Zona Pellucida Genes and Proteins: Essential Players in Mammalian Oogenesis and Fertility
Abstract
:1. Introduction
2. Mammalian Oogenesis
2.1. Meiosis during Oogenesis
2.2. Oocyte and Follicle Growth
2.3. Meiotic Maturation of Oocytes
2.4. Ultrastructural Changes during Oocyte Growth
3. ZP Gene Expression
3.1. Transcription of ZP Genes during Oocyte Growth
3.2. Location and Size of mZP and hZP Genes
3.3. Conservation of ZP Genes
3.4. Cis-Acting Sequences Regulate Transcription
3.5. Trans-Acting Factors Regulate Transcription
4. ZP Protein Characteristics
4.1. mZP and hZP Proteins
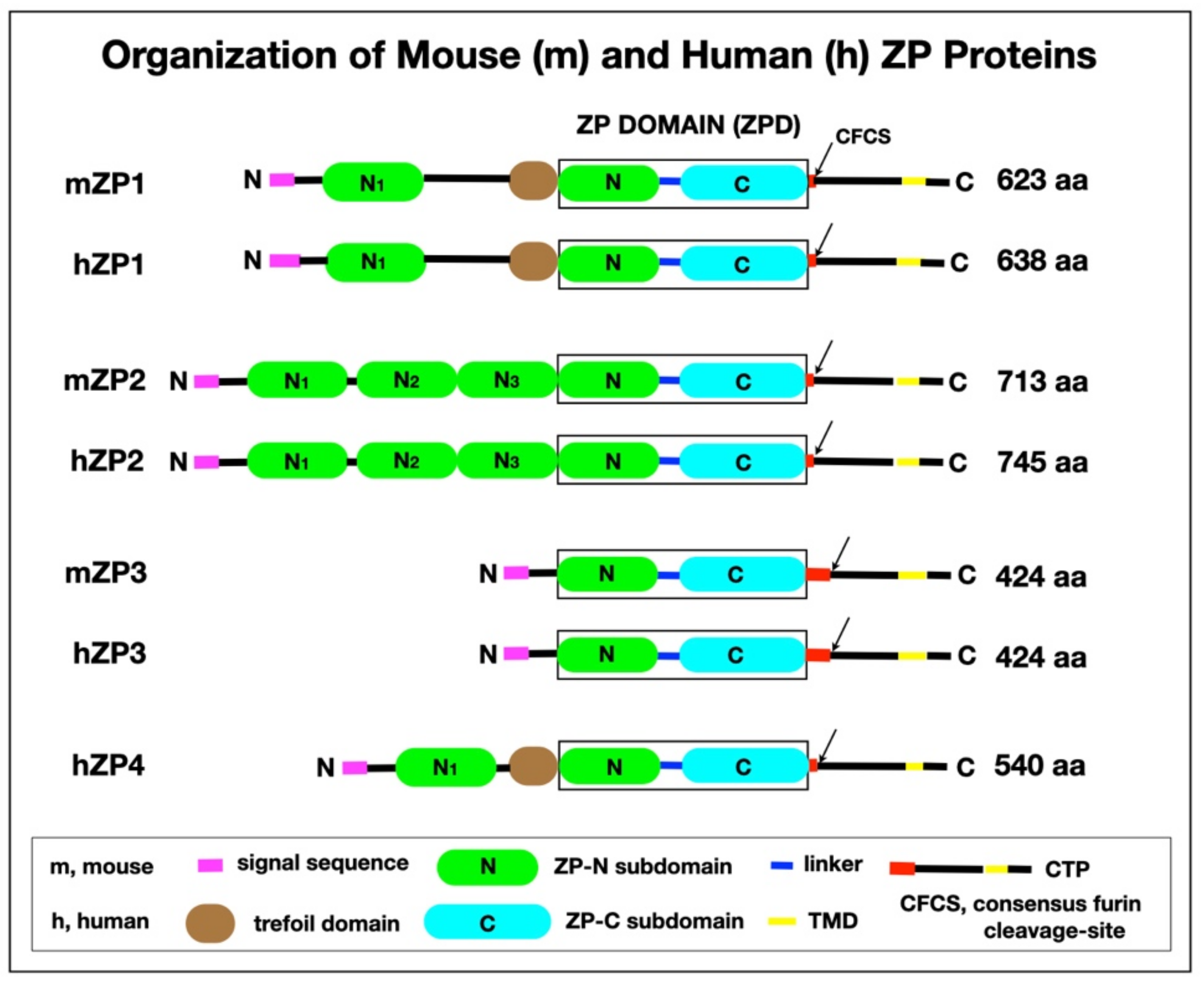
4.2. ZPD of ZP Proteins
4.3. Proteolytic Processing of ZP Proteins
4.4. Secretion of ZP Proteins
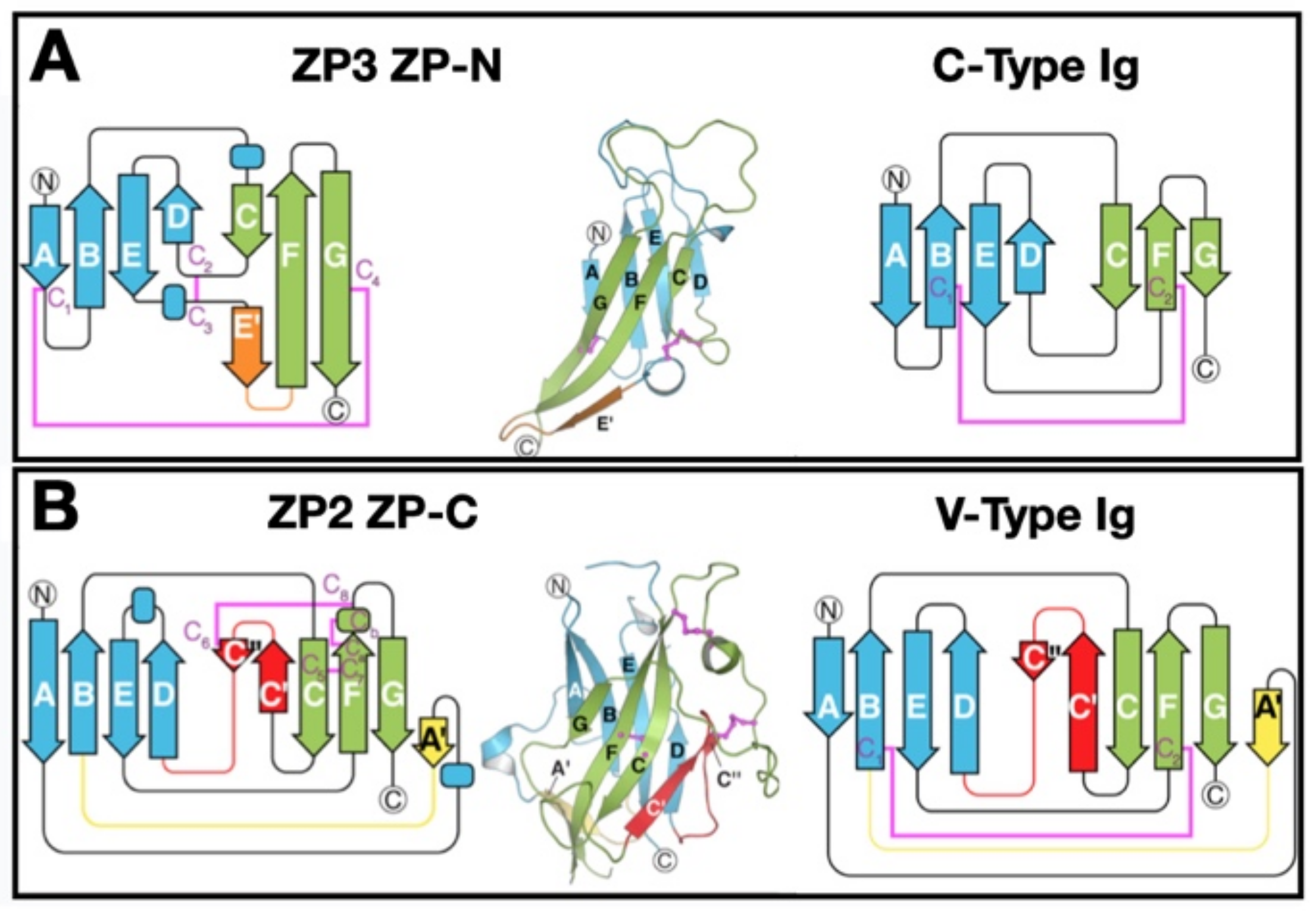
5. ZP Protein 3-Dimensional Structure
5.1. ZPD 3-Dimensional Structure
5.2. ZP Protein Arrangement
5.3. ZP Proteins as Functional Amyloids
6. ZP Fibril Assembly and Arrangement
6.1. Sequence Elements Regulate ZP Protein Polymerization
6.2. ZP Fibril Arrangement in Layers
7. mZP Genes and Female Fertility
7.1. mZP2 and mZP3 Homozygous Nulls Are Infertile
7.2. mZP3 Heterozygous Nulls Are Fertile
7.3. mZP1 Homozygous Nulls Exhibit Reduced Fertility
8. hZP Genes and Female Fertility
8.1. Infertile Women and Mutant hZP1 Genes
8.2. Infertile Women and Mutant hZP2, hZP3, or hZP4 Genes
9. Summary Points
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014; pp. 1090–1117. [Google Scholar]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 24, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Litscher, E.S.; Wassarman, P.M. (Eds.) Extracellular Matrix and Egg Coats; Academic Press: Oxford, UK, 2018. [Google Scholar]
- Wassarman, P.M. Zona pellucida glycoproteins. Annu. Rev. Biochem. 1988, 57, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M. Zona pellucida glycoproteins. J. Biol. Chem. 2008, 283, 24285–24289. [Google Scholar] [CrossRef] [Green Version]
- Litscher, E.S.; Wassarman, P.M. Zona pellucida proteins, fibrils, and matrix. Annu. Rev. Biochem. 2020, 89, 695–715. [Google Scholar] [CrossRef]
- Jovine, L.; Darie, C.C.; Litscher, E.S.; Wassarman, P.M. Zona pellucida domain proteins. Annu. Rev. Biochem. 2005, 74, 83–114. [Google Scholar] [CrossRef] [Green Version]
- Plaza, S.; Chanut-Delalande, H.; Fernandes, I.; Wassarman, P.M.; Payre, F. From A to Z: Apical structures and zona pellucida-domain proteins. Trends Cell Biol. 2010, 20, 524–532. [Google Scholar] [CrossRef]
- Litscher, E.S.; Wassarman, P.M. A Guide to Zona Pellucida Domain Proteins; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Bokhove, M.; Jovine, L. Structure of zona pellucida module proteins. Curr. Top. Dev. Biol. 2018, 130, 413–442. [Google Scholar] [PubMed]
- Short, R.V. The discovery of the ovaries. In The Ovary. I. General Aspects; Zuckerman, S., Weir, B.J., Eds.; Academic Press: New York, NY, USA, 1977; pp. 1–41. [Google Scholar]
- von Baer, K.E. De Ovi Mammalium et Hominis Genesi [On the Genesis of the Ovum of Mammals and of Man]; Leopold Voss: Leipzig, Germany, 1827. [Google Scholar]
- Austin, C.R.; Short, R.V. (Eds.) Reproduction in Mammals: I. Germ Cells and Fertilization; Cambridge University Press: London, UK, 1972. [Google Scholar]
- Zuckerman, S.; Baker, T.G. The development of the ovary and the process of oogenesis. In The Ovary. I. General Aspects; Zuckerman, S., Weir, B.J., Eds.; Academic Press: New York, NY, USA, 1977; pp. 42–112. [Google Scholar]
- Wassarman, P.M. The mammalian ovum. In The Physiology of Reproduction; Knobil, E., Neill, J., Eds.; Raven Press: New York, NY, USA, 1988; Volume 1, pp. 69–102. [Google Scholar]
- Wassarman, P.M.; Litscher, E.S. The mouse egg’s zona pellucida. Curr. Top. Dev. Biol. 2018, 130, 331–356. [Google Scholar]
- Sorensen, R.A. Cinemicrography of mouse oocyte maturation utilizing Nomarski differential-interference microscopy. Amer. J. Anat. 1973, 136, 265–276. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Josefowicz, W.J. Oocyte development in the mouse: An ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J. Morphol. 1978, 156, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.M.; Goodenough, D.A. Diverse functions of vertebrate gap junctions. Trends Cell Biol. 1998, 8, 477–483. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc. Natl. Acad. Sci. USA 1980, 77, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.-F.; Dean, J. Oocyte development: Molecular biology of the zona pellucida. Vitam. Horm. 1992, 47, 115–159. [Google Scholar]
