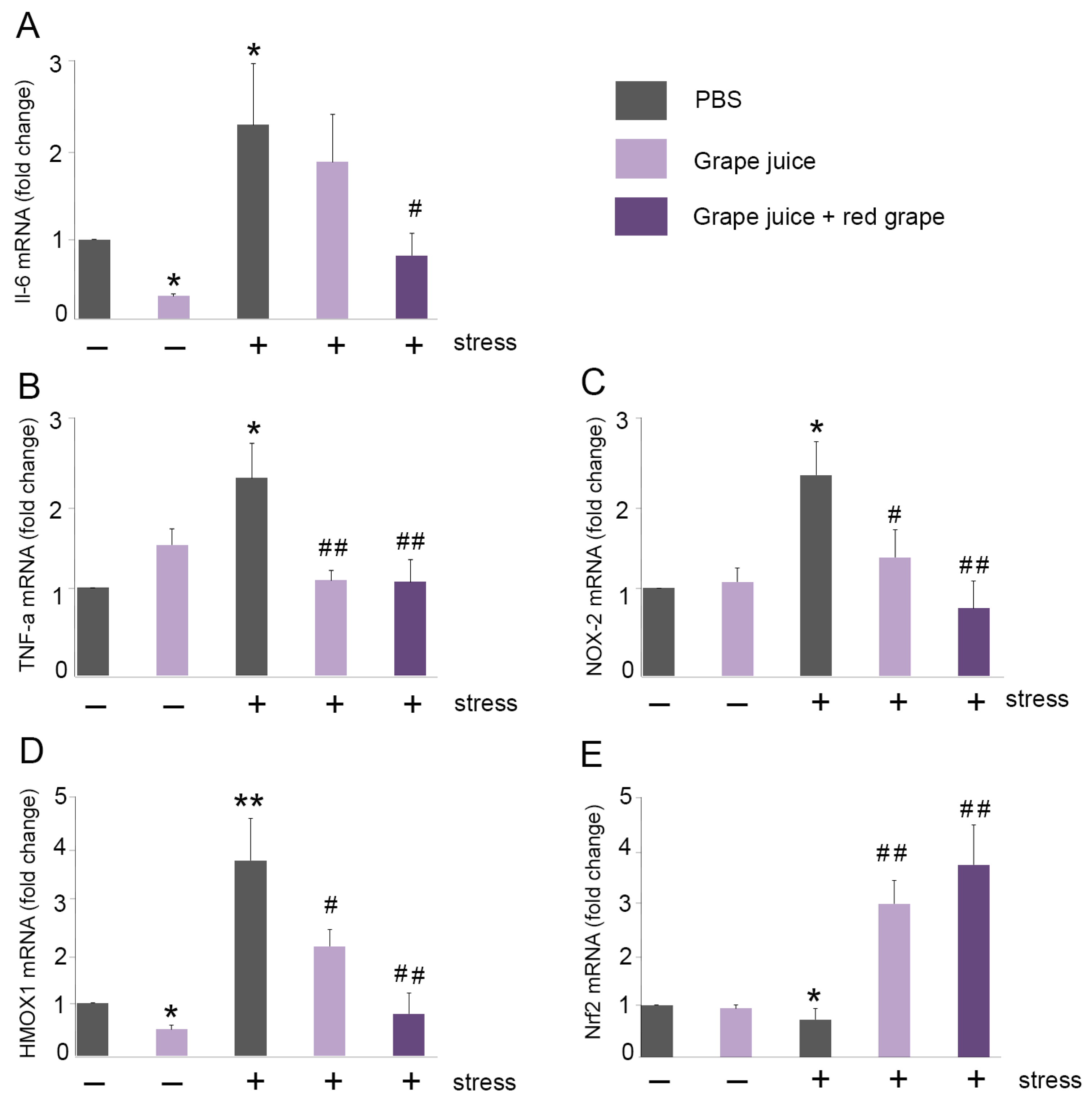
3.6. Oral Administration of Grape Juice Supplemented with Red Grape Polyphenols Exerts an Antioxidant Effect in the Brain of Stressed Mice
3.7. Preventive Treatment with Grape Juice Enriched with Red Grape Polyphenols Increases Antioxidant Enzymes Activity in the Brain
3.8. Preventive Treatment with Red Grape Polyphenol-Enriched Grape Juice Prevents Lipid Peroxidation in the Brain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villain, N.; Dubois, B. Alzheimer’s Disease Including Focal Presentations. Semin. Neurol. 2019, 39, 213–226. [Google Scholar] [CrossRef]
- Heemels, M.-T. Neurodegenerative diseases. Nat. Cell Biol. 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of Wine Consumption with Alzheimer’s Disease. Nutrients 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- Dugger, B.N.; Perl, D.P.; Carlson, G.A. Neurodegenerative Disease Transmission and Transgenesis in Mice. Cold Spring Harb. Perspect. Biol. 2017, 9, a023549. [Google Scholar] [CrossRef] [Green Version]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative Diseases: Might Citrus Flavonoids Play a Protective Role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef] [Green Version]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Reale, M.; Kamal, M.A.; Velluto, L.; Gambi, D.; Di Nicola, M.; Greig, N.H. Relationship between inflammatory mediators, Abeta levels and ApoE genotype in Alzheimer disease. Curr. Alzheimer Res. 2012, 9, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Cauchi, R.J.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Bartolome, B.; Penalvo, J.L.; Perez-Matute, P.; Motilva, M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients 2020, 12, 3082. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Hernández, J.; Zaldívar-Machorro, V.J.; Villanueva-Porras, D.; Vega-Ávila, E.; Chavarría, A. A Potential Alternative against Neurodegenerative Diseases: Phytodrugs. Oxidative Med. Cell. Longev. 2016, 2016, 8378613. [Google Scholar] [CrossRef] [Green Version]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef] [Green Version]
- Bobadilla, M.; García-Sanmartín, J.; Martínez, A. Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases. Antioxidants 2021, 10, 46. [Google Scholar] [CrossRef]
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and Vegetable Juices and Alzheimer’s Disease: The Kame Project. Am. J. Med. 2006, 119, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4531–4538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Tang, J.; Barrett, D.M.; Sablani, S.S.; Anderson, N.; Powers, J.R. Thermal pasteurization of ready-to-eat foods and vegetables: Critical factors for process design and effects on quality. Crit. Rev. Food Sci. Nutr. 2017, 57, 2970–2995. [Google Scholar] [CrossRef]
