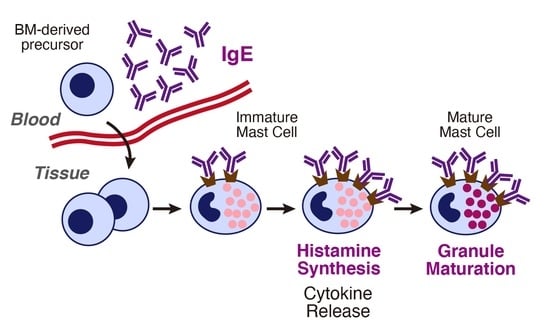
Roles of IgE and Histamine in Mast Cell Maturation
Abstract
:1. Introduction
2. Roles of IgE in Urticaria
3. Effects of IgE in the Absence of Antigens
3.1. Effects of IgE in the Absence of Antigens (Monomeric IgE Responses)
3.2. Possible Molecular Mechanisms of IgE-Mediated FcεRI Activation
3.3. Differences between Monomeric IgE Responses and IgE-Mediated Antigen Stimulation
3.4. Antigen-Independent Roles of IgE in Cutaneous Mast Cells
4. Histamine Synthesis in Mast Cells
4.1. The Rate-Limiting Enzyme for Histamine Synthesis: Histidine Decarboxylase
4.2. Gene Targeting of Hdc
5. Histamine-Mediated Granule Maturation of Mast Cells
6. Pro-Inflammatory Roles of Granule Components
6.1. Pathophysiological Role of Granule Components
6.2. IgE-Independent Degranulation
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef]
- Tsai, M.; Galli, S.J. IgE and Mast Cells in Allergic Disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast Cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef]
- Kitamura, Y. Heterogeneity of Mast Cells and Phenotypic Change between Subpopulations. Annu. Rev. Immunol. 1989, 7, 59–76. [Google Scholar] [CrossRef]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, S.; Xu, J.; Zhang, X.; Han, D.; Liu, J.; Xia, M.; Yi, L.; Shen, Q.; Xu, S.; et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity 2018, 49, 640.e5–653.e5. [Google Scholar] [CrossRef] [Green Version]
- Hatanaka, K.; Kitamura, Y.; Nishimune, Y. Local Development of Mast Cells from Bone Marrow-Derived Precursors in the Skin of Mice. Blood 1979, 53, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Weitzmann, A.; Naumann, R.; Dudeck, A.; Zerjatke, T.; Gerbaulet, A.; Roers, A. Mast Cells Occupy Stable Clonal Territories in Adult Steady-State Skin. J. Invest. Dermatol. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Marichal, T.; Galli, S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012, 33, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Boulet, L.P.; Chapman, K.R.; Côté, J.; Kalra, S.; Bhagat, R.; Swystun, V.A.; Laviolette, M.; Cleland, L.D.; Deschesnes, F.; Su, J.Q.; et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am. J. Respir. Crit. Care Med. 1997, 155, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Fleming, H.E.; Wong, H.H.; Liu, J.T.; Su, J.Q.; Reimann, J.; Fick, R.B., Jr.; Boushey, H.A. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am. J. Respir. Crit. Care Med. 1997, 155, 1828–1834. [Google Scholar] [CrossRef] [Green Version]
- MacGlashan, D.W.; Bochner, B.S.; Adelman, D.C.; Jardieu, P.M.; Togias, A.; McKenzie-White, J.; Sterbinsky, S.A.; Hamilton, R.G.; Lichtenstein, L.M. Down-Regulation of Fc(Epsilon)RI Expression on Human Basophils during in Vivo Treatment of Atopic Patients with Anti-IgE Antibody. J. Immunol. 1997, 158, 1438–1445. [Google Scholar]
- Deza, G.; Bertolín-Colilla, M.; Sánchez, S.; Soto, D.; Pujol, R.M.; Gimeno, R.; Giménez-Arnau, A.M. Basophil FcɛRI expression is linked to time to omalizumab response in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2018, 141, 2313.e1–2316.e1. [Google Scholar] [CrossRef] [Green Version]
- Kessel, A.; Helou, W.; Bamberger, E.; Sabo, E.; Nusem, D.; Panassof, J.; Toubi, E. Elevated serum total IgE--a potential marker for severe chronic urticaria. Int. Arch. Allergy Immunol. 2010, 153, 288–293. [Google Scholar] [CrossRef]