- Rankin, T.; Dean, J. The molecular genetics of the zona pellucida: Mouse mutations and infertility. Mol. Hum. Reprod. 1996, 2, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Lira, S.A.; Kinloch, R.A.; Mortillo, S.; Wassarman, P.M. An upstream region of the mouse ZP3 gene directs expression of firefly luciferase specifically to growing oocytes in transgenic mice. Proc. Natl. Acad. Sci. USA 1990, 87, 7215–7219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.-F.; Chamow, S.M.; Dean, J. Oocyte-specific expression of mouse Zp-2: Developmental regulation of the zona pellucida genes. Mol. Cell. Biol. 1990, 10, 1507–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringette, M.J.; Chamberlin, M.E.; Baur, A.W.; Sobieski, D.A.; Dean, J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zone pellucida. Dev. Biol. 1988, 127, 287–295. [Google Scholar] [CrossRef]
- Roller, R.J.; Kinloch, R.A.; Hiraoka, B.Y.; Li, S.S.-L.; Wassarman, P.M. Gene expression durting mammalian oogenesis and early embryogenesis: Quantification of three messenger-RNAs abundant in fully-grown mouse oocytes. Development 1989, 106, 251–261. [Google Scholar] [CrossRef]
- Philpott, M.; Ringuette, M.J.; Dean, J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev. Biol. 1987, 121, 568–575. [Google Scholar] [CrossRef]
- Epifano, O.; Liang, L.F.; Familiari, M.; Moos, M.C.; Dean, J. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development 1995, 121, 1947–1956. [Google Scholar] [CrossRef]
- Lunsford, R.D.; Jenkins, N.A.; Kozak, C.A.; Liang, L.-F.; Silan, C.M.; Copeland, N.G.; Dean, J. Genomic mapping of murine Zp-2 and Zp-3, two oocyte-specific loci encoding zona pellucida proteins. Genomics 1990, 6, 184–187. [Google Scholar] [CrossRef]
- Chamberlin, M.E.; Dean, J. Genomic organization of a sex specific gene: The primary sperm receptor of the mouse zona pellucida. Dev. Biol. 1989, 131, 207–214. [Google Scholar] [CrossRef]
- Chamberlin, M.E.; Dean, J. Human homolog of the mouse sperm receptor. Proc. Natl. Acad. Sci. USA 1990, 87, 6014–6018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.D.; Hibler, D.W.; Fontenot, G.K.; Hsu, K.T.; Yurowicz, E.C.; Sacco, A.G. Cloning and characterization of zona pellucida genes and cDNAs from a variety of mammalian species: The ZPA, ZPB and ZPC gene families. DNA Seq. 1994, 4, 361–393. [Google Scholar] [CrossRef]
- Epifano, O.; Liang, L.-F.; Dean, J. Mouse Zp1 encodes a zona pellucida protein homologous to egg envelope proteins in mammals and fish. J. Biol. Chem. 1995, 270, 27254–27258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo-Rico, M.J.; Moros-Nicolás, C.; Pérez-Crespo, M.; Laguna-Barraza, R.; Gutiérrez-Adán, A.; Veryrunes, F.; Ballesta, J.; Laudet, V.; Chevret, P.; Avilés, M. ZP4 is present in murine zona pellucida and is not responsible for the specific gamete interaction. Front. Cell Dev. Biol. 2021, 8, 626679. [Google Scholar] [CrossRef] [PubMed]
- Ringuette, M.J.; Sobieski, D.A.; Chamow, S.M.; Dean, J. Oocyte-specific gene expression: Molecular characterization of a cDNA coding for ZP-3, the sperm receptor of the mouse zona pellucida. Proc. Natl. Acad. Sci. USA 1986, 83, 4341–4345. [Google Scholar] [CrossRef] [Green Version]
- Kinloch, R.A.; Roller, R.J.; Fimiani, C.M.; Wassarman, D.A.; Wassarman, P.M. Primary structure of the mouse sperm receptor’s polypeptide chain determined by genomic cloning. Proc. Natl. Acad. Sci. USA 1988, 85, 6409–6413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinloch, R.A.; Ruiz-Seiler, B.; Wassarman, P.M. Genomic organization and polypeptide primary structure of zona pellucida glycoprotein, hZP3, the hamster sperm receptor. Dev. Biol. 1990, 142, 414–421. [Google Scholar] [CrossRef]
- Liang, L.-F.; Dean, J. Conservation of mammalian secondary sperm receptor genes enables the promoter of the human gene to function in mouse oocytes. Dev. Biol. 1993, 156, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Millar, S.E.; Lader, E.; Liang, L.-F.; Dean, J. Oocyte-specific factors bind a conserved upstream sequence required for mouse zona pellucida promoter activity. Mol. Cell. Biol. 1991, 11, 6197–6204. [Google Scholar] [CrossRef] [Green Version]
