Investigation of Microstructure and Corrosion Resistance of Ti-Al-V Titanium Alloys Obtained by Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
3. Results
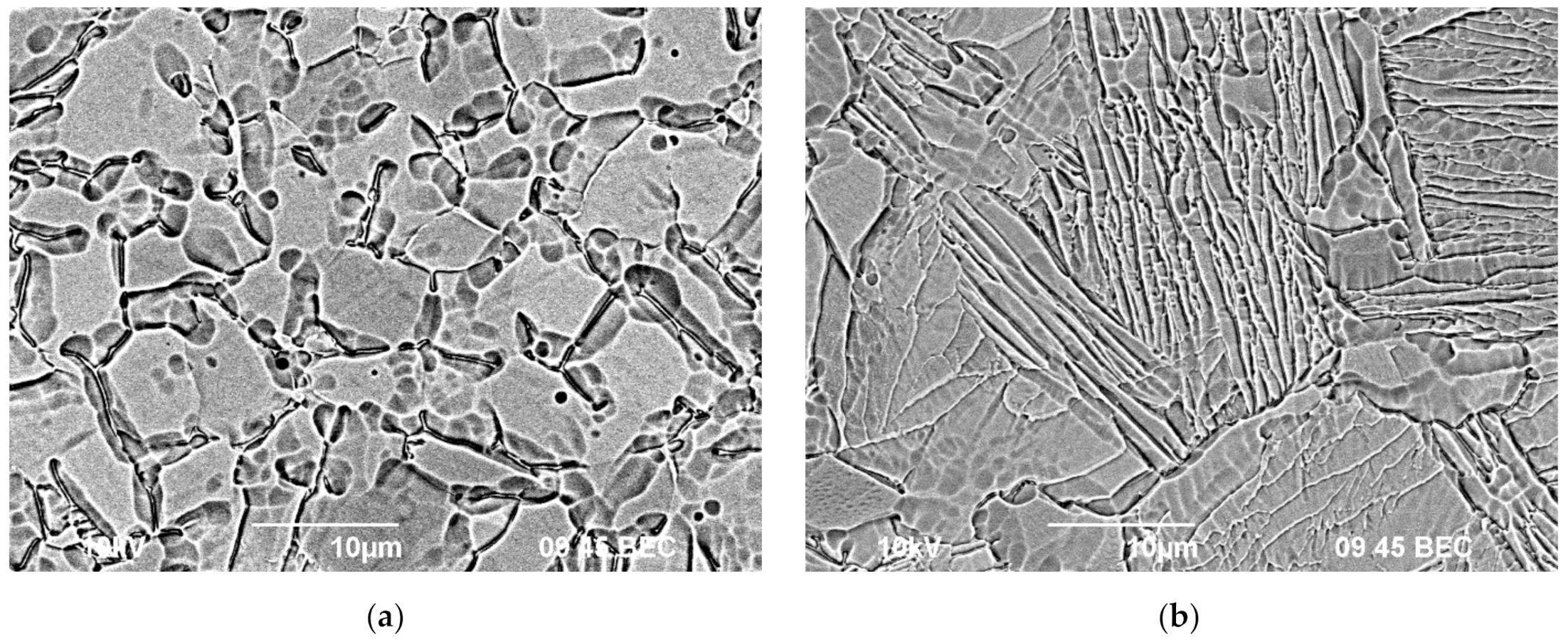
3.1. Microstructure and Mechanical Properties Investigation
3.2. Corrosion Resistance Investigations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lütjering, G.; Williams, J. Titanium, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 442. [Google Scholar] [CrossRef]
- Gorynin, I.V. Titanium alloys for marine application. Mater. Sci. Eng. A 1999, 263, 112–116. [Google Scholar] [CrossRef]
- Oryshchenko, A.S.; Gorynin, I.V.; Leonov, V.P.; Kudryavtsev, A.S.; Mikhailov, V.I.; Chudakov, E.V. Marine titanium alloys: Present and future. Inorg. Mater. Appl. Res. 2015, 6, 571–579. [Google Scholar] [CrossRef]
- Leonov, V.P.; Gorynin, I.V.; Kudryavtsev, A.S.; Ivanova, L.A.; Travin, V.V.; Lysenko, L.V. Titanium alloys in steam turbine construction. Inorg. Mater. Appl. Res. 2015, 6, 580–590. [Google Scholar] [CrossRef]
- Chevrot, T. Pressure Effects on the Holt-Salt Stress-Corrosion Cracking of Titanium Alloys. Ph.D. Thesis, Cranfield University, Bedford, UK, 1994. [Google Scholar]
- Pustode, M.D.; Raja, V.S.; Paulose, N. The stress-corrosion cracking suspeptibility of near-α titanium alloy IMI 834 in presence of hot salt. Corros. Sci. 2014, 82, 191–196. [Google Scholar] [CrossRef]
- Ciszaka, C.; Popa, I.; Brossard, J.-M.; Monceau, D.; Chevalier, S. NaCl induced corrosion of Ti-6Al-4V alloy at high temperature. Corros. Sci. 2016, 110, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Pustode, M.D.; Raja, V.S. Hot salt stress corrosion cracking behavior of Ti-6242S alloy. Metall. Mater. Trans. A 2015, 46, 6081–6089. [Google Scholar] [CrossRef]
- Myers, J.R.; Hall, J.A. Hat salt corrosion cracking of titanium alloys: An improved model for the mechanism. Corrosion 1977, 33, 252–257. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Kudryavtsev, E.; Kostjuchenko, S.; Malysheva, S.; Salishchev, G. Strength and ductility-related properties of ultrafine grained two-phase titanium alloy produced by warm multiaxial forging. Mater. Sci. Eng. A 2012, 536, 190–196. [Google Scholar] [CrossRef]
- Gornakova, A.S.; Straumal, A.B.; Khodos, I.I.; Gnesin, I.B.; Mazilkin, A.A.; Afonikova, N.S.; Straumal, B.B. Effect of composition, annealing temperature, and high pressure torsion on structure and hardness of Ti-V and Ti-V-Al alloys. J. Appl. Phys. 2019, 128, 082522. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Mosleh, A.O.; Mestre-Rinn, P.; Kotov, A.D.; Sitkina, M.N.; Bazlov, A.I.; Louzguine-Luzgin, D.V. High-strength titanium-based alloy for low-temperature superplastic forming. Metall. Mater. Trans. A 2021, 52, 293–302. [Google Scholar] [CrossRef]