- Altrichter, S.; Fok, J.S.; Jiao, Q.; Kolkhir, P.; Pyatilova, P.; Romero, S.M.; Scheffel, J.; Siebenhaar, F.; Steinert, C.; Terhorst-Molawi, D.; et al. Total IgE as a Marker for Chronic Spontaneous Urticaria. Allergy Asthma Immunol. Res. 2021, 13, 206–218. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Latiff, A.H.A.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Bedrikow, R.B.; et al. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef]
- Altrichter, S.; Peter, H.-J.; Pisarevskaja, D.; Metz, M.; Martus, P.; Maurer, M. IgE Mediated Autoallergy against Thyroid Peroxidase—A Novel Pathomechanism of Chronic Spontaneous Urticaria? PLoS ONE 2011, 6, e14794. [Google Scholar] [CrossRef]
- Cugno, M.; Asero, R.; Ferrucci, S.; Lorini, M.; Carbonelli, V.; Tedeschi, A.; Marzano, A.V. Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy 2018, 73, 2408–2411. [Google Scholar] [CrossRef]
- Hatada, Y.; Kashiwakura, J.; Hayama, K.; Fujisawa, D.; Sasaki-Sakamoto, T.; Terui, T.; Ra, C.; Okayama, Y. Significantly high levels of anti-dsDNA immunoglobulin E in sera and the ability of dsDNA to induce the degranulation of basophils from chronic urticaria patients. Int. Arch. Allergy Immunol. 2013, 161 (Suppl. 2), 154–158. [Google Scholar] [CrossRef]
- Schmetzer, O.; Lakin, E.; Topal, F.A.; Preusse, P.; Freier, D.; Church, M.K.; Maurer, M. IL-24 Is a Common and Specific Autoantigen of IgE in Patients with Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. 2018, 142, 876–882. [Google Scholar] [CrossRef] [Green Version]
- Straesser, M.D.; Oliver, E.; Palacios, T.; Kyin, T.; Patrie, J.; Borish, L.; Saini, S.S.; Lawrence, M.G. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J. Allergy Clin. Immunol. Pract. 2018, 6, 1386–1388.e1. [Google Scholar] [CrossRef]
- Kaplan, A.; Ledford, D.; Ashby, M.; Canvin, J.; Zazzali, J.L.; Conner, E.; Veith, J.; Kamath, N.; Staubach, P.; Jakob, T.; et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J. Allergy Clin. Immunol. 2013, 132, 101–109. [Google Scholar] [CrossRef]
- Maurer, M.; Rosén, K.; Hsieh, H.-J.; Saini, S.; Grattan, C.; Gimenéz-Arnau, A.; Agarwal, S.; Doyle, R.; Canvin, J.; Kaplan, A.; et al. Omalizumab for the Treatment of Chronic Idiopathic or Spontaneous Urticaria. N. Eng. J. Med. 2013, 368, 924–935. [Google Scholar] [CrossRef] [Green Version]
- Gould, H.J.; Sutton, B.J.; Beavil, A.J.; Beavil, R.L.; McCloskey, N.; Coker, H.A.; Fear, D.; Smurthwaite, L. The biology of IGE and the basis of allergic disease. Annu. Rev. Immunol. 2003, 21, 579–628. [Google Scholar] [CrossRef]
- Cheng, L.E.; Hartmann, K.; Roers, A.; Krummel, M.F.; Locksley, R.M. Perivascular Mast Cells Dynamically Probe Cutaneous Blood Vessels to Capture Immunoglobulin E. Immunity 2013, 38, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Conroy, M.C.; Adkinson, N.F., Jr.; Lichtenstein, L.M. Measurement of IgE on human basophils: Relation to serum IgE and anti-IgE-induced histamine release. J. Immunol. 1977, 118, 1317–1321. [Google Scholar]
- Stallman, P.J.; Aalberse, R.C. Estimation of basophil-bound IgE by quantitative immunofluorescence microscopy. Int. Arch. Allergy Appl. Immunol. 1977, 54, 9–18. [Google Scholar] [CrossRef]
- Furuichi, K.; Rivera, J.; Isersky, C. The receptor for immunoglobulin E on rat basophilic leukemia cells: Effect of ligand binding on receptor expression. Proc. Natl. Acad. Sci. USA 1985, 82, 1522–1525. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.; MacGlashan, D., Jr. IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol. Lett. 1996, 52, 129–134. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Lantz, C.S.; Oettgen, H.C.; Katona, I.M.; Fleming, T.; Miyajima, I.; Kinet, J.P.; Galli, S.J. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: Evidence for a novel amplification mechanism in IgE-dependent reactions. J. Exp. Med. 1997, 185, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Kubo, S.; Matsuoka, K.; Taya, C.; Kitamura, F.; Takai, T.; Yonekawa, H.; Karasuyama, H. Drastic up-regulation of Fcepsilonri on mast cells is induced by IgE binding through stabilization and accumulation of Fcepsilonri on the cell surface. J. Immunol. 2001, 167, 3427–3434. [Google Scholar] [CrossRef] [Green Version]