- Lira, S.A.; Schickler, M.; Wassarman, P.M. Cis-acting DNA elements involved in oocyte-specific expression of mouse sperm receptor gene mZP3 are located close to the gene’s transcription start-site. Mol. Reprod. Dev. 1993, 36, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-F.; Soyal, S.M.; Dean, J. FIGalpha, a germ cell specific transcription factor involved in the coordinate expression of zona pellucida genes. Development 1997, 124, 4939–4947. [Google Scholar] [CrossRef] [PubMed]
- Soyal, S.M.; Amleh, A.; Dean, J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 2000, 127, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.E.; Lader, E.S.; Dean, J. ZAP-1 DNA binding activity is first detected at the onset of zona pellucida gene expression in embryonic mouse oocytes. Dev. Biol. 1993, 158, 410–413. [Google Scholar] [CrossRef]
- Schickler, M.; Lira, S.A.; Kinloch, R.A.; Wassarman, P.M. A mouse oocyte-specific protein that binds to a region of mZP3 promoter involved in regulating oocyte-specific expression of the mZP3 gene. Mol. Cell. Biol. 1992, 12, 120–127. [Google Scholar]
- Orkin, S.H. Globin gene regulation and switching: Circa 1990. Cell 1990, 63, 665–672. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Structure and function of the zona pellucida: Identification and characterization of the proteins of the mouse oocyte’s zona pellucida. Dev. Biol. 1980, 76, 185–202. [Google Scholar] [CrossRef]
- Shimizu, S.; Tsuji, M.; Dean, J. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J. Biol. Chem. 1983, 258, 5858–5863. [Google Scholar] [CrossRef]
- Hughes, D.C.; Barratt, C.L. Identification of the true orthologue of the mouse ZP1 gene: Evidence for greater complexity in the mammalian zona pellucida. Biochem. Biophys. Acta 1999, 1447, 303–306. [Google Scholar] [CrossRef]
- Lefievre, L.; Conner, S.J.; Salpekar, A.; Olufaowobi, O.; Ashton, P.; Pavlovic, B.; Lenton, W.; Afnan, M.; Brewis, I.A.; Monk, M.; et al. Four zona pellucida glycoproteins are expressed in the human. Hum. Reprod. 2004, 19, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, S.J.; Lefievre, L.; Hughes, D.C.; Barratt, C.L. Cracking the egg: Increased complexity in the zona pellucida. Hum. Reprod. 2005, 20, 1148–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.K. The human egg’s zona pellucida. Curr. Top. Dev. Biol. 2018, 130, 379–411. [Google Scholar]
- Goudet, G.; Mugnier, S.; Callebaut, I.; Monget, P. Phylogenetic analysis and identification of pseudogenes reveal a progressive loss of zona pellucida genes during evolution of vertebrates. Biol. Reprod. 2008, 78, 796–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litscher, E.S.; Wassarman, P.M. Evolution, structure, and synthesis of vertebrate egg-coat proteins. Trends Dev. Biol. 2014, 8, 65–76. [Google Scholar]
- Killingback, E.E.; Swanson, W.J. Egg coat proteins across metazoan evolution. Curr. Top. Dev. Biol. 2018, 130, 443–488. [Google Scholar]
- Thim, L. Trefoil peptides: From structure to function. Cell. Mol. Life Sci. 1997, 53, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.M.; Salzmann, G.S.; Roller, R.J.; Wassarman, P.M. Biosynthesis of the major zona pellucida glycoprotein secreted by oocytes during mammalian oogenesis. Cell 1982, 31, 749–759. [Google Scholar] [CrossRef]
- Salzmann, G.S.; Greve, J.M.; Roller, R.J.; Wassarman, P.M. Biosynthesis of the sperm receptor during oogenesis in the mouse. EMBO J. 1983, 2, 1451–1456. [Google Scholar] [CrossRef]
- Boja, E.S.; Hoodbhoy, T.; Fales, H.M.; Dean, J. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 2003, 278, 34189–34202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easton, R.L.; Patankar, M.S.; Lattanzio, F.A.; Leaven, T.H.; Morris, H.R.; Clark, G.F.; Dell, A. Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd(a) antigen. J. Biol. Chem. 2000, 275, 7731–7742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell, A.; Chalabi, S.; Easton RLHaslam, S.M.; Sutton-Smith, M.; Patankar, M.S.; Lattanzio, F.; Panicao, M.; Morris, H.R.; Clark, G.F. Murine and human zona pellucida 3 derived from mouse eggs express identical O-glycans. Proc. Natl. Acad. Sci. USA 2003, 100, 15631–15636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, P.C.; Chiu, P.C.; Lee, C.L.; Chang, L.Y.; Panico, M.; Morris, H.R.; Haslam, S.M.; Khoo, K.H.; Clark, G.F.; Yeung, W.S.; et al. Human sperm binding is mediated by the sialyl-Lewis(X) oligosaccharide on the zona pellucida. Science 2011, 333, 1761–1764. [Google Scholar] [CrossRef] [Green Version]
- Claw, K.G.; Swanson, W.J. Evolution of the egg: New findings and challenges. Annu. Rev. Genom. Hum. Genet. 2012, 13, 109–125. [Google Scholar] [CrossRef]