- Leonov, V.P.; Chudakov, E.V.; Malinkina, Y.Y. The influence of microadditives of ruthenium on the structure, corrosive-mechanical strength, and fractography of destruction of pseudo-alpha-titanium alloys. Inorg. Mater. Appl. Res. 2017, 8, 556–565. [Google Scholar] [CrossRef]
- Malinkina, Y.Y.; Chudakov, E.V.; Leonov, V.P. The influence of ruthenium on structure, corrosion and mechanical properties, and fatigue characteristics of titanium α-alloys in corrosive environment. Inorg. Mater. Appl. Res. 2017, 8, 906–913. [Google Scholar] [CrossRef]
- Chuvildeev, V.N.; Kopylov, V.I.; Nokhrin, A.V.; Tryaev, P.V.; Tabachkova, N.Y.; Chegurov, M.K.; Kozlova, N.A.; Mikhaylov, A.S.; Ershova, A.V.; Gryaznov, M.Y.; et al. Effect of severe plastic deformation realized by rotary swaging on the mechanical properties and corrosion resistance of near-α-titanium alloy Ti-2.5Al-2.6Zr. J. Alloys Compd. 2019, 785, 1233–1244. [Google Scholar] [CrossRef]
- Tokita, M. Spark Plasma Sintering (SPS) Method, Systems, and Applications. In Handbook of Advanced Ceramics, 2nd ed.; Somiya, S., Ed.; Academic Press: London, UK, 2013. [Google Scholar] [CrossRef]
- Olevsky, E.; Dudina, D. Field-Assisted Sintering; Springer: Basel, Switzerland, 2018; p. 425. [Google Scholar] [CrossRef]
- Chuvildeev, V.N.; Panov, D.V.; Boldin, M.S.; Nokhrin, A.V.; Blagoveshchensky, Y.V.; Sakharov, N.V.; Shotin, S.V.; Kotkov, D.N. Structure and properties of advanced materials obtained by Spark Plasma Sintering. Acta Astronaut. 2015, 109, 172–176. [Google Scholar] [CrossRef]
- Asl, M.S.; Namini, A.S.; Motallebzadeh, A.; Azadbeh, M. Effect of sintering temperature on microstructure and mechanical properties of spark plasma sintered titanium. Mater. Chem. Phys. 2018, 203, 266–273. [Google Scholar] [CrossRef]
- Zadra, M.; Casari, F.; Girardini, L.; Molinari, A. Microstructure and mechanical properties of cp-titanium produced by spark plasma sintering. Powder Metall. 2008, 51, 59–65. [Google Scholar] [CrossRef]
- Ertorer, O.; Topping, T.D.; Li, Y.; Moss, W.; Lavernia, E.J. Nanostrucutred Ti consolidated via Spark Plasma Sintering. Metall. Mater. Trans. A 2011, 42, 964–973. [Google Scholar] [CrossRef] [Green Version]
- Namini, A.S.; Dilawary, S.A.A.; Motallebzadeh, A.; Asl, M.S. Effect of TiB2 addition on the elevated temperature tribological behavior of spark plasma sintered Ti matrix composite. Compos. B Eng. 2019, 172, 271–280. [Google Scholar] [CrossRef]
- Kozlík, J.; Becker, H.; Strásky, J.; Harcuba, P.; Janeček, M. Manufacturing of fine-grained titanium by cryogenic milling and spark plasma sintering. Mater. Sci. Eng. A 2020, 772, 138783. [Google Scholar] [CrossRef]
- Mahundla, M.R.; Matizamhuka, W.R.; Shongwe, M.B. The effect of densification of hardness of Ti, Ti-6Al-4V, Ti-34Nb-25Zr alloy produced by spark plasma sintering. Mater. Today Proc. 2021, 38, 605–608. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, H.; Wang, T.; Huang, X.; Li, Y.; Wu, J.; Chen, H. High-strength Ti-6Al-4V with ultrafine-grained structure fabricated by high energy ball milling and spark plasma sintering. Mater. Sci. Eng. A 2013, 585, 408–414. [Google Scholar] [CrossRef]