- Asai, K.; Kitaura, J.; Kawakami, Y.; Yamagata, N.; TSAI, M.; Carbone, D.P.; Liu, F.T.; Galli, S.J.; Kawakami, T. Regulation of Mast Cell Survival by IgE. Immunity 2001, 14, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Kalesnikoff, J.; Huber, M.; Lam, V.; Damen, J.E.; Zhang, J.; Siraganian, R.P.; Krystal, G. Monomeric IgE Stimulates Signaling Pathways in Mast Cells That Lead to Cytokine Production and Cell Survival. Immunity 2001, 14, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Kitaura, J.; Song, J.; Tsai, M.; Asai, K.; Maeda-Yamamoto, M.; Mócsai, A.; Kawakami, Y.; Liu, F.-T.; Lowell, C.A.; Barisas, B.G.; et al. Evidence That IgE Molecules Mediate a Spectrum of Effects on Mast Cell Survival and Activation via Aggregation of the FcεRI. Proc. Natl. Acad. Sci. USA 2003, 100, 12911–12916. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Takasu, Y.; Mikura, S.; Satoh, N.; Ichikawa, A. Antigen-Independent Induction of Histamine Synthesis by Immunoglobulin E in Mouse Bone Marrow–Derived Mast Cells. J. Exp. Med. 2002, 196, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Lam, V. IgE Alone Stimulates Mast Cell Adhesion to Fibronectin via Pathways Similar to Those Used by IgE + Antigen but Distinct from Those Used by Steel Factor. Blood 2003, 102, 1405–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, V.; Mihara, S.; Fensome-Green, A.; Bolsover, S.; Cockcroft, S. Monomeric IgE Stimulates NFAT Translocation into the Nucleus, a Rise in Cytosol Ca2+, Degranulation, and Membrane Ruffling in the Cultured Rat Basophilic Leukemia-2H3 Mast Cell Line. J. Immunol. 2004, 172, 4048–4058. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Hori, M.; Tanaka, A.; Matsuda, H.; Karaki, H.; Ozaki, H. IgE Alone-Induced Actin Assembly Modifies Calcium Signaling and Degranulation in RBL-2H3 Mast Cells. Am. J. Physiol. -Cell Physiol. 2004, 286, C256–C263. [Google Scholar] [CrossRef]
- Kitaura, J.; Kinoshita, T.; Matsumoto, M.; Chung, S.; Kawakami, Y.; Leitges, M.; Wu, D.; Lowell, C.A.; Kawakami, T. IgE- and IgE+Ag-Mediated Mast Cell Migration in an Autocrine/Paracrine Fashion. Blood 2005, 105, 3222–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitaura, J.; Xiao, W.; Maeda-Yamamoto, M.; Kawakami, Y.; Lowell, C.A.; Kawakami, T. Early Divergence of Fc Epsilon Receptor I Signals for Receptor Up-Regulation and Internalization from Degranulation, Cytokine Production, and Survival. J. Immunol. 2004, 173, 4317–4323. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Mikura, S.; Hashimoto, E.; Sugimoto, Y.; Ichikawa, A. Ca2+ Influx-Mediated Histamine Synthesis and IL-6 Release in Mast Cells Activated by Monomeric IgE. Eur. J. Immunol. 2005, 35, 460–468. [Google Scholar] [CrossRef]
- Kohno, M.; Yamasaki, S.; Tybulewicz, V.L.J.; Saito, T. Rapid and Large Amount of Autocrine IL-3 Production Is Responsible for Mast Cell Survival by IgE in the Absence of Antigen. Blood 2005, 105, 2059–2065. [Google Scholar] [CrossRef] [Green Version]
- Sakanaka, M.; Kurimune, Y.; Yamada, K.; Hyodo, N.; Natsuhara, M.; Ichikawa, A.; Furuta, K.; Tanaka, S. Down-Modulation of Antigen-Induced Activation of Murine Cultured Mast Cells Sensitized with a Highly Cytokinergic IgE Clone. Immunol. Lett. 2016, 174, 1–8. [Google Scholar] [CrossRef]