- Wassarman, P.M. Fertilization in mammals. Sci. Am. 1988, 255, 78–84. [Google Scholar] [CrossRef]
- Bork, P.; Sander, C. A large domain common to sperm receptors (Zp2 and Zp3) and TGF-β type III receptor. FEBS Lett. 1992, 300, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Bokhove, M.; Nishimura, K.; Brunati, M.; Han, L.; de Sanctis, D.; Rampoldi, L.; Jovine, L. A structured interdomain linker directs self-polymerization of human uromodulin. Proc. Natl. Acad. Sci. USA 2016, 113, 1552–1557. [Google Scholar] [CrossRef] [Green Version]
- Wilburn, D.B.; Swanson, W.J. The “ZP domain” is not one, but likely two independent domains. Mol. Reprod. Dev. 2017, 84, 284–285. [Google Scholar] [CrossRef]
- Callebaut, I.; Mornon, J.P.; Monget, P. Isolated ZP-N domains constitute the N-terminal extensions of zona pellucida proteins. Bioinformatics 2007, 23, 1871–1874. [Google Scholar] [CrossRef]
- Jovine, L.; Qi, H.; Williams, Z.; Litscher, E.S.; Wassarman, P.M. The ZP domain is a conserved module for protein polymerization. Nat. Cell Biol. 2002, 4, 457–461. [Google Scholar] [CrossRef]
- Jovine, L.; Janssen, W.G.; Litscher, E.S.; Wassarman, P.M. The PLAC1-homology region of the ZP domain is sufficient for protein polymerization. BMC Biochem. 2006, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Goodyear, R.J.; Richardson, G.P. Structure, function, and development of the tectorial membrane: An extracellular matrix essential for hearing. Curr. Top. Dev. Biol. 2018, 130, 217–244. [Google Scholar]
- Schaeffer, C.; Santambrogio, S.; Perucca, S.; Casari, G.; Rampoldi, L. Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol. Biol. Cell 2009, 20, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Killick, R.; Legan, P.K.; Malenczak, C.; Richardson, G.P. Molecular cloning of chick beta-tectorin, an extracellular matrix molecule of the inner ear. J. Cell Biol. 1995, 129, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Legan, P.K.; Rau, A.; Keen, J.N.; Richardson, G.P. The mouse tectorins. Molecular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J. Biol. Chem. 1997, 272, 8791–8801. [Google Scholar] [CrossRef] [Green Version]
- Spargo, S.C.; Hope, R.M. Evolution and nomenclature of the zona pellucida gene family. Biol. Reprod. 2003, 68, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.; Paton, I.R.; Hughes, D.C.; Burt, D.W. Isolation and mapping the chicken zona pellucida genes: An insight into the evolution of orthologous genes in different species. Mol. Reprod. Dev. 2005, 70, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-M.; Tian, H.-F.; Hu, Q.-M.; Meng, Y.; Xiao, H.-B. Evolution and multiple origins of zona pellucida genes in vertebrates. Biol. Open 2018, 7, bio036137. [Google Scholar] [CrossRef] [Green Version]
- Jovine, L.; Qi, H.; Williams, Z.; Litscher, E.S.; Wassarman, P.M. Features that affect secretion and assembly of zona pellucida glycoproteins during mammalian oogenesis. In Gamete Biology; Gupta, S.K., Koyama, K., Murray, J.F., Eds.; Nottingham University Press: Nottingham, UK, 2007; pp. 187–216. [Google Scholar]
- Zhao, M.; Gold, L.; Dorward, H.; Liang, L.; Hoodbhoy, T.; Boja, E.; Fales, H.M.; Dean, J. Mutation of a conserved hydrophobic patch prevents incorporation of ZP3 into the zona pellucida surrounding mouse eggs. Mol. Cell. Biol. 2003, 23, 8982–8991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovine, L.; Qi, H.; Williams, Z.; Litscher, E.S.; Wassarman, P.M. A duplicated motif controls assembly of zona pellucida domain proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 5922–5927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Williams, Z.; Wassarman, P.M. Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3. Mol. Biol. Cell 2002, 13, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Hoodbhoy, T.; Aviles, M.; Baibakov, B.; Epifano, O.; Jiménez-Movilla, M.; Gauthier, L.; Dean, J. ZP2 and ZP3 traffic independently within oocytes prior to assembly into the extracellular zona pellucida. Mol. Cell. Biol. 2006, 26, 7991–7998. [Google Scholar] [CrossRef] [Green Version]
- Wassarman, P.M.; Qi, H.; Litscher, E.S. Mutant female mice carrying a single mZP3 allele produce eggs with a thin zona pellucida, but reproduce normally. Proc. R. Soc. Lond. Biol. Sci. 1997, 264, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Wassarman, P.M. Secretion of zona pellucida glycoprotein mZP2 by growing oocytes from mZP3+/+ and mZP3-/- mice. Dev. Genet. 1999, 25, 95–102. [Google Scholar] [CrossRef]