- Chandra, T.; Ionescu, M.; Mantovani, D. Mechanical properties and wear resistance of TiC or B4C reinforced Ti-6Al-4V prepared by Spark Plasma Sintering. Mater. Sci. Forum. 2012, 706–709, 222–227. [Google Scholar] [CrossRef]
- Garbiec, D.; Siwak, P.; Mrόz, A. Effect of compaction pressure and heating rate on microstructure and mechanical properties of spark plasma sintered Ti6Al4V alloy. Arch. Civil Mech. Eng. 2016, 16, 702–707. [Google Scholar] [CrossRef]
- Rajadurai, M.; Annamalai, A.R. Effect of various sintering methods on microstructures and mechanical properties of titanium and its alloy (Ti-Al-V-X): A review. Russ. J. Non-Ferr. Metals. 2017, 58, 434–448. [Google Scholar] [CrossRef]
- Adegbenjo, A.O.; Olubambi, P.A.; Potgieter, J.H.; Shongwe, M.B.; Ramakokovhu, M. Spark plasma sintering of graphitized multi-walled carbon nanotube reinforced Ti6Al4V. Mater. Des. 2017, 128, 119–129. [Google Scholar] [CrossRef]
- Falodun, O.E.; Obadele, B.A.; Oke, S.R.; Maja, M.E.; Olubambi, P.A. Synthesis of Ti-6Al-4V alloy with nano-TiN microstructure via spark plasma technique. IOP Conf. Series: Mater. Sci. Eng. 2017, 272, 012029. [Google Scholar] [CrossRef] [Green Version]
- Babu, N.K.; Kallip, K.; Leparoux, M.; AlOgab, K.A.; Talari, M.K.; Alqahtani, N.M. High strength Ti-6Al-4V alloy fabricated by high-energy cube milling using calcium as process control agent (PCA) and spark plasma sintering. Int. J. Manuf. Technol. 2017, 93, 445–453. [Google Scholar] [CrossRef]
- Falodun, O.E.; Obadele, B.A.; Oke, S.R.; Ige, O.O.; Olubambi, P.A.; Lethabane, M.L.; Bhero, S.W. Influence of spark plasma sintering on microstructure and wear behavior of Ti-6Al-4V reinforced with nanosized TiN. Trans. Nonferrous Met. Soc. China 2018, 28, 47–54. [Google Scholar] [CrossRef]
- Kgoete, F.M.; Popoola, A.P.I.; Fayomi, O.S.I. Influence of spark plasma sintering on microstructure and corrosion behaviour of Ti-6Al-4V alloy reinforced with micron-sized Si3N4 powder. Def. Technol. 2018, 14, 403–407. [Google Scholar] [CrossRef]
- Kgoete, F.M.; Popoola, A.P.I.; Fayomi, O.S.I.; Adebiyi, I.D. Influence of Si3N4 on Ti-6Al-4V via spark plasma sintering: Microstructure, corrosion and thermal stability. J. Alloys Compd. 2018, 763, 322–328. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Popoola, O.M. Consolidation of Ti6Al4V alloy and refractory nitride nanoparticles by spark plasma sintering method: Microstructure, mechanical, corrosion and oxidation characteristics. Mater. Sci. Eng. A 2020, 774, 138920. [Google Scholar] [CrossRef]