- James, L.C.; Roversi, P.; Tawfik, D.S. Antibody Multispecificity Mediated by Conformational Diversity. Science 2003, 299, 1362–1367. [Google Scholar] [CrossRef] [Green Version]
- Bax, H.J.; Bowen, H.; Dodev, T.S.; Sutton, B.J.; Gould, H.J. Mechanism of the Antigen-Independent Cytokinergic SPE-7 IgE Activation of Human Mast Cells in Vitro. Sci. Rep. 2015, 5, 9538-7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, H.J.; Bowen, H.; Beavil, R.L.; Chung, R.; Ward, M.; Davies, A.M.; Dodev, T.S.; McDonnell, J.M.; Beavil, A.J.; Sutton, B.J.; et al. IgE Trimers Drive SPE-7 Cytokinergic Activity. Sci. Rep. 2017, 7, 8164. [Google Scholar] [CrossRef] [Green Version]
- Shade, K.-T.C.; Conroy, M.E.; Washburn, N.; Kitaoka, M.; Huynh, D.J.; Laprise, E.; Patil, S.U.; Shreffler, W.G.; Anthony, R.M. Sialylation of Immunoglobulin E Is a Determinant of Allergic Pathogenicity. Nature 2020, 582, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Sharma, B.B.; Yu, L.; Zuberi, R.; Weng, I.-C.; Kawakami, Y.; Kawakami, T.; Hsu, D.K.; Liu, F.-T. Role of Galectin-3 in Mast Cell Functions: Galectin-3-Deficient Mast Cells Exhibit Impaired Mediator Release and Defective JNK Expression. J. Immunol. 2006, 177, 4991–4997. [Google Scholar] [CrossRef] [Green Version]
- Bambouskova, M.; Polakovicova, I.; Halova, I.; Goel, G.; Draberova, L.; Bugajev, V.; Doan, A.; Utekal, P.; Gardet, A.; Xavier, R.J.; et al. New Regulatory Roles of Galectin-3 in High-Affinity IgE Receptor Signaling. Mol. Cell Biol. 2016, 36, 1366–1382. [Google Scholar] [CrossRef] [Green Version]
- Niki, T.; Tsutsui, S.; Hirose, S.; Aradono, S.; Sugimoto, Y.; Takeshita, K.; Nishi, N.; Hirashima, M. Galectin-9 Is a High Affinity IgE-Binding Lectin with Anti-Allergic Effect by Blocking IgE-Antigen Complex Formation. J. Biol. Chem. 2009, 284, 32344–32352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, S.M. Histamine-releasing factors. Curr. Opin. Immunol. 1996, 8, 778–783. [Google Scholar] [CrossRef]
- Xie, L.; Schroeder, J.T.; Langdon, J.M.; Sora-Scott, R.S.; Kawakami, T.; MacDonald, S.M. Human IgE+ and IgE− Are Not Equivalent to Mouse Highly Cytokinergic IgE. J. Allergy Clin. Immunol. 2008, 121, 1027–1033. [Google Scholar] [CrossRef]
- Kashiwakura, J.; Ando, T.; Matsumoto, K.; Kimura, M.; Kitaura, J.; Matho, M.H.; Zajonc, D.M.; Ozeki, T.; Ra, C.; MacDonald, S.M.; et al. Histamine-Releasing Factor Has a Proinflammatory Role in Mouse Models of Asthma and Allergy. J. Clin. Invest. 2012, 122, 218–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, M.; Helgason, C.D.; Damen, J.E.; Liu, L.; Humphries, R.K.; Krystal, G. The Src Homology 2-Containing Inositol Phosphatase (SHIP) Is the Gatekeeper of Mast Cell Degranulation. Proc. Natl. Acad. Sci. USA 1998, 95, 11330–11335. [Google Scholar] [CrossRef] [Green Version]
- Sly, L.M.; Kalesnikoff, J.; Lam, V.; Wong, D.; Song, C.; Omeis, S.; Chan, K.; Lee, C.W.K.; Siraganian, R.P.; Rivera, J.; et al. IgE-Induced Mast Cell Survival Requires the Prolonged Generation of Reactive Oxygen Species. J. Immunol. 2008, 181, 3850–3860. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Furuta, K.; Teshima, R.; Shirata, N.; Sugimoto, Y.; Ichikawa, A.; Tanaka, S. Critical Role of Protein Kinase C BetaII in Activation of Mast Cells by Monomeric IgE. J. Biol. Chem. 2005, 280, 38976–38981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holowka, D.; Calloway, N.; Cohen, R.; Gadi, D.; Lee, J.; Smith, N.L.; Baird, B. Roles for Ca2+ mobilization and its regulation in mast cell functions. Front. Immunol. 2012, 3, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froghi, S.; Grant, C.R.; Tandon, R.; Quaglia, A.; Davidson, B.; Fuller, B. New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice. Clin. Rev. Allergy Immunol. 2021, 60, 271–292. [Google Scholar] [CrossRef]
