Copper Oxide Films Deposited by Microwave Assisted Alkaline Chemical Bath
Abstract
:1. Introduction
2. Physical-Chemical Analysis
Solubility and Species Distribution Diagrams
3. Experimental Procedure
4. Results and Discussion
4.1. X-ray Analysis
4.2. Morphology Analysis (SEM)
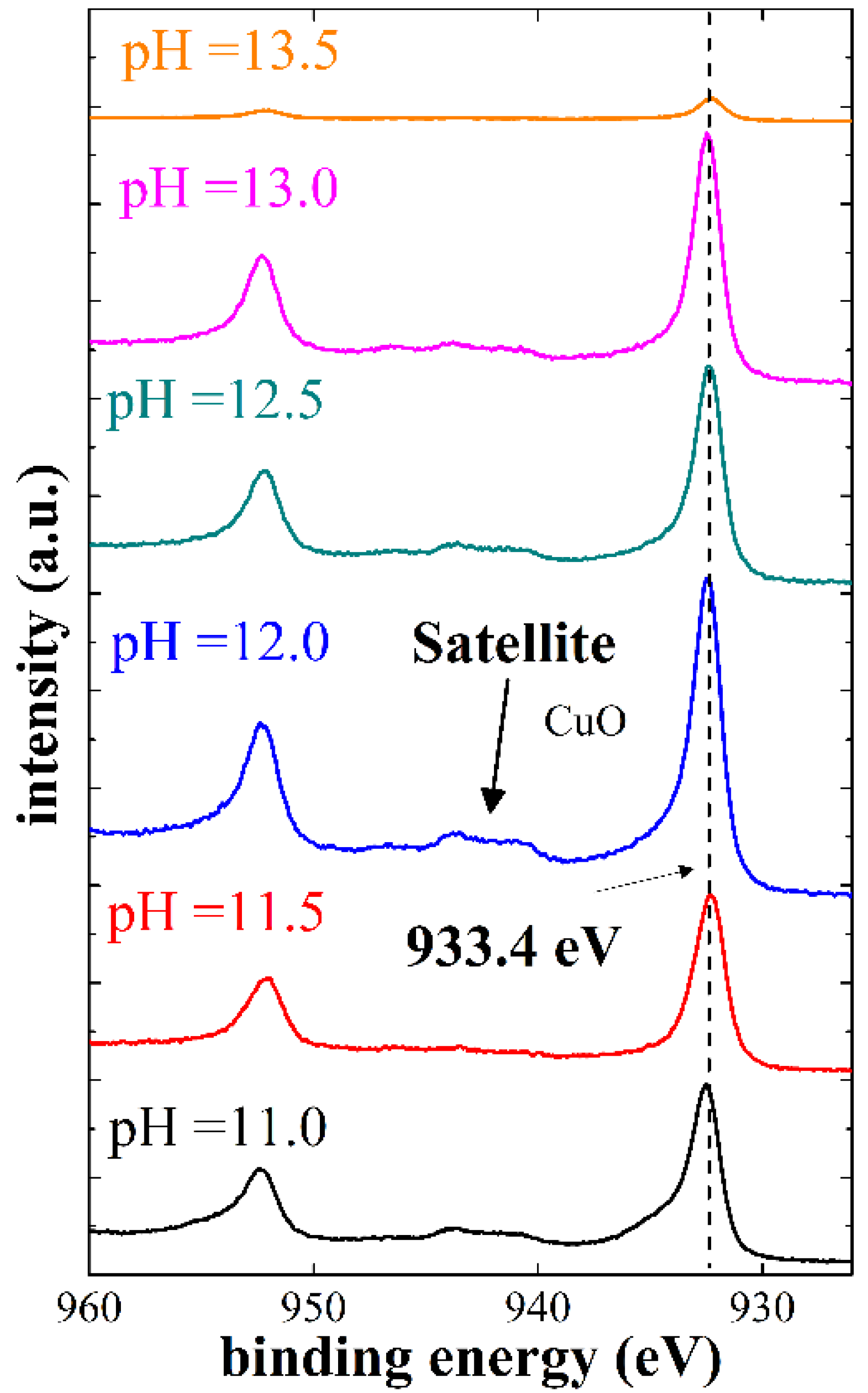
4.3. X-ray Photoelectron Spectroscopy (XPS)
4.4. Optical Properties and Band-Gap Energy Determination
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, T.; Yamaguchi, H.; Okabe, K.; Masumi, T. Single-crystal growth and characterization of Cu2O and CuO. J. Mater. Sci. 1998, 33, 3555–3566. [Google Scholar] [CrossRef]
- Ghisen, J. Electronic-structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Venkateswari, P.; Thirunavukkarasu, P.; Ramamurthy, M.; Balaji, M.; Chandrasekaran, J. Optimization and characterization of CuO thin films for P–N junction diode application by JNSP technique. Optik 2017, 140, 476–484. [Google Scholar] [CrossRef]
- Dolai, S.; Sarangi, S.N.; Hussain, S.; Bhar, R.; Pal, A.K. Magnetic properties of nanocrystalline nickel incorporated CuO thin films. J. Magn. Magn. Mater. 2019, 479, 59–66. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Neyvasagam, K.; Chandramohan, R.; Valanarasu, S.; Benial, A.M.F. Studies on chemical bath deposited CuO thin films for solar cells application. J. Mater. Sci. Mater. Electron. 2015, 26, 8489–8496. [Google Scholar]
- Shinde, S.; Yadav, H.; Ghodake, G.; Kadam, A.; Kumbhar, V.; Yang, J.; Hwang, K.; Jagadale, A.; Kumar, S.; Kim, D. Using chemical bath deposition to create nanosheet-like CuO electrodes for supercapacitor applications. Colloids Surf. B 2019, 181, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Paul, S.; Karmakar, A.; Yi, R.; Dalapati, G.K.; Chattopadhyay, S. Chemical bath deposited (CBD) CuO thin films on n-silicon substrate for electronic and optical applications: Impact of growth time. Appl. Surf. Sci. 2017, 418, 380–387. [Google Scholar] [CrossRef]
- Balaram, N.; Reddy, V.R.; Reddy, P.S.; Janardhanam, V.; Choi, C.J. Microstructural, chemical states and electrical properties of Au/CuO/n-InP heterojunction with a cupric oxide interlayer. Vacuum 2018, 152, 15–24. [Google Scholar] [CrossRef]
- Hajipour, P.; Bahrami, A.; Eslami, A.; Hosseini-Abari, A. Chemical bath synthesis of CuO-GO-Ag nanocomposites with enhanced antibacterial properties. J. Alloys Compd. 2020, 821, 153456. [Google Scholar] [CrossRef]