- Bustillos, J.; Zhang, C.; Loganathan, A.; Boesl, B.; Agarwal, A. Ultralow densification of a titanium alloy by Spark Plasma Sintering. Adv. Eng. Mater. 2020, 22, 2000076. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.M.; Cai, Y.J.; Luo, X.C.; Li, Z.J.; Liu, X.B.; Wang, Z.; Li, Y.Y.; Yang, C. Bimorphic microstructure in Ti-6Al-4V alloy manipulated by spark plasma sintering and in-situ press forging. Scr. Mater. 2021, 193, 43–48. [Google Scholar] [CrossRef]
- Wang, L.; Ma, H.; Fan, Q.; Yao, J.; Shen, X.; Zhang, S.; Zhou, Y.; Peng, Y.; Gao, Y.; Wang, D. Simultaneously enhancing strength and ductility of Ti-6Al-4V alloy with the hierarchical structure via a novel thermal annealing treatment. Mater. Charact. 2021, 176, 111112. [Google Scholar] [CrossRef]
- Chuvildeev, V.N.; Nokhrin, A.V.; Kopylov, V.I.; Boldin, M.S.; Vostokov, M.M.; Gryaznov, M.Y.; Tabachkova, N.Y.; Tryaev, P.V. Spark plasma sintering for high-speed diffusion bonding of the ultrafine-grained near-α Ti-5Al-2V alloy with high strength and corrosion resistance for nuclear engineering. J. Mater. Sci. 2019, 54, 14926–14949. [Google Scholar] [CrossRef]
- Yang, J.; Wang, G.; Jiao, X.; Gu, Y.; Liu, Q.; Li, Y. Current-assisted diffusion bonding of extruded Ti-22Al-25Nb alloy by Spark Plasma Sintering: Interfacial microstructure and mechanical properties. J. Mater. Eng. Perform. 2018, 27, 3035–3043. [Google Scholar] [CrossRef]
- Ozerov, M.; Klimova, M.; Sokolovsky, V.; Stepanov, N.; Popov, A.; Boldin, M.; Zherebtsov, S. Evolution of microstructure and mechanical properties of Ti/TiB metal-matrix composite during isothermal multiaxial forging. J. Alloys Compd. 2019, 770, 840–848. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Ozerov, M.; Klimova, M.; Moskovskikh, D.; Stepanov, N.; Salishchev, G. Mechanical behavior and microstructure evolution of a Ti-15Mo/TiB titanium-matrix composite during hot deformation. Metals 2019, 9, 1175. [Google Scholar] [CrossRef] [Green Version]
- Illarionov, A.G.; Vodolazskiy, F.V.; Barannikova, N.A.; Kosmatskiy, Y.I.; Khudorozhkova, Y.V. Influence of phase composition on thermal expansion of Ti-0.4Al, Ti-2.2Al-2.5Zr and Ti-3Al-2.5V alloys. J. Alloys Compd. 2021, 857, 158049. [Google Scholar] [CrossRef]
- Andreev, P.V.; Gudz, D.A.; Smetanina, K.E. Surface treatment of titanium alloys for the X-ray diffraction study. AIP Conf. Proc. 2020, 2315, 03001. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.M. X-ray line broadening from field aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Bakhmetyev, A.M.; Bykh, O.A.; Sandler, N.G.; Tryaev, P.V.; Ershova, A.V.; Mikhailov, A.S.; Sozinov, A.N. Hot-salt corrosion of alloys PT-7M, 42XHM, Inconel 690 and Incoloy 800. Appl. Solid State Chem. 2019, 1, 23–31. [Google Scholar] [CrossRef]
- Chuvildeev, V.N.; Kopylov, V.I.; Nokhrin, A.V.; Tryaev, P.V.; Kozlova, N.A.; Tabachkova, N.Y.; Lopatin, Y.G.; Ershova, А.V.; Mikhaylov, А.S.; Gryaznov, М.Y.; et al. Study of mechanical properties and corrosive resistance of ultrafine-grained a-titanium alloy Ti-5Al-2V. J. Alloys Compd. 2017, 723, 354–367. [Google Scholar] [CrossRef]