- Gonzalez-Espinosa, C.; Odom, S.; Olivera, A.; Hobson, J.P.; Martinez, M.E.C.; Oliveira-dos-Santos, A.; Barra, L.; Spiegel, S.; Penninger, J.M.; Rivera, J. Preferential Signaling and Induction of Allergy-Promoting Lymphokines Upon Weak Stimulation of the High Affinity IgE Receptor on Mast Cells. J. Exp. Med. 2003, 197, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Leach, S.; Liu, W.; Ralston, E.; Scheffel, J.; Zhang, W.; Lowell, C.A.; Rivera, J. Molecular Editing of Cellular Responses by the High-Affinity Receptor for IgE. Science 2014, 343, 1021–1025. [Google Scholar] [CrossRef] [Green Version]
- Bryce, P.J.; Miller, M.L.; Miyajima, I.; Tsai, M.; Galli, S.J.; Oettgen, H.C. Immune Sensitization in the Skin Is Enhanced by Antigen-Independent Effects of IgE. Immunity 2004, 20, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.F.; West, G.B. The presence of histamine in tissue mast cells. J. Physiol. 1953, 120, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Schayer, R.W. The origin and fate of histamine in the body. In Wolstenholme GEW; O’Connor, C.M., Ed.; Ciba Foundation Symposium on Histamine. J. and A. Churchill Ltd.: London, UK, 1956; pp. 183–188. [Google Scholar]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.S.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef] [Green Version]
- MacGlashan, D. Histamine: A mediator of inflammation. J. Allergy Clin. Immunol. 2003, 112, S53–S59. [Google Scholar] [CrossRef]
- Schaper-Gerhardt, K.; Roßbach, K.; Nikolouli, E.; Werfel, T.; Gutzmer, R.; Mommert, S. The Role of the Histamine H4 receptor in Atopic Dermatitis and Psoriasis. Br. J. Pharmacol. 2020, 177, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Thurmond, R.L.; Gelfand, E.W.; Dunford, P.J. The Role of Histamine H1 and H4 Receptors in Allergic Inflammation: The Search for New Antihistamines. Nat. Rev. Drug Discov. 2008, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, Y.; Hirasawa, N. The Antagonism of Histamine H1 and H4 Receptors Ameliorates Chronic Allergic Dermatitis via Anti-pruritic and Anti-inflammatory Effects in NC/Nga Mice. Allergy 2012, 67, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, A.; Sugimoto, Y.; Tanaka, S. Molecular Biology of Histidine Decarboxylase and Prostaglandin Receptors. Proc. Jpn. Acad. Ser. B 2010, 86, 848–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Nemoto, K.; Yamamura, E.; Ohmura, S.; Ichikawa, A. Degradation of the 74 KDa Form of L-Histidine Decarboxylase via the Ubiquitin-Proteasome Pathway in a Rat Basophilic/Mast Cell Line (RBL-2H3). FEBS Lett. 1997, 417, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Furuta, K.; Nakayama, K.; Sugimoto, Y.; Ichikawa, A.; Tanaka, S. Activation of Histidine Decarboxylase through Post-Translational Cleavage by Caspase-9 in a Mouse Mastocytoma P-815. J. Biol. Chem. 2007, 282, 13438–13446. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Nemoto, K.; Yamamura, E.; Ichikawa, A. Intracellular Localization of the 74- and 53-KDa Forms Of l-Histidine Decarboxylase in a Rat Basophilic/Mast Cell Line, RBL-2H3. J. Biol. Chem. 1998, 273, 8177–8182. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Deai, K.; Konomi, A.; Takahashi, K.; Yamane, H.; Sugimoto, Y.; Ichikawa, A. Expression Of L-Histidine Decarboxylase in Granules of Elicited Mouse Polymorphonuclear Leukocytes. Eur. J. Immunol. 