- Muiva, C.; Juma, A.; Lepodise, L.M.; Maabong, K.; Letsholathebe, D. Surfactant assisted chemical bath deposition based synthesis of 1-D nanostructured CuO thin films from alkaline baths. Mater. Sci. Semicond. Process. 2017, 67, 69–74. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Watheyb, B.; Westmana, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Tierney, J.P.; Lidstrom, P. (Eds.) Microwave Assisted Organic Synthesis; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–22. [Google Scholar]
- Mohapatra, R.K.; Das, P.K.; Pradhan, M.K.; El-Ajaily, M.M.; Das, D.; Salem, H.F.; Mahanta, U.; Badhei, G.; Parhi, P.K.; Maihub, A.A.; et al. Recent advances in urea and thiourea based metal complexes: Biological sensor, optical and corrosion inhibition studies. Comments Inorg. Chem. 2019, 39, 127–187. [Google Scholar] [CrossRef]
- Bates, R.G.; Pinching, G.D. Dissociation Constant of Aqueous Ammonia at 0 to 50 from E. mf Studies of the Ammonium Salt of a Weak Acid. J. Am. Chem. Soc. 1950, 72, 1393. [Google Scholar] [CrossRef]
- Trevani, L.N.; Roberts, J.C.; Tremaine, P.R. Trevani, Roberts, and Tremaine, Copper (II)–Ammonia complexation equilibria in aqueous solutions at temperatures from 30 to 250 °C by visible spectroscopy. J. Solut. Chem. 2001, 30, 585–622. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants; Plenum Press: New York, NY, USA, 1974; Volume 4: Inorganic Complexes, ISBN 978-1-4757-5508-4. [Google Scholar] [CrossRef]
- John, A. Dean, Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Patnaik, P. Dean’s Analytical Chemistry Handbook, 2nd ed.; McGrawHill: New York, NY, USA, 2004. [Google Scholar]
- González-Panzo, I.J.; Martín-Várguez, P.E.; Oliva, A.I. Physicochemical conditions for ZnS films deposited by chemical bath. J. Electrochem. Soc. 2014, 161, D181–D189. [Google Scholar] [CrossRef]
- Galeazzi, R.; Gonzalez-Panzo, I.J.; Díaz-Becerril, T.; Morales, C.; Rosendo, E.; Silva, R.; Romano-Trujillo, R.; Coyopol, A.; Nieto-Caballero, F.G.; Treviño-Yarce, L. Physicochemical conditions for ZnO films deposited by microwave chemical bath deposition. RSC Adv. 2018, 8, 8662–8670. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, J.; Su, Q.; Shi, J.; Liu, Y.; Du, G. Nanoleaf-on-sheet CuO/graphene composites: Microwave-assisted assemble and excellent electrochemical performances for lithium ion batteries. Electrochim. Acta 2014, 125, 615–621. [Google Scholar] [CrossRef]
