Synthesis of Ziziphus spina-christi (Jujube) Root Methanol Extract Loaded Functionalized Silver Nanoparticle (ZS-Ag-NPs); Physiochemical Characterization and Effect of ZS-Ag-NPs on Adipocyte Maturation, Adipokine and Vascular Smooth Muscle Cell Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Preparation and Composition Analysis
2.1.1. Preparation of Ziziphus spina-christi (L.) (Jujube, Sidhr) Root Methanol Extract
2.1.2. GC-MS Analysis of Ziziphus spina-christi Methanol Root Extract
2.2. Synthesis of Ziziphus spina-christi Root Methanol Extract Loaded Silver Nanoparticles
Characterization of Silver Nanoparticles (ZS-Ag-NPs)
2.3. Biology
2.3.1. Chemicals
2.3.2. hMSCs Culture and Adipocyte Differentiation
2.3.3. Cytotoxicity Analysis
2.3.4. Experimental Design
2.3.5. Interaction of ZS-Ag-NPs Treated Adipokines with HUVECs
2.3.6. Oil Red’O and Nile Red Staining Analysis to Determine Lipid Accumulation Levels
2.3.7. Analysis of Mitochondrial Membrane Potential Using JC-1 Staining
2.3.8. Propidium Iodide Staining for Nuclear Damage Analysis in HUVECs
2.3.9. Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.3.10. Quantification of Protein Using ELISA
2.4. Statistical Analysis
3. Results
3.1. GC-MS Analysis of Ziziphus spina-christi Root Methanol Extract
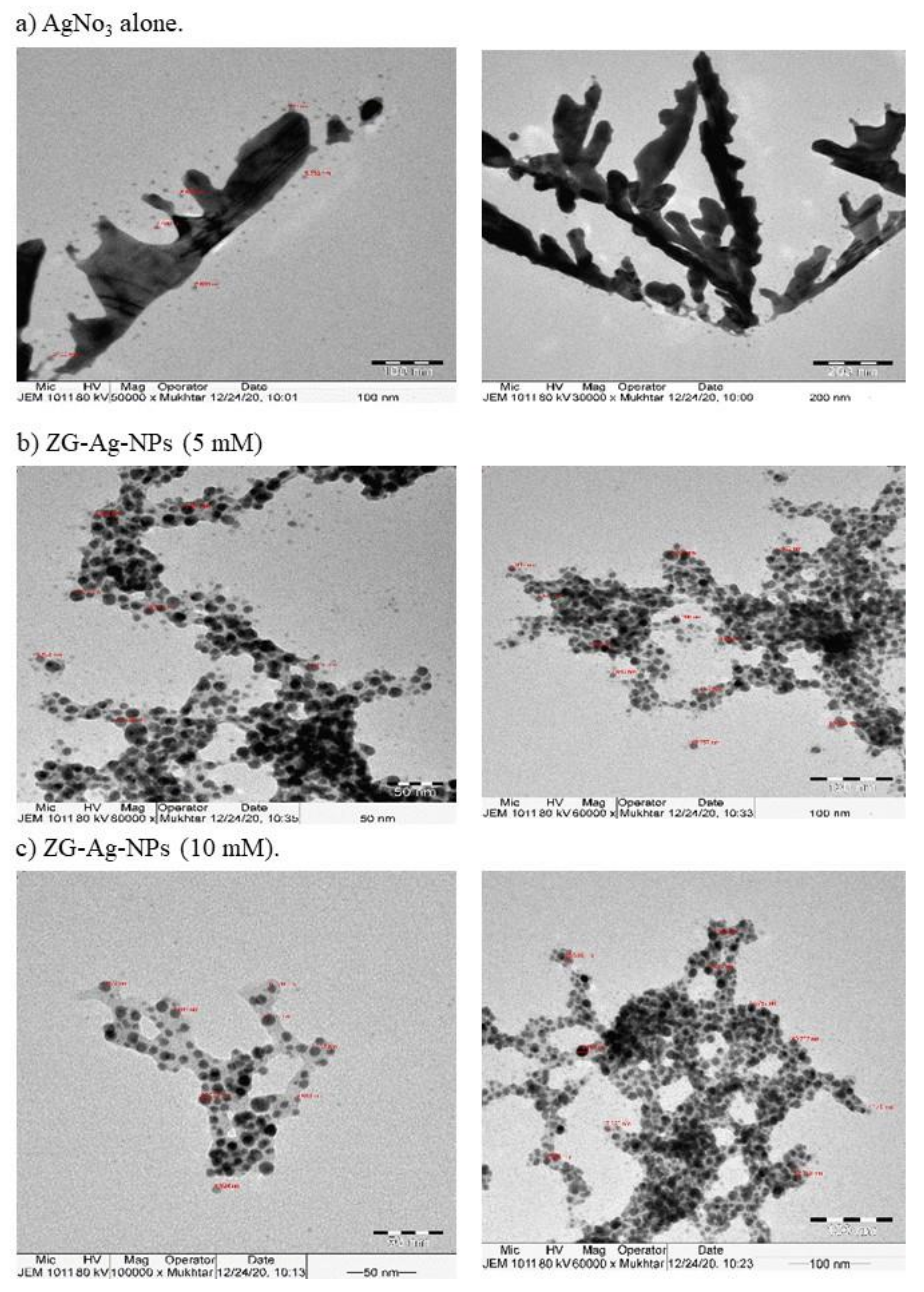
3.2. Characterization of Silver Nanoparticles
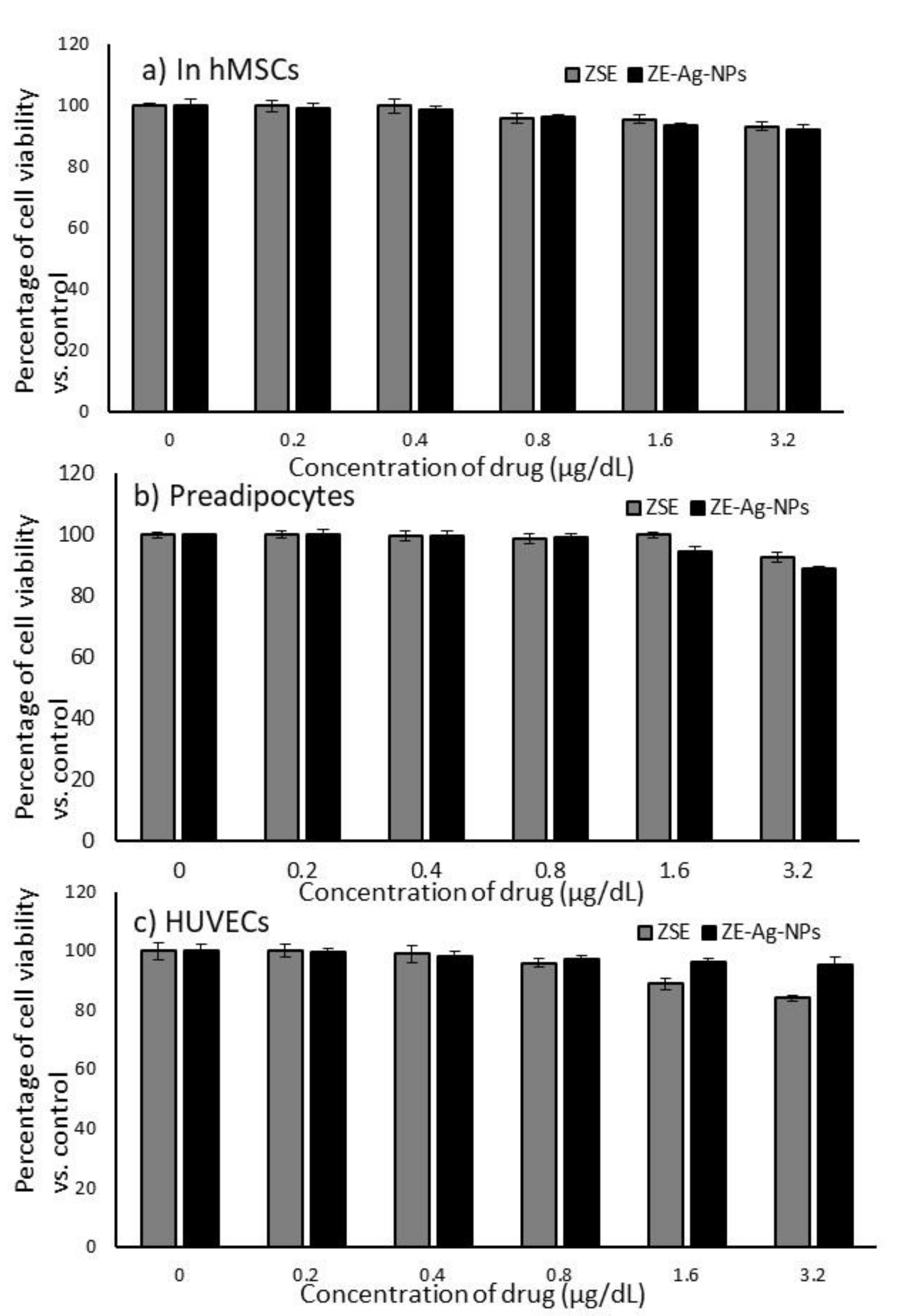
3.3. Cytotoxic Effects of ZSE and ZS-Ag-NPs on hMSCs, Preadipocytes and HUVECs