- Campos-Gimnez, E.; Fontannaz, P.; Trisconi, M.-J.; Kilinc, T.; Gimenez, C.; Andrieux, P. Determination of Vitamin B12 in Food Products by Liquid Chromatography/UV Detection with Immunoaffinity Extraction: Single-Laboratory Validation. J. AOAC Int. 2008, 91, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The evaluation of chemical, antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Choi, H.-I.; Lee, H.-W.; Eom, T.-M.; Lim, S.-A.; Ha, H.-Y.; Seol, I.-C.; Kim, Y.-S.; Oh, D.-S.; Yoo, H.-R. A traditional Korean multiple herbal formulae (Yuk-Mi-Jihwang-Tang) attenuates acute restraint stress-induced brain tissue oxidation. Drug Chem. Toxicol. 2017, 40, 125–133. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef]
- Ochoa-Callejero, L.; García-Sanmartín, J.; Martínez-Herrero, S.; Rubio-Mediavilla, S.; Narro-Íñiguez, J.; Martínez, A. Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci. Rep. 2017, 7, 17488. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levonen, A.L.; Inkala, M.; Heikura, T.; Jauhiainen, S.; Jyrkkänen, H.K.; Kansanen, E.; Määttä, K.; Romppanen, E.; Turunen, P.; Rutanen, J.; et al. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Vieira, F.N.; Lourenço, S.; Fidalgo, L.G.; Santos, S.A.O.; Silvestre, A.J.D.; Jerónimo, E.; Saraiva, J.A. Long-Term Effect on Bioactive Components and Antioxidant Activity of Thermal and High-Pressure Pasteurization of Orange Juice. Molecules 2018, 23, 2706. [Google Scholar] [CrossRef] [Green Version]
- Vergne, M.J.; Patras, A.; Bhullar, M.S.; Shade, L.M.; Sasges, M.; Rakariyatham, K.; Pan, C.; Xiao, H. UV-C Irradiation on the Quality of Green Tea: Effect on Catechins, Antioxidant Activity, and Cytotoxicity. J. Food Sci. 2018, 83, 1258–1264. [Google Scholar] [CrossRef]
- Tarazona-Diaz, M.P.; Aguayo, E. Influence of acidification, pasteurization, centrifugation and storage time and temperature on watermelon juice quality. J. Sci. Food Agric. 2013, 93, 3863–3869. [Google Scholar] [CrossRef]
- Capanoglu, E.; de Vos, R.C.; Hall, R.D.; Boyacioglu, D.; Beekwilder, J. Changes in polyphenol content during production of grape juice concentrate. Food Chem. 2013, 139, 521–526. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yuan, W.; Lin, Y.-L.; Liu, R.-S.; Juan, Y.-C.; Sun, W.-H.; Tsay, H.J.; Huang, H.-C.; Lee, Y.-C.; Liu, H.-K. Evaluation of the In VivoTherapeutic Effects of Radix Paeoniae Rubra Ethanol Extract with the Hypoglycemic Activities Measured from Multiple Cell-Based Assays. Evid. Based Complement. Altern. Med. 2016, 2016, 3262790. [Google Scholar] [CrossRef] [Green Version]
- Tarazona-Díaz, M.P.; Alacid, F.; Carrasco, M.; Martínez, I.; Aguayo, E. Watermelon Juice: Potential Functional Drink for Sore Muscle Relief in Athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. High pressure processing and thermal pasteurization of strawberry purée: Quality parameters and shelf life evaluation during cold storage. J. Food Sci. Technol. 2017, 54, 832–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-S.; Luo, H.; Wang, P.; Tang, L.; Yu, J.; Huang, T.; Cox, S.; Gao, W. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem. Toxicol. 