2004, 34, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, H.; Tanaka, S.; Terui, T.; Hori, Y.; Makabe-Kobayashi, Y.; Pejler, G.; Tchougounova, E.; Hellman, L.; Gertsenstein, M.; Hirasawa, N.; et al. Mice Lacking Histidine Decarboxylase Exhibit Abnormal Mast Cells. FEBS Lett. 2001, 502, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Ohtsu, H.; Kuramasu, A.; Tanaka, S.; Terui, T.; Hirasawa, N.; Hara, M.; Makabe-Kobayashi, Y.; Yamada, N.; Yanai, K.; Sakurai, E.; et al. Plasma Extravasation Induced by Dietary Supplemented Histamine in Histamine-Free Mice. Eur. J. Immunol. 2002, 32, 1698–1708. [Google Scholar] [CrossRef]
- Morin, F.; Singh, N.; Mdzomba, J.B.; Dumas, A.; Pernet, V.; Vallières, L. Conditional Deletions of Hdc Confirm Roles of Histamine in Anaphylaxis and Circadian Activity but Not in Autoimmune Encephalomyelitis. J. Immunol. 2021, 206, 2029–2037. [Google Scholar] [CrossRef]
- Yang, X.D.; Ai, W.; Asfaha, S.; Bhagat, G.; Friedman, R.A.; Jin, G.; Park, H.; Shykind, B.; Diacovo, T.G.; Falus, A.; et al. Histamine Deficiency Promotes Inflammation-Associated Carcinogenesis through Reduced Myeloid Maturation and Accumulation of CD11b+Ly6G+ Immature Myeloid Cells. Nat. Med. 2010, 17, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Takai, J.; Ohtsu, H.; Sato, A.; Uemura, S.; Fujimura, T.; Yamamoto, M.; Moriguchi, T. Lipopolysaccharide-Induced Expansion of Histidine Decarboxylase-Expressing Ly6G+ Myeloid Cells Identified by Exploiting Histidine Decarboxylase BAC-GFP Transgenic Mice. Sci. Rep. 2019, 9, 15603–15612. [Google Scholar] [CrossRef]
- Humphries, D.E.; Wong, G.W.; Friend, D.S.; Gurish, M.F.; Qiu, W.T.; Huang, C.; Sharpe, A.H.; Stevens, R.L. Heparin Is Essential for the Storage of Specific Granule Proteases in Mast Cells. Nature 1999, 400, 769–772. [Google Scholar] [CrossRef]
- Forsberg, E.; Pejler, G.; Ringvall, M.; Lunderius, C.; Tomasini-Johansson, B.; Kusche-Gullberg, M.; Eriksson, I.; Ledin, J.; Hellman, L.; Kjellen, L. Abnormal Mast Cells in Mice Deficient in a Heparin-Synthesizing Enzyme. Nature 1999, 400, 773–776. [Google Scholar] [CrossRef]
- Nakazawa, S.; Sakanaka, M.; Furuta, K.; Natsuhara, M.; Takano, H.; Tsuchiya, S.; Okuno, Y.; Ohtsu, H.; Nishibori, M.; Thurmond, R.L.; et al. Histamine Synthesis Is Required for Granule Maturation in Murine Mast Cells. Eur. J. Immunol. 2014, 44, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Levi-Schaffer, F.; Austen, K.F.; Gravallese, P.M.; Stevens, R.L. Coculture of Interleukin 3-Dependent Mouse Mast Cells with Fibroblasts Results in a Phenotypic Change of the Mast Cells. Proc. Natl. Acad. Sci. USA 1986, 83, 6485–6488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, H.; Nakazawa, S.; Okuno, Y.; Shirata, N.; Tsuchiya, S.; Kainoh, T.; Takamatsu, S.; Furuta, K.; Taketomi, Y.; Naito, Y.; et al. Establishment of the Culture Model System That Reflects the Process of Terminal Differentiation of Connective Tissue-Type Mast Cells. FEBS Lett. 2008, 582, 1444–1450. [Google Scholar] [CrossRef] [Green Version]
- Wernersson, S.; Pejler, G. Mast Cell Secretory Granules: Armed for Battle. Nat. Rev. Immunol. 2014, 14, 1–17. [Google Scholar] [CrossRef]
- Rabenstein, D.L.; Bratt, P.; Peng, J. Quantitative Characterization of the Binding of Histamine by Heparin. Biochemistry 1998, 37, 14121–14127. [Google Scholar] [CrossRef]
- Schneider, E.; Machavoine, F.; Pleau, J.-M.; Bertron, A.-F.; Thurmond, R.L.; Ohtsu, H.; Watanabe, T.; Schinkel, A.H.; Dy, M. Organic Cation Transporter 3 Modulates Murine Basophil Functions by Controlling Intracellular Histamine Levels. J. Exp. Med. 2005, 202, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Fesus, L.; Szucs, E.F.; Barrett, K.E.; Metcalfe, D.D.; Folk, J.E. Activation of Transglutaminase and Production of Protein-bound g-Glutamylhistamine in Stimulated Mouse Mast Cells. J. Biol. Chem. 1985, 260, 13771–13778. [Google Scholar] [CrossRef]