- Monné, M.; Han, L.; Schwend, T.; Burendahl, S.; Jovine, L. Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats. Nature 2008, 456, 653–657. [Google Scholar] [CrossRef]
- Han, L.; Monné, M.; Okumura, H.; Schwend, T.; Cherry, A.L.; Flot, D.; Matsuda, T.; Jovine, L. Insights into egg coat assembly and egg-sperm interaction from the X-ray structure of full-length ZP3. Cell 2010, 143, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.J.; Hu, Y.; Zhu, J.; Woodruff, T.K.; Jardetzky, T.S. Structure of betaglycan zona pellucida (ZP)-C domain provides insights into ZP-mediated protein polymerization and TGF-β binding. Proc. Natl. Acad. Sci. USA 2011, 108, 5232–5236. [Google Scholar] [CrossRef] [Green Version]
- Diestel, U.; Resch, M.; Meinhardt, K.; Weiler, S.; Hellmann, T.V.; Mueller, T.D.; Nickel, J.; Eichler, J.; Muller, Y.A. Identification of a novel TGF-β-binding site in the zona pellucida C-terminal (ZP-C) domain of TGF- β-Receptor-3 (TGFR-3). PLoS ONE 2013, 8, e67214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, I.; Sadat, A.I.; Hosseini, H.; Dioguardi, E.; Nishimura, K.; Han, L.; Villa, A.; de Sanctis, D.; Jovine, L. Structural basis of egg coat-sperm recognition at fertilization. Cell 2017, 169, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Bokhove, M.; Croci, R.; Zamora-Caballero, S.; Han, L.; Letarte, M.; de Sanctis, D.; Jovine, L. Structural basis of the human endoglin-BMP9 interaction: Insights into BMP signaling and HHT1. Cell Rep. 2017, 19, 1917–1928. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, K.; Dioguardi, E.; Nishio, S.; Villa, A.; Han, L.; Matsuda, T.; Jovine, L. Molecular basis of egg coat cross-linking sheds light on ZP1-associated female infertility. Nature Comm. 2019, 10, 3086. [Google Scholar] [CrossRef] [Green Version]
- Stsiapanava, A.; Xu, C.; Brunati, M.; Zamora-Caballero, S.; Schaeffer, C.; Bokhove, M.; Han, L.; Hebert, H.; Carroni, M.; Yasumasu, S.; et al. Cryo-EM structure of native human uromodulin, a zona pellucida module polymer. EMBO J. 2020, 39, e106807. [Google Scholar] [CrossRef] [PubMed]
- Monné, M.; Jovine, L. A structural view of egg coat architecture and function in fertilization. Biol. Reprod. 2011, 85, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.F.; Barclay, A.N. The immunoglobulin superfamily—Domains for cell surface recognition. Annu. Rev. Immunol. 1988, 6, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.G.; Thornton, J.M. The Greek key motif: Extraction, classification and analysis. Protein Eng. 1993, 6, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Legan, P.K.; Lukashkina, V.A.; Goodyear, R.J.; Lukashkin, A.N.; Verhoeven, K.; Van Camp, G.; Russell, I.J.; Richardson, G.P. A deafness mutation isolates a second role for the tectorial membrane in hearing. Nat. Neurosci. 2005, 8, 1035–1042. [Google Scholar] [CrossRef]
- Eisenberg, D.S.; Sawaya, M.R. Structural studies of amyloid proteins at the molecular level. Annu. Rev. Biochem. 2017, 86, 69–95. [Google Scholar] [CrossRef] [Green Version]
- Tsolis, A.C.; Papandreou, N.C.; Iconomidou, V.A.; Hamodrakas, S.J. A consensus method for the prediction of “aggregation-prone” peptides in globular proteins. PLoS ONE 2013, 8, e54175. [Google Scholar] [CrossRef] [Green Version]
- Louros, N.N.; Iconomidou, V.A.; Giannelou, P.; Hamodrakas, S.J. Structural analysis of peptide-analogues of human zona pellucida ZP1 protein with amyloidogenic properties: Insights into mammalian zona pellucida formation. PLoS ONE 2013, 8, e73258. [Google Scholar] [CrossRef] [Green Version]
- Egge, N.; Muthusubramanian, A.; Cornwall, G.A. Amyloid properties of the mouse egg zona pellucida. PLoS ONE 2015, 10, e129907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid—From bacteria to humans. Trends Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Movilla, M.; Dean, J. ZP2 and ZP3 cytoplasmic tails prevent premature interactions and ensure incorporation into the zona pellucida. J. Cell Sci. 2011, 124, 940–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litscher, E.S.; Wassarman, P.M. Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry 1999, 38, 12280–12287. [Google Scholar] [CrossRef]
- Williams, Z.; Wassarman, P.M. Secretion of mouse ZP3, the sperm receptor, requires cleavage of its polypeptide at a consensus furin cleavage-site. Biochemistry 2001, 40, 929–937. [Google Scholar] [CrossRef]