2008, 46, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, F.J.; Criado, M.N.; Belda-Galbis, C.M.; Esteve, M.J.; Rodrigo, D. Stevia rebaudiana Bertoni as a natural antioxidant/antimicrobial for high pressure processed fruit extract: Processing parameter optimization. Food Chem. 2014, 148, 261–267. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Haiar, J.M.; Watt, M.J.; Newton, S.S. Restraint stress differentially regulates inflammation and glutamate receptor gene expression in the hippocampus of C57BL/6 and BALB/c mice. Stress 2017, 20, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Sulakhiya, K.; Patel, V.K.; Saxena, R.; Dashore, J.; Srivastava, A.K.; Rathore, M. Effect of Beta vulgaris Linn. leaves extract on anxiety- and depressive-like behavior and oxidative stress in mice after acute restraint stress. Pharmacogn. Res. 2016, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Garg, R.; Gaur, V.; Kumar, P. Possible role of NO modulators in protective effect of trazodone and citalopram (antidepressants) in acute immobilization stress in mice. Indian J. Exp. Boil. 2010, 48, 1131–1135. [Google Scholar]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Agúndez, J.A.G.; García-Martín, E.; Martínez, C.; Benito-León, J.; Millán-Pascual, J.; Díaz-Sánchez, M.; Calleja, P.; Pisa, D.; Turpín-Fenoll, L.; Alonso-Navarro, H.; et al. Heme Oxygenase-1 and 2 Common Genetic Variants and Risk for Multiple Sclerosis. Sci. Rep. 2016, 6, 20830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, M.D.C.P.; Viana, A.C.; Pereira, G.E.; Nassur, R.D.; Lima, M.D.S. Whole, concentrated and reconstituted grape juice: Impact of processes on phenolic composition, “foxy” aromas, organic acids, sugars and antioxidant capacity. Food Chem. 2021, 343, 128399. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Deiana, M.; Spissu, Y.; Azara, E.; Fadda, A.; Serra, P.A.; D’hallewin, G.; Pisano, M.; Serreli, G.; Orrù, G.; et al. Antioxidant, Antimicrobial, and Other Biological Propertiof Pompia Juice. Molecules 2020, 25, 3186. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef]
- Danesi, F.; Ferguson, L.R. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef] [Green Version]
- Musazzi, L.; Tornese, P.; Sala, N.; Popoli, M. Acute stress is not acute: Sustained enhancement of glutamate release after acute stress involves readily releasable pool size and synapsin I activation. Mol. Psychiatry 2016, 22, 1226–1227. [Google Scholar] [CrossRef]
- Musazzi, L.; Tornese, P.; Sala, N.; Popoli, M. What Acute Stress Protocols Can Tell Us about PTSD and Stress-Related Neuropsychiatric Disorders. Front. Pharmacol. 2018, 9, 758. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, M.; Castilla-Ortega, E.; Pedraza, C.; Blanco, E.; Hurtado-Guerrero, I.; Barbancho, M.Á.; Chun, J.; Rodríguez-De-Fonseca, F.; Estivill-Torrús, G.; Núñez, L.J.S. Chronic Immobilization in the malpar1Knockout Mice Increases Oxidative Stress in the Hippocampus. Int. J. Neurosci. 2012, 122, 583–589. [Google Scholar] [CrossRef]