- Folk, J.E. Transglutaminases. Annu. Rev. Biochem. 1980, 49, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Vowinckel, J.; Stahlberg, S.; Paulmann, N.; Bluemlein, K.; Grohmann, M.; Ralser, M.; Walther, D.J. Histaminylation of Glutamine Residues Is a Novel Posttranslational Modification Implicated in G-protein Signaling. FEBS Lett. 2012, 586, 3819–3824. [Google Scholar] [CrossRef] [Green Version]
- Mackey, E.; Thelen, K.M.; Bali, V.; Fardisi, M.; Trowbridge, M.; Jordan, C.L.; Moeser, A.J. Perinatal Androgens Organize Sex Differences in Mast Cells and Attenuate Anaphylaxis Severity into Adulthood. Proc. Natl. Acad. Sci. USA 2020, 117, 23751–23761. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.; Alenina, N.; Andrade-Navarro, M.A.; Santos, R.A. Mas and Its Related G Protein–Coupled Receptors, Mrgprs. Pharmacol. Rev. 2014, 66, 1080–1105. [Google Scholar] [CrossRef] [PubMed]
- Ali, H. Emerging Roles for MAS-Related G Protein-Coupled Receptor-X2 in Host Defense Peptide, Opioid, and Neuropeptide-Mediated Inflammatory Reactions. Adv. Immunol. 2017, 136, 123–162. [Google Scholar] [CrossRef] [PubMed]
- Kühn, H.; Kolkhir, P.; Babina, M.; Düll, M.; Frischbutter, S.; Fok, J.S.; Jiao, Q.; Metz, M.; Scheffel, J.; Wolf, K.; et al. Mas-Related G Protein–Coupled Receptor X2 and Its Activators in Dermatologic Allergies. J. Allergy Clin. Immunol. 2020, 147, 456–469. [Google Scholar] [CrossRef]
- Molino, M.; Barnathan, E.S.; Numerof, R.; Clark, J.; Dreyer, M.; Cumashi, A.; Hoxie, J.A.; Schechter, N.; Woolkalis, M.; Brass, L.F. Interactions of Mast Cell Tryptase with Thrombin Receptors and PAR-2. J. Biol. Chem. 1997, 272, 4043–4049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, H.; Schmidt, V.A.; Derian, C.K.; Jesty, J.; Bahou, W.F. Mitogenic Responses Mediated Through the Proteinase-Activated Receptor-2 Are Induced by Expressed Forms of Mast Cell α- or β-Tryptases. Blood 1997, 90, 3914–3922. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Sinnamon, M.J.; Lyng, G.D.; Glickman, J.N.; Wang, X.; Xing, W.; Krilis, S.A.; Blumberg, R.S.; Adachi, R.; Lee, D.M.; et al. Essential Role for Mast Cell Tryptase in Acute Experimental Colitis. Proc. Natl. Acad. Sci. USA 2011, 108, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Dahlin, J.S.; Feinstein, R.; Bankova, L.G.; Xing, W.; Shin, K.; Gurish, M.F.; Hallgren, J. Mouse Mast Cell Protease-6 and MHC Are Involved in the Development of Experimental Asthma. J. Immunol. 2014, 193, 4783–4789. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Daubeuf, F.; Pejler, G.; Åbrink, M.; Frossard, N. Mast Cells Contribute to Bleomycin-Induced Lung Inflammation and Injury in Mice through a Chymase/Mast Cell Protease 4-Dependent Mechanism. J. Immunol. 2014, 192, 1847–1854. [Google Scholar] [CrossRef] [Green Version]
- Waern, I.; Jonasson, S.; Hjoberg, J.; Bucht, A.; Abrink, M.; Pejler, G.; Wernersson, S. Mouse Mast Cell Protease 4 Is the Major Chymase in Murine Airways and Has a Protective Role in Allergic Airway Inflammation. J. Immunol. 2009, 183, 6369–6376. [Google Scholar] [CrossRef] [Green Version]
- Waern, I.; Lundequist, A.; Pejler, G.; Wernersson, S. Mast Cell Chymase Modulates IL-33 Levels and Controls Allergic Sensitization in Dust-Mite Induced Airway Inflammation. Mucosal Immunol. 2013, 6, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.; Wedemeyer, J.; Metz, M.; Piliponsky, A.M.; Weller, K.; Chatterjea, D.; Clouthier, D.E.; Yanagisawa, M.M.; Tsai, M.; Galli, S.J. Mast Cells Promote Homeostasis by Limiting Endothelin-1-Induced Toxicity. Nature 2004, 432, 512–516. [Google Scholar] [CrossRef]