- Zhao, M.; Gold, L.; Ginsberg, A.M.; Liang, L.-F.; Dean, J. Conserved furin cleavage-site not essential for secretion and integration of ZP3 into the extracellular coat of transgenic mice. Mol. Cell. Biol. 2002, 22, 3111–3120. [Google Scholar] [CrossRef] [Green Version]
- Mosesson, M.W.; Siebenlist, K.R.; Meh, D.A. The structure and biological features of fibrinogen and fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 11–30. [Google Scholar] [CrossRef]
- Handford, P.A.; Downing, A.K.; Reinhardt, D.P.; Sakai, L.Y. Fibrillin: From domain structure to supramolecular assembly. Matrix Biol. 2000, 19, 457–470. [Google Scholar] [CrossRef]
- Gamblin, T.C.; Berry, R.W.; Binder, L.I. Modeling tau polymerization in vitro: A review and synthesis. Biochemistry 2003, 42, 15009–15017. [Google Scholar] [CrossRef]
- Greve, J.M.; Wassarman, P.M. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J. Mol. Biol. 1985, 181, 253–264. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Mortillo, S. Structure of the mouse egg extracellular coat, the zona pellucida. Int. Rev. Cytol. 1991, 130, 85–110. [Google Scholar]
- Wassarman, P.M.; Liu, C.; Litscher, E.S. Constructing the mouse egg zona pellucida: Some new pieces of an old puzzle. J. Cell Sci. 1996, 109, 2001–2004. [Google Scholar] [CrossRef]
- Phillips, D.M.; Shalgi, R.M. Surface architecture of the mouse and hamster zona pellucida and oocyte. J. Ultrastruct. Res. 1980, 72, 1–12. [Google Scholar] [CrossRef]
- Familiari, G.; Relucenti, M.; Heyn, R.; Micara, G.; Correr, S. 3-dimensional structure of the zona pellucida at ovulation. Microsc. Res. Tech. 2006, 69, 415–426. [Google Scholar] [CrossRef]
- Keefe, D.; Tran, P.; Pellegrini, C.; Oldenbourg, R. Polarized light microscopy and digital image processing indentify a multilaminar structure of the hamster zona pellucida. Hum. Reprod. 1997, 12, 1250–1252. [Google Scholar] [CrossRef] [Green Version]
- El-Mestrah, M.; Castle, P.E.; Borossa, G.; Kan, F.W.K. Sucellular distribution of ZP1, ZP2, and ZP3 glycoproteins during folliculogenesis and demonstration of their topographical disposition within the zona matrix of mouse ovarian oocytes. Biol. Reprod. 2002, 66, 866–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelletier, C.; Keefe, D.L.; Trimarchi, J.R. Noninvasive polarized light microscopy quantitatively distinguishes the multilaminar structure of the zona pellucida of living human eggs and embryos. Fertil. Steril. 2004, 81, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.-B.; Nelson, L.M.; Dean, J. Inhibition of zona pellucida gene expression by antisense oligonucleotides injected into mouse oocytes. J. Biol. Chem. 1995, 270, 849–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, T.L.; Talbot, P.; Lee, E.; Dean, J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development 1999, 126, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Rankin, T.; O’Brien, M.; Lee, E.; Wigglesworth, K.; Eppig, J.; Dean, J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development 2001, 128, 1119–1126. [Google Scholar] [CrossRef]
- Liu, C.; Litscher, E.S.; Mortillo, S.; Sakai, Y.; Kinloch, R.A.; Stewart, C.L.; Wassarman, P.M. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc. Natl. Acad. Sci. USA 1996, 93, 5431–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, T.; Familiari, M.; Lee, E.; Ginsberg, A.; Dwyer, N.; Blanchette-Mackie, J.; Drago, J.; Westphal, H.; Dean, J. Mice homozygous for an insertional mutation in the ZP3 gene lacks a zona pellucida and are infertile. Development 1996, 122, 2903–2910. [Google Scholar] [CrossRef]
- Wassarman, P.M.; Liu, C.; Chen, J.; Qi, H.; Litscher, E.S. Ovarian development in mice bearing homozygous and heterozygous null mutations in zona pellucida glycoprotein gene mZP3. Histol. Histopathol. 1998, 13, 293–300. [Google Scholar] [PubMed]
- Wassarman, P.M.; Litscher, E.S. Influence of the zona pellucida of the mouse egg on folliculogenesis and fertility. Int. J. Dev. Biol. 2012, 56, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matzuk, M.M.; Burns, K.H.; Viveiros, M.M.; Eppig, J.J. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science 2002, 296, 2178–2180. [Google Scholar] [CrossRef]
- Wassarman, P.M. Channels of communication in the ovary. Nat. Cell Biol. 2002, 4, s7–s9. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef]