- Kowalczuk, K.; Stryjecka-Zimmer, M. The influence of oxidative stress on the level of malondialdehyde (MDA) in different areas of the rabbit brain. Ann. Univ. Maiae Curie Sklodowska Med. 2002, 57, 160–164. [Google Scholar]


| Code | Natural Extract | Concentration |
|---|---|---|
| Mec1 | MecobalActive® | 0.25 mg/unit |
| Mec2 | MecobalActive® | 0.40 mg/unit |
| Mec3 | MecobalActive® | 0.50 mg/unit |
| Gra1 | Red grape | 180 mg/unit |
| Gra2 | Red grape | 350 mg/unit |
| Gra3 | Red grape | 500 mg/unit |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession Number |
|---|---|---|---|
| NOX-2 | GCTGGGATCACAGGAATTGT | CTTCCAAACTCTCCGCAGTC | NM_007807 |
| HMOX-1 | TGCTCGAATGAACACTCTGG | TAGCAGGCCTCTGACGAAGT | NM_010442 |
| IL-6 | ATGGATGCTACCAAACTGGAT | TGAAGGACTCTGGCTTTGTCT | NM_031168 |
| TNF-alpha | CCACCACGCTCTTCTGTCTA | CACTTGGTGGTTTGCTACGA | NM_001278601 |
| Nrf-2 | AGCGAGCAGGCTATCTCCTA | TCTTGCCTCCAAAGGATGTC | NM_010902 |
| GAPDH | CATGGCCTTCCGTGTTCCTA | GCGGCACGTCAGATCCA | NM_008084 |
| VitB12 (µg/100 g) | |||
|---|---|---|---|
| Grape Juice | Mec1 | Mec3 | |
| Added amount | 75 | 150 | |
| Expected amount | 75 | 150 | |
| Obtained value | 0 | 67 | 117 |
| Lost amount | 8 | 33 | |
| % loss | 10. 7% | 22.0% | |
| Polyphenols (mg/Kg) | |||
|---|---|---|---|
| Grape Juice | Gra1 | Gra3 | |
| Added amount | 500.0 | 1250.0 | |
| Expected amount | 846.4 | 1596.4 | |
| Obtained value | 346.4 | 601.3 | 1124.0 |
| Lost amount | 245.1 | 472.4 | |
| % loss | 28.96% | 29.59% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobadilla, M.; Hernández, C.; Ayala, M.; Alonso, I.; Iglesias, A.; García-Sanmartín, J.; Mirpuri, E.; Barriobero, J.I.; Martínez, A. A Grape Juice Supplemented with Natural Grape Extracts Is Well Accepted by Consumers and Reduces Brain Oxidative Stress. Antioxidants 2021, 10, 677. https://doi.org/10.3390/antiox10050677
Bobadilla M, Hernández C, Ayala M, Alonso I, Iglesias A, García-Sanmartín J, Mirpuri E, Barriobero JI, Martínez A. A Grape Juice Supplemented with Natural Grape Extracts Is Well Accepted by Consumers and Reduces Brain Oxidative Stress. Antioxidants. 2021; 10(5):677. https://doi.org/10.3390/antiox10050677
Chicago/Turabian StyleBobadilla, Miriam, Carlos Hernández, María Ayala, Ixone Alonso, Ana Iglesias, Josune García-Sanmartín, Eduardo Mirpuri, José Ignacio Barriobero, and Alfredo Martínez. 2021. "A Grape Juice Supplemented with Natural Grape Extracts Is Well Accepted by Consumers and Reduces Brain Oxidative Stress" Antioxidants 10, no. 5: 677. https://doi.org/10.3390/antiox10050677
APA StyleBobadilla, M., Hernández, C., Ayala, M., Alonso, I., Iglesias, A., García-Sanmartín, J., Mirpuri, E., Barriobero, J. I., & Martínez, A. (2021). A Grape Juice Supplemented with Natural Grape Extracts Is Well Accepted by Consumers and Reduces Brain Oxidative Stress. Antioxidants, 10(5), 677. https://doi.org/10.3390/antiox10050677