- Ratochka, I.; Lykova, O.; Mishin, I.; Naydenkin, E. Superplastic deformation behavior of Ti-4Al-2V alloy governed by its structure and precipitation phase evolution. Mater. Sci. Eng. A 2018, 731, 577–582. [Google Scholar] [CrossRef]
- Ratochka, I.V.; Lykova, O.N.; Naidenkin, E.V. Influence of low-temperature annealing time on the evolution of the structure and mechanical properties of a titanium Ti-Al-V alloy in the submicrocrystalline state. Phys. Met. Metallogr. 2015, 116, 302–308. [Google Scholar] [CrossRef]
- American Society for Metals. Metals Handbook: Fractography and Atlas of Fractographs, 8th ed.; Boyer, H.E., Ed.; American Society for Metals: Metals Park, OH, USA, 1974; p. 499. [Google Scholar]
- Nokhrin, A.V.; Boldin, M.S.; Lantsev, E.A.; Vostokov, M.M.; Gryaznov, M.Y.; Kopylov, V.I.; Chuvildeev, V.N. Spark plasma sintering for high-rate diffusion welding of a UFG titanium alloy PT3V. IOP Conf. Ser. Mater. Sci. Eng. 2019, 558, 012029. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Ukhina, A.V.; Dudina, D.V.; Anisimov, A.G.; Mali, V.I.; Batraev, I.S. Carbon uptake during Spark Plasma Sintering: Investigation through the analysis of the carbide “footprint” in a Ni-W alloy. RSC Adv. 2015, 5, 80228–80237. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Ukhina, A.V.; Anisimov, A.G.; Mali, V.I.; Esikov, M.A.; Batraev, I.S.; Kuznechik, O.O.; Pilinevich, L.P. Reactivity of materials towards carbon of graphite foil during Spark Plasma Sintering: A case study using Ni-W powders. Mater. Lett. 2016, 168, 62–67. [Google Scholar] [CrossRef]
- Lantsev, E.A.; Malekhonova, N.V.; Nokhrin, A.V.; Chuvildeev, V.N.; Boldin, M.S.; Blagoveshchenskiy, Y.V.; Andreev, P.V.; Smetanina, K.E.; Isaeva, N.V.; Shotin, S.V. Influence of oxygen on densification kinetics of WC nanopowders during SPS. Ceram. Int. 2021, 47, 4294–4309. [Google Scholar] [CrossRef]
- Lantsev, E.A.; Malekhonova, N.V.; Nokhrin, A.V.; Chuvildeev, V.N.; Boldin, M.S.; Andreev, P.V.; Smetanina, K.E.; Blagoveshchenskiy, Y.V.; Isaeva, N.V.; Murashov, A.A. Spark plasma sintering of fine-grained WC hard alloys with ultra-low cobalt content. J. Alloys Compd. 2021, 857, 157535. [Google Scholar] [CrossRef]
- Kasyanov, D.Y.; Tikhonov, S.A.; Vornovskikh, A.A.; Yavetskiy, R.P.; Dobrotvorskaya, M.V.; Doroshenko, A.G.; Shichalin, O.O.; Papynov, E.K.; Ustinov, A.Y. Influence of carbon contamination on transparency of reactive SPSed Nd3+:YAG ceramics. J. Phys. Conf. Ser. 2020, 1461, 012187. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B. Elimination of oxide films during Spark Plasma Sintering of metallic powders: A case study using partially oxidized nickel. Adv. Powder Technol. 2017, 28, 641–647. [Google Scholar] [CrossRef]