- Schneider, L.A.; Schlenner, S.M.; Feyerabend, T.B.; Wunderlin, M.; Rodewald, H.R. Molecular Mechanism of Mast Cell Mediated Innate Defense against Endothelin and Snake Venom Sarafotoxin. J. Exp. Med. 2007, 204, 2629–2639. [Google Scholar] [CrossRef]
- Oschatz, C.; Maas, C.; Lecher, B.; Jansen, T.; Björkqvist, J.; Tradler, T.; Sedlmeier, R.; Burfeind, P.; Cichon, S.; Hammerschmidt, S.; et al. Mast Cells Increase Vascular Permeability by Heparin-Initiated Bradykinin Formation In Vivo. Immunity 2011, 34, 258–268. [Google Scholar] [CrossRef]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE Mediated Mast Cell Activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Lagunoff, D.; Martin, T.W.; Read, G. Agents That Release Histamine from Mast Cells. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 331–351. [Google Scholar] [CrossRef]
- Ferry, X.; Brehin, S.; Kamel, R.; Landry, Y. G Protein-Dependent Activation of Mast Cell by Peptides and Basic Secretagogues. Peptides 2002, 23, 1507–1515. [Google Scholar] [CrossRef]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-Independent Activation of Mast Cell Is Mediated by Mrg Receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-Related Gene X2 on Mast Cells Is Upregulated in the Skin of Patients with Severe Chronic Urticaria. J. Allergy Clin. Immunol. 2014, 134, 622.e9–633.e9. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Han, S.; Zylka, M.J.; Simon, M.I.; Anderson, D.J. A Diverse Family of GPCRs Expressed in Specific Subsets of Nociceptive Sensory Neurons. Cell 2001, 106, 619–632. [Google Scholar] [CrossRef] [Green Version]
- Zylka, M.J.; Dong, X.; Southwell, A.L.; Anderson, D.J. Atypical Expansion in Mice of the Sensory Neuron-Specific Mrg G Protein-Coupled Receptor Family. Proc. Natl. Acad. Sci. USA 2003, 100, 10043–10048. [Google Scholar] [CrossRef] [Green Version]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a Mast-Cell-Specific Receptor Crucial for Pseudo-Allergic Drug Reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Sato, H.; Sakamaki, K.; Kamada, M.; Okuno, Y.; Fukuishi, N.; Furuta, K.; Tanaka, S. Suppression of IgE-Independent Degranulation of Murine Connective Tissue-Type Mast Cells by Dexamethasone. Cells 2019, 8, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House Dust Mites Activate Nociceptor–Mast Cell Clusters to Drive Type 2 Skin Inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Byrne, M.C.; Paranavitana, N.C.; Aronow, B.; Siefring, J.E.; D’Souza, M.; Horton, H.F.; Quilliam, L.A.; Williams, D.A. Rac2, a Hematopoiesis-Specific Rho GTPase, Specifically Regulates Mast Cell Protease Gene Expression in Bone Marrow-Derived Mast Cells. Mol. Cell. Biol. 2002, 22, 7645–7657. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, S.; Furuta, K. Roles of IgE and Histamine in Mast Cell Maturation. Cells 2021, 10, 2170. https://doi.org/10.3390/cells10082170
Tanaka S, Furuta K. Roles of IgE and Histamine in Mast Cell Maturation. Cells. 2021; 10(8):2170. https://doi.org/10.3390/cells10082170
Chicago/Turabian StyleTanaka, Satoshi, and Kazuyuki Furuta. 2021. "Roles of IgE and Histamine in Mast Cell Maturation" Cells 10, no. 8: 2170. https://doi.org/10.3390/cells10082170
APA StyleTanaka, S., & Furuta, K. (2021). Roles of IgE and Histamine in Mast Cell Maturation. Cells, 10(8), 2170. https://doi.org/10.3390/cells10082170