- Oruç, Ç.; Altındal, A. Structural and dielectric properties of CuO nanoparticles. Ceram. Int. 2017, 43, 10708–10714. [Google Scholar] [CrossRef]
- Khan, M.A.; Nayan, N.; Ahmad, M.K.; Soon, C.F.; Shadiullah, S. Surface study of CuO nanopetals by advanced nanocharacterization techniques with enhanced optical and catalytic properties. Nanomaterials 2020, 10, 1298. [Google Scholar] [CrossRef]
- Mark, C. Biesinger, Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar]
- Svintsitskiy, D.; Stadnichenko, A.; Demidov, D.; Koscheev, S.; Boronin, A. Investigation of oxygen states and reactivities on a nanostructured cupric oxide surface. Appl. Surf. Sci. 2011, 257, 8542–8549. [Google Scholar] [CrossRef]
- Li, Z.; Tong, K.; Shi, R.; Shen, Y.; Zhang, Y.; Yao, Z.; Fan, J.; Thwaites, M.; Shao, G. Reactive plasma deposition of high quality single phase CuO thin films suitable for metal oxide solar cells. J. Alloys Compd. 2016, 695, 3116–3123. [Google Scholar] [CrossRef]
- Tahir, D.; Tougaard, S. Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. J. Phys. Condens. Matter. 2012, 24, 175002. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Yang, G.; Li, J.; Zhang, J.; Zhang, J.; Xue, D. Room-temperature ferromagnetism of flowerlike CuO nanostructures. J. Phys. Chem. C 2010, 114, 18347–18351. [Google Scholar] [CrossRef]
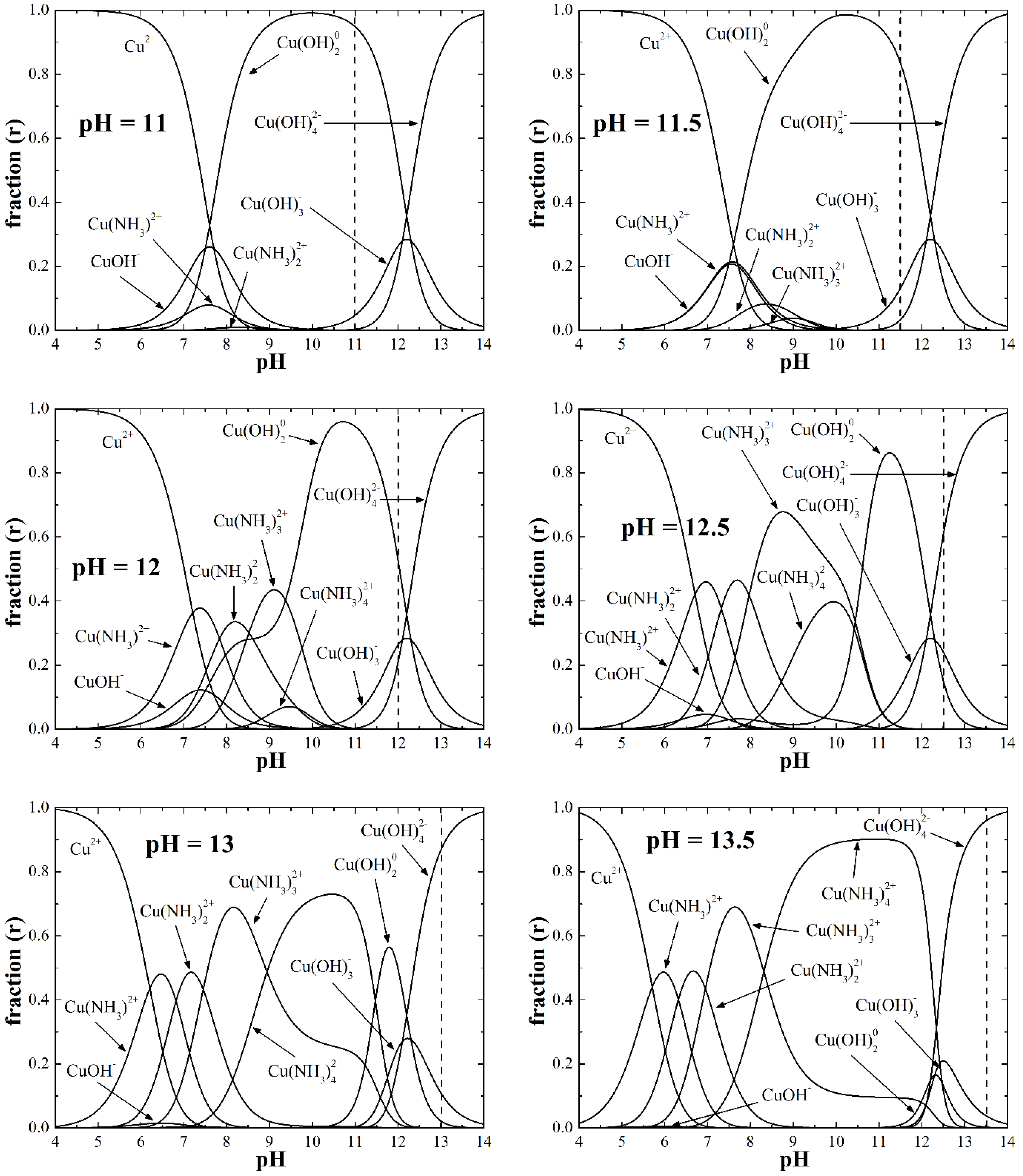
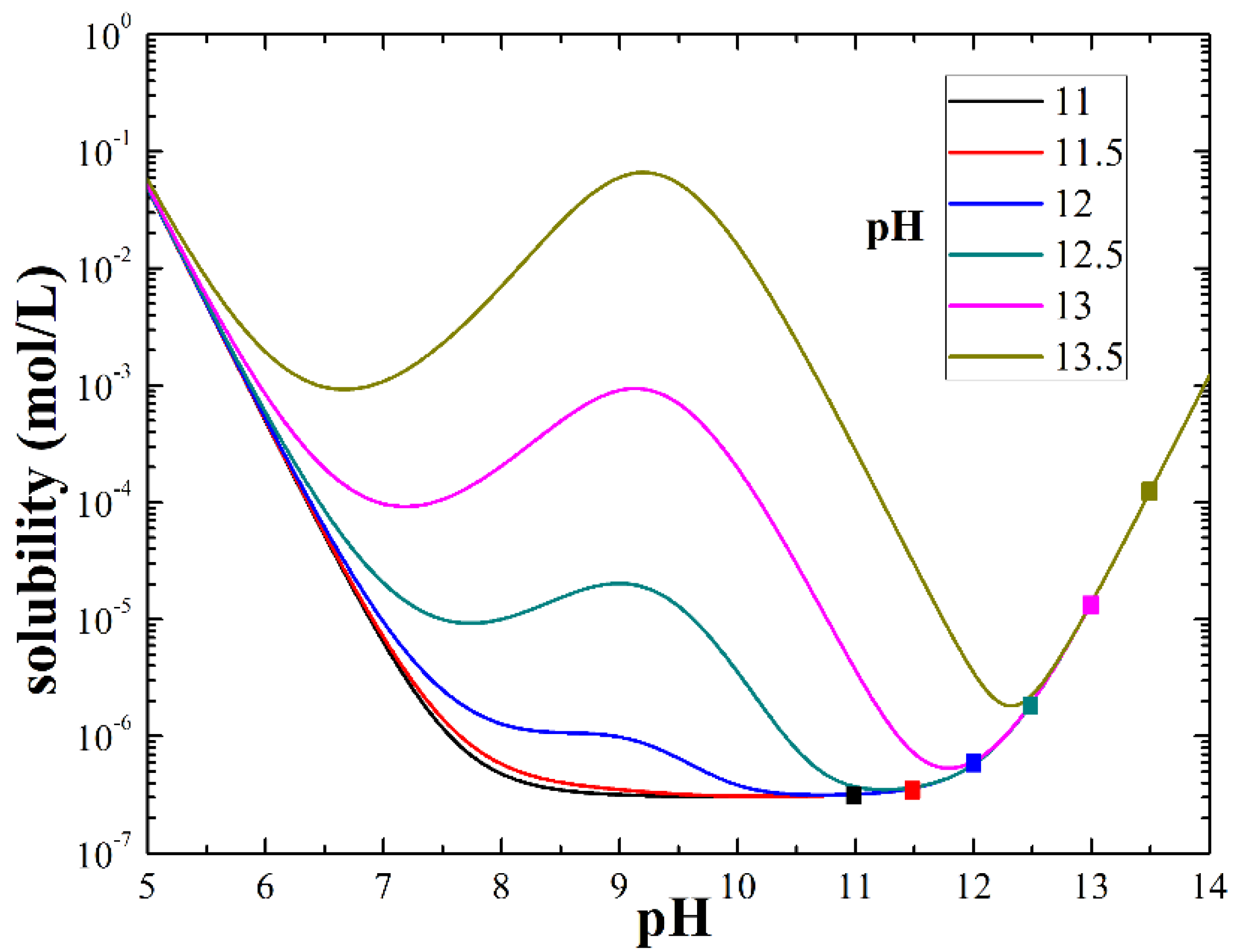

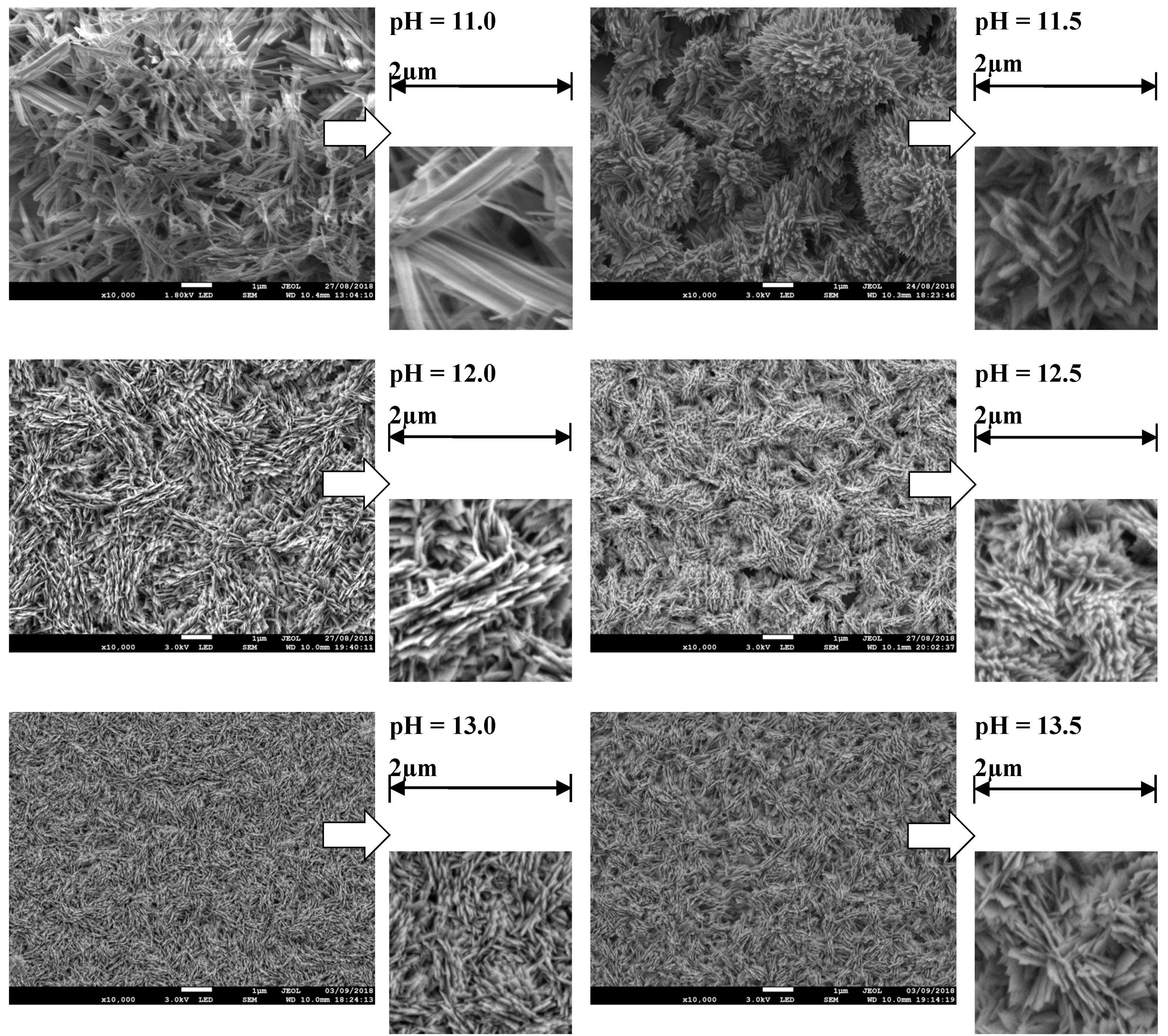
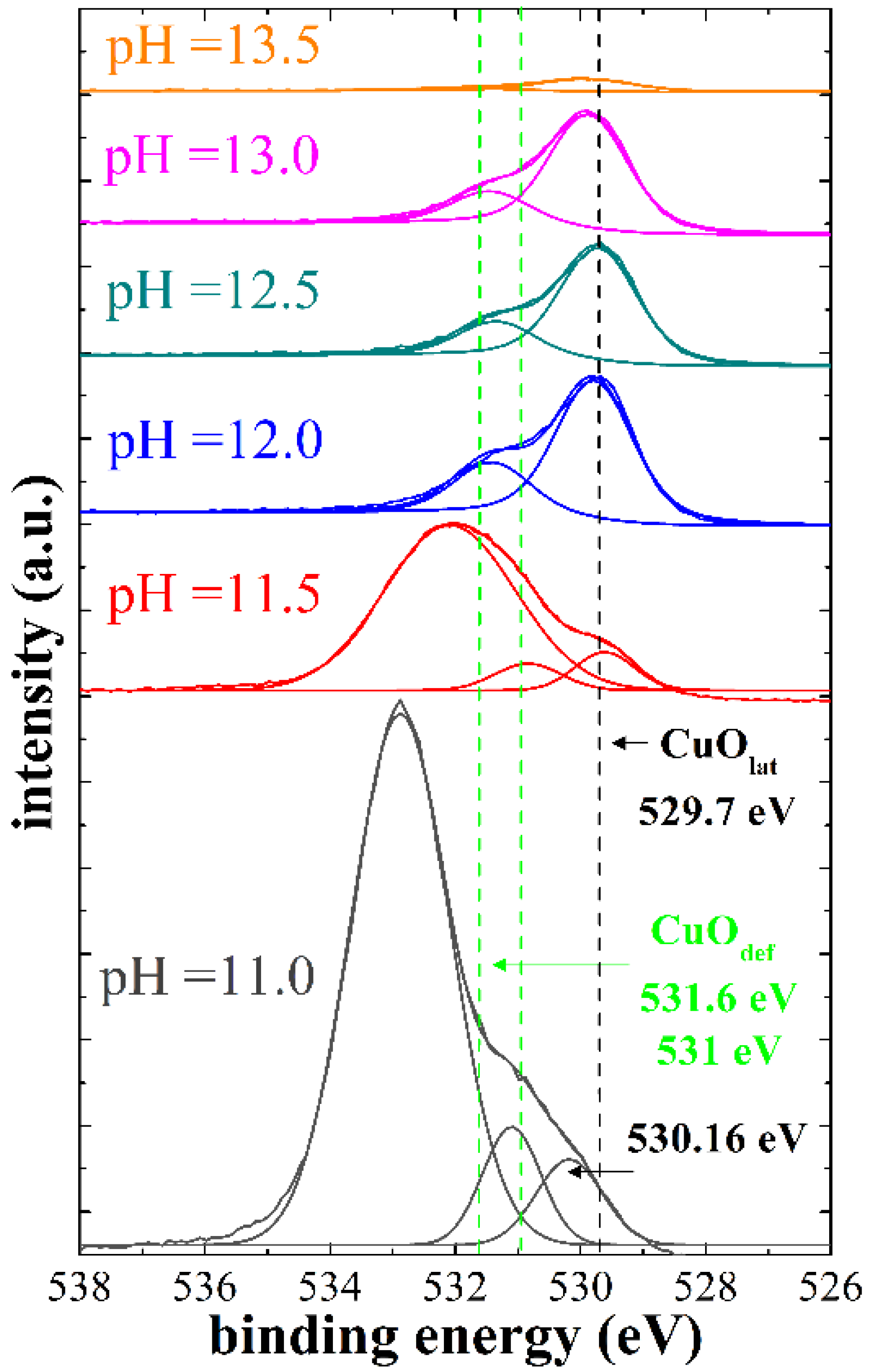

| pH Value (Concentration) | pH = 11 (at%) | pH = 11.5 (at%) | pH = 12 (at%) | pH = 12.5 (at%) | pH = 13 (at%) | pH = 13.5 (at%) | |
|---|---|---|---|---|---|---|---|
| O1s | CuOlat + | 25.65 | 37.85 | 34.82 | 39.75 | 39.58 | 47.59 |
| CuOdef ++ | 31.21 | 13.18 | 13.06 | 12.41 | 11.48 | 10.81 | |
| Total O | 56.86 | 51.03 | 47.88 | 52.16 | 51.06 | 58.40 | |
| Total Cu | Cu2p3/2 | 43.14 | 48.97 | 52.12 | 47.84 | 48.94 | 41.60 |
| Cu/O ratio | 0.76 | 0.96 | 1.09 | 0.92 | 0.96 | 0.71 | |
| pH Value | 11 | 11.5 | 12 | 12.5 | 13 | 13.5 |
|---|---|---|---|---|---|---|
| Eg (eV) | -- | 1.68 | 1.2 | 1.16 | 1.34 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeazzi Isasmendi, R.; Gonzalez Panzo, I.J.; Morales-Ruiz, C.; Romano Trujillo, R.; Rosendo, E.; García, I.; Coyopol, A.; García-Salgado, G.; Silva-González, R.; Oliva Arias, I.; et al. Copper Oxide Films Deposited by Microwave Assisted Alkaline Chemical Bath. Crystals 2021, 11, 968. https://doi.org/10.3390/cryst11080968