- Carabatsos, M.J.; Sellitto, C.; Goodenough, D.A.; Albertini, D.F. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev. Biol. 2000, 226, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Kidder, G.M.; Mhawi, A. Gap junctions and ovarian folliculogenesis. Reproduction 2002, 123, 613–620. [Google Scholar] [CrossRef]
- Gittens, J.E.; Barr, K.J.; Vanderhyden, B.C.; Kidder, G.M. Interplay between paracrine signaling and gap junctional communication in ovarian follicles. J. Cell Sci. 2005, 118, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.M.; Goodenough, D.A.; Li, E.; Paul, D.L. Female infertility in mice lacking connexin-37. Nature 1997, 385, 525–529. [Google Scholar] [CrossRef]
- Ackert, C.L.; Gittens, J.E.; O’Brien, M.J.; Eppig, J.J.; Kidder, G.M. Intercellular communication via connexin-43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 2001, 233, 258–270. [Google Scholar] [CrossRef] [Green Version]
- El-Hayek, S.; Yang, Q.; Abbassi, L.; FitzHarris, G.; Clarke, H.J. Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr. Biol. 2018, 28, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Männikkö, M.; Törmälä, R.M.; Tuuri, T.; Haltia, A.; Martikainen, H.; Ala-Kokko, L.; Tapanainen, J.S.; Lakkakorpi, J.T. Association between sequence variations in genes encoding human zona pellucida glycoproteins and fertilization failure in IVF. Hum. Reprod. 2005, 20, 1578–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pökkylä, R.M.; Lakkakorpi, J.T.; Noujua-Huttunen, S.H.; Tapanainen, J.S. Sequence variations in human ZP genes as potential modifiers of zona pellucida architecture. Fertil. Steril. 2011, 95, 2669–2672. [Google Scholar] [CrossRef]
- Margalit, M.; Paz, G.; Yavetz, H.; Yogev, L.; Amit, A.; Hevlin-Schwartz, T.; Gupta, S.K.; Kleiman, S.E. Genetic and physiological study of morphologically abnormal human zona pellucida. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 70–76. [Google Scholar] [CrossRef]
- Sun, L.; Fang, X.; Chen, Z.; Zhang, H.; Zhang, Z.; Zhou, P.; Xue, T.; Peng, X.; Zhu, Q.; Yin, M.; et al. Compound heterozygous ZP1 mutations cause empty follicle syndrome in infertile sisters. Hum. Mut. 2019, 40, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Li, R.; Li, D.; Zheng, L.; Ou, S.; Zhao, H.; Zhang, Q.; Wang, W. Novel mutation in the ZP1 gene and clinical implications. J. Assist. Reprod. Genet. 2019, 36, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Luan, X.; Peng, Y.; Chen, T.; Su, S.; Zhang, C.; Wang, Z.; Cheng, L.; Zhang, X.; Wang, Y.; et al. Novel zona pellucida gene variants identified in patients with oocyte anomalies. Fertil. Steril. 2017, 107, 1364–1369. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, X.; Sun, X.; Ma, L.; Yin, Y.; He, G.; Zhang, Y.; Zhou, J.; Cao, L.; Liu, J.; et al. A novel homozygous nonsense mutation in zona pellucida 1 (ZP1) causes human empty follicle syndrome. J. Assist. Reprod. Genet. 2021, 38, 1459–1468. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, C.; Zhang, X.; Zhang, H.; Lu, Q.; Wang, C.; Hu, Y.; Ling, X.; Zhang, J.; Huo, R. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J. Cell. Mol. Med. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Zhou, Z.; Ni, C.; Wu, L.; Chen, B.; Xu, Y.; Zhang, S.; Mu, J.; Li, B.; Yan, Z.; Fu, J.; et al. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum. Genet. 2019, 138, 327–337. [Google Scholar] [CrossRef]
- Zhang, Z.; Shangguan, T.; Li, Y.-Y.; He, W. Infertility due to lack of zona pellucida caused by a compound heterozygous mutation in ZP1 gene. Reprod. Dev. Med. 2020, 2, 183–186. [Google Scholar]
- Huang, H.-L.; Lv, C.; Zhao, Y.-C.; Li, W.; He, X.-M.; Li, P.; Sha, A.-G.; Tian, X.; Papasian, C.J.; Deng, H.-W.; et al. Mutant ZP1 in familial infertility. N. Engl. J. Med. 2014, 370, 1220–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, C.; Huang, H.-L.; Wang, Y.; Peng, T.-L.; Tan, H.-J.; Zeng, M.-H.; Quan, R.-P.; Deng, H.-W.; Xiao, H.-M. Mutant ZP1 impedes incorporation of ZP3 and ZP4 in the zona pellucida, resulting in zona absence and female infertility in rats. Biol. Reprod. 2021, 104, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zeng, Y.; Chen, H.; Zhou, Z.; Fu, J.; Sang, Q.; Wang, L.; Sun, X.; Chen, B.; Xu, C. A novel homozygous variant in ZP2 causes abnormal zona pellucida formation and female infertility. J. Assist. Reprod. Genet. 2021, 38, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, K.; Bai, D.; Yin, J.; Tang, Y.; Chi, F.; Zhang, L.; Wang, Y.; Pan, J.; Liang, S.; et al. Dosage effects of ZP2 and ZP3 heterogeneous mutations cause human infertility. Hum. Genet. 2017, 136, 1822–1827. [Google Scholar] [CrossRef]