- Larikov, L.N.; Isaychev, V.I. Diffusion in Metals and Alloys; Naukova Dumka: Kiev, Ukraine, 1987; p. 510. (In Russian) [Google Scholar]
- Chaudhari, R.; Bauri, R. Microstructure and mechanical properties of titanium processed by Spark Plasma Sintering (SPS). Metallogr. Microstruct. Anal. 2014, 3, 30–35. [Google Scholar] [CrossRef]
- Ozerov, M.S.; Klimenko, D.N.; Rtishcheva, L.P.; Kopylov, V.N.; Stepanov, N.D.; Zherebtsov, S.V. Effect of carbon on microstructure and mechanical properties of titanium. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1014, 012039. [Google Scholar] [CrossRef]
- Krupa, D.; Jezierska, E.; Baszkiewicz, J.; Wierzchon, T.; Barcz, A.; Gawlik, G.; Jagielski, J.; Sobczak, J.; Bilinski, A.; Larisch, B. Effect of carbon ion implantation on the structure and corrosion resistance of OT-4-0 titanium alloy. Surf. Coat. Tech. 1999, 114, 250–259. [Google Scholar] [CrossRef]
















| Alloy | Initial Powders | SPS Mode | Material Characteristics | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vh (°С/min) | T (°C) | P (MPa) | t (min) | XRD Analysis | ρ (%) | d (μm) | Hv | σb (MPa) | δ (%) | ||||
| Ti | R0 < 75 μm, purity > 98% | 50 | 1200 | 50 | 5 | α + β | 99.8 | - | ~391 | ~500 | ~4.2 | [19] | |
| 1350 | - | ~98 | ~360 | ~390 | ~2.5 | ||||||||
| Ti | Grade 1 | - | 900 | 60 | 5 | - - | ~100 | ~15 | ~145 | ~450 | 38–39 | [20] | |
| Grade 3 | ~100 | ~12 | ~240 | ~700 | ~18 | ||||||||
| Ti | Grade 2 + CM 2 (8 h) | 50 | 900 | 80 | 5 | - | 99.16 | - | ~244 | 776 | 29.3 | [21] | |
| 50 | 850 | 80 | 3 | 99.2 | - | ~247 | 838 | 26.4 | |||||
| 100 | 850 | 80 | 3 | 99.2 | - | ~247 | 823 | 19 | |||||
| 50 | 850 | 40 | 3 | 98.48 | - | ~240 | 758 | 18.4 | |||||
| Ti | - | - | 1050 | 50 | 5 | α-Ti | 97.9 | 40–50 | 291 | - | - | [22] | |
| Ti | Grade 4 (20–60 μm) + CM 2 (8 h) | 100 | 800 | 80 | 3 | - | ~99 | ~4.2 | ~260 | - | - | [23] | |
| Ti | 99.8% (HEBM 2) | 100 | 1200 | 50 | 10 | - | ~97 | 341 | - | - | [24] | ||
| Ti-6Al-4V | HEBM 2 | - | - | - | - | - | ~100 | - | ~370 | - | - | ||
| Ti-6Al-4V | HEBM 2 | 0 h | 100 | 850 | 50 | 4 | α + β | 87.4 | ~15 | - | ~1200 | ~40 | [25] |
| 10 h | 99.3 | ~0.87 | - | 1663 | 20 | ||||||||
| 30 h | - | ~0.58 | - | 1735 | 10 | ||||||||
| Ti-6Al-4V | R0 ~ 13.4 μm | 293 | 900 | 70 | 10 | - | ~97.5 | 10–20 | ~4 | ~1050 | ~10 | [26] | |
| Ti-6Al-4V | purity 99.9% | 200 | 1000 | 5 | 5 | α + β | 94.58 | - | 293 | 1169 1 | - | [27,28] | |
| 25 | 98.87 | 120.44 | 346 | 1281 1 | |||||||||
| 50 | 98.65 | 110.19 | 348 | 1310 1 | |||||||||
| 300 | 1000 | 5 | 5 | 93.45 | 115.49 | 325 | 1287 1 | ||||||
| 25 | 100.0 | 143.57 | 352 | 1406 1 | |||||||||
| 50 | 97.74 | 86.94 | 373 | 1394 1 | |||||||||
| 400 | 1000 | 5 | 5 | 96.16 | - | 318 | 1310 1 | ||||||
| 25 | 99.55 | 118.23 | 345 | 1414 1 | |||||||||
| 50 | 99.32 | 84.31 | 352 | 1394 1 | |||||||||
| Ti-6Al-4V | Pure Ti-6–4 | 100 | 1000 | 50 | 5 | α + β | ~99.6 | - | ~360 | - | - | [29] | |