Galeazzi Isasmendi R, Gonzalez Panzo IJ, Morales-Ruiz C, Romano Trujillo R, Rosendo E, García I, Coyopol A, García-Salgado G, Silva-González R, Oliva Arias I, et al. Copper Oxide Films Deposited by Microwave Assisted Alkaline Chemical Bath. Crystals. 2021; 11(8):968. https://doi.org/10.3390/cryst11080968
Chicago/Turabian StyleGaleazzi Isasmendi, Reina, Isidro Juvenal Gonzalez Panzo, Crisóforo Morales-Ruiz, Román Romano Trujillo, Enrique Rosendo, Iván García, Antonio Coyopol, Godofredo García-Salgado, Rutilo Silva-González, Iván Oliva Arias, and et al. 2021. "Copper Oxide Films Deposited by Microwave Assisted Alkaline Chemical Bath" Crystals 11, no. 8: 968. https://doi.org/10.3390/cryst11080968
APA StyleGaleazzi Isasmendi, R., Gonzalez Panzo, I. J., Morales-Ruiz, C., Romano Trujillo, R., Rosendo, E., García, I., Coyopol, A., García-Salgado, G., Silva-González, R., Oliva Arias, I., & Tabasco Novelo, C. (2021). Copper Oxide Films Deposited by Microwave Assisted Alkaline Chemical Bath. Crystals, 11(8), 968. https://doi.org/10.3390/cryst11080968