- Chen, T.; Bian, Y.; Liu, X.; Zhao, S.; Wu, K.; Yan, L.; Li, M.; Yang, Z.; Liu, H.; Zhao, H.; et al. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am. J. Hum. Genet. 2017, 101, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhu, D.; Liu, Z.; Ren, X.; Yang, X.; Li, D.; Luo, Y.; Peng, X.; Zhou, X.; Jia, W.; et al. A novel mutation in ZP3 causes empty follicle syndrome and abnormal zona pellucida formation. J. Assist. Reprod. Genet. 2021, 38, 251–259. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Liu, Q.; Liu, W.; Yan, X.; Zhu, X.; Zhou, D.; Tian, Y.; Zhang, F.; Li, N.; et al. Mutations in ZP4 are associated with abnormal zona pellucida and female infertility. J. Clin. Pathol. 2021, in press. [Google Scholar] [CrossRef]




| ZPD 2 | ZP-N 3 | ZP-C 4 | |
|---|---|---|---|
| 1 mZP1 | 271 (271–541) | 99 | 149 |
| 1 hZP1 | 270 (279–548) | 99 | 148 |
| mZP2 | 267 (364–630) | 96 | 148 |
| hZP2 | 267 (371–637) | 96 | 148 |
| mZP3 | 260 (45–304) | 96 | 135 |
| hZP3 | 259 (45–303) | 97 | 133 |
| hZP4 | 275 (188–462) | 99 | 151 |
| ZP Protein | Polypeptide Length (aa) | Single Sequence (aa) | ZP Domain (aa) | Consensus Furin Cleavage-Site (aa) | Transmembrance Domain (aa) | Trefoil Domain (aa) |
|---|---|---|---|---|---|---|
| mZP1 | 623 | 1–20 | 271–542 | 545–548 | 591–611 | 225–266 |
| hZP1 | 638 | 1–25 | 279–549 | 552–555 | 602–622 | 234–274 |
| mZP2 | 713 | 1–34 | 364–630 | 632–635 | 684–703 | - |
| hZP2 | 745 | 1–38 | 371–637 | 639–642 | 717–736 | - |
| mZP3 | 424 | 1–22 | 45–304 | 350–353 | 387–409 | - |
| hZP3 | 424 | 1–22 | 45–303 | 349–352 | 388–408 | - |
| hZP4 | 540 | 1–19 | 188–462 | 463–466 | 505–526 | 141–183 |
| Genotype | Fertility | Zona Pellucida | References |
|---|---|---|---|
| Wild-type | Fertile | Normal | - |
| ZP1-/- | Reduced Fertility | Abnormal | [118] |
| ZP2-/- | Infertile | None | [119] |
| ZP3-/- | Infertile | None | [120,121] |
| ZP3+/- | Fertile | Thin | [83] |
| hZP1 Mutations | Location of Mutation | Status of Zona Pellucida | References |
|---|---|---|---|
| G57Dfs*9 | exon-1, SC in NI befroe TD | none | [136] |
| R61C | exon-1, NI befroe TD | none (?) | [137] |
| W83R | exon-2, NI befroe TD | abnormal/none | [138] |
| E67>X | exon-2, SC in NI befroe TD | none | [139] |
| RI09H | exon-3, NI befroe TD | none | [140] |
| H701fs*52 | exon-3, SC between NI and TD | none | [141] |
| Q292>X | exon-5, SC in ZP | none | [142] |
| I386>X | exon-7, SC between ZP-N and ZP-C(linker) | none | [142] |
| I390fs404X | exon-7, SC between ZP-N and ZP-C(linker) | none | [143,144] |
| I390Tfs*16 | exon-7, SC between ZP-N and ZP-C(linker) | none | [136,137] |
| R410W | exon-7, between ZP-N and ZP-C(linker) | none | [141] |
| W471>X | exon-8, SC in ZP-C | abnormal/none | [138] |
| C478>X | exon-9, SC in ZP-C | none | [141] |
| V570M | exon-11, between CFCS and EHP | none | [141] |
| D592Gfs*29 | exon-12, SC between CFCS and TMD | none | [141] |
| hZP2Mutations | |||
| C372S | exon-11, ZP-N | thin/none | [141] |
| Q412Rfs*17 | exon-11, ZP-N | thin | [145] |
| R533S | exon-15, ZP-C | normal/none | [138] |
| C566R | exon-16, ZP-C | abnormal/none | [138] |
| R698>X | exon-19, SC between CFCS and TMD | very thin/none | [146] |
| hZP3Mutations | |||
| A134T | exon-2, ZP-N | none | [140,147] |
| S173C | exon-3, ZP-C | none | [148] |
| R255G | exon-5, ZP-C | none | [141] |
| R349L>X | exon-8, SC at CFCS | very thin/none | [146] |
| hZP4Mutations | |||
| D100N | exon-3, NI | thin, irregular | [149] |
| V444L | exon-10, ZP-C | thin, irregular | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassarman, P.M.; Litscher, E.S. Zona Pellucida Genes and Proteins: Essential Players in Mammalian Oogenesis and Fertility. Genes 2021, 12, 1266. https://doi.org/10.3390/genes12081266
Wassarman PM, Litscher ES. Zona Pellucida Genes and Proteins: Essential Players in Mammalian Oogenesis and Fertility. Genes. 2021; 12(8):1266. https://doi.org/10.3390/genes12081266
Chicago/Turabian StyleWassarman, Paul M., and Eveline S. Litscher. 2021. "Zona Pellucida Genes and Proteins: Essential Players in Mammalian Oogenesis and Fertility" Genes 12, no. 8: 1266. https://doi.org/10.3390/genes12081266
APA StyleWassarman, P. M., & Litscher, E. S. (2021). Zona Pellucida Genes and Proteins: Essential Players in Mammalian Oogenesis and Fertility. Genes, 12(8), 1266. https://doi.org/10.3390/genes12081266