| HEBM 2 + 1% MWCNT 3 | α + β + CNT + TiC | ~99 | - | ~380 | - | - | |||||||
| HEBM 2 + 2% MWCNT 3 | ~98.2 | - | ~395 | - | - | ||||||||
| HEBM 2 + 3% MWCNT 3 | ~98 | - | ~400 | - | - | ||||||||
| Ti-6Al-4V | R0 ~ 25 μm, purity > 99.9% | 100 | 1000 | 50 | 10 | α + β | 97–99 | - | 389 | - | - | [30] | |
| Ti-6Al-4V | R0 ~ 65 μm | 100 | 1250 | 50 | 15 | α + β | 99.8 | - | 450 | 1057 | 15 | [31] | |
| Ti-6Al-4V | R0 ~ 25 μm, purity > 99.9% | 100 | 1000 | 50 | 10 | α + β | 99.54 | - | 389 | - | - | [32] | |
| Ti-6Al-4V | R0 ~ 45–90 μm | 100 | 1000 | 50 | 6 | α + β | 98.6 | - | 325.46 | - | - | [33,34] | |
| Ti-6Al-4V | R0 ~ 60 μm, purity > 99% | 100 | 1000 | 30 | 10 | α + β | 98.22 | - | 3.25 GPa | 1060 | - | [35] | |
| Ti-6Al-4V | - | - | 650 | 555 | - | α-Ti | 98.9 | 11.7 | 3.7 GPa | - | - | [36] | |
| Ti-6Al-4V | R0 ~ 75–150 μm | 100 | 1050 | 30 | 5 | α + β | - | - | - | 1240 | 19.5 | [37] | |
| Ti-6Al-4V | R0 ~ 53–106 μm | 100 | 900 | 50 | 5 | α + β | - | - | - | 844 | 12 | [38] | |
| Alloys | Elements, wt.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ti | Al | V | Zr | Si | Fe | O | H | N | C | |
| Ti (VT1-0) | base | - | - | - | 0.01 | 0.14 | 0.13 | 0.006 | 0.038 | 0.021 |
| Ti5Al2V (PT-3V) | base | 4.4 | 1.95 | 0.15 | 0.02 | 0.06 | 0.13 | 0.0049 | 0.039 | 0.015 |
| Ti6Al4V (VT-6) | base | 6.24 | 4.05 | - | - | 0.12 | 0.12 | 0.006 | 0.020 | 0.016 |
| Alloy | Zone I | Zone II | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Est, mV | Ecor, mV | icor, mA/cm2 | A | C | Est, mV | Ecor, mV | icor, mA/cm2 | A | C | |
| Ti (VT1-0) | −444 | −442 | 1.34 | 121 | 71 | −456 | −458 | 1.16 | 120 | 74 |
| Ti5Al2V (PT-3V) | −462 | −460 | 1.51 | 77 | 102 | −468 | −467 | 1.17 | 85 | 95 |
| Ti6Al4V (VT-6) | −485 | −484 | 1.72 | 78 | 100 | −494 | −492 | 1.19 | 83 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokhrin, A.; Andreev, P.; Boldin, M.; Chuvil’deev, V.; Chegurov, M.; Smetanina, K.; Gryaznov, M.; Shotin, S.; Nazarov, A.; Shcherbak, G.; et al. Investigation of Microstructure and Corrosion Resistance of Ti-Al-V Titanium Alloys Obtained by Spark Plasma Sintering. Metals 2021, 11, 945. https://doi.org/10.3390/met11060945
Nokhrin A, Andreev P, Boldin M, Chuvil’deev V, Chegurov M, Smetanina K, Gryaznov M, Shotin S, Nazarov A, Shcherbak G, et al. Investigation of Microstructure and Corrosion Resistance of Ti-Al-V Titanium Alloys Obtained by Spark Plasma Sintering. Metals. 2021; 11(6):945. https://doi.org/10.3390/met11060945
Chicago/Turabian StyleNokhrin, Aleksey, Pavel Andreev, Maksim Boldin, Vladimir Chuvil’deev, Mikhail Chegurov, Ksenia Smetanina, Mikhail Gryaznov, Sergey Shotin, Artem Nazarov, Gleb Shcherbak, and et al. 2021. "Investigation of Microstructure and Corrosion Resistance of Ti-Al-V Titanium Alloys Obtained by Spark Plasma Sintering" Metals 11, no. 6: 945. https://doi.org/10.3390/met11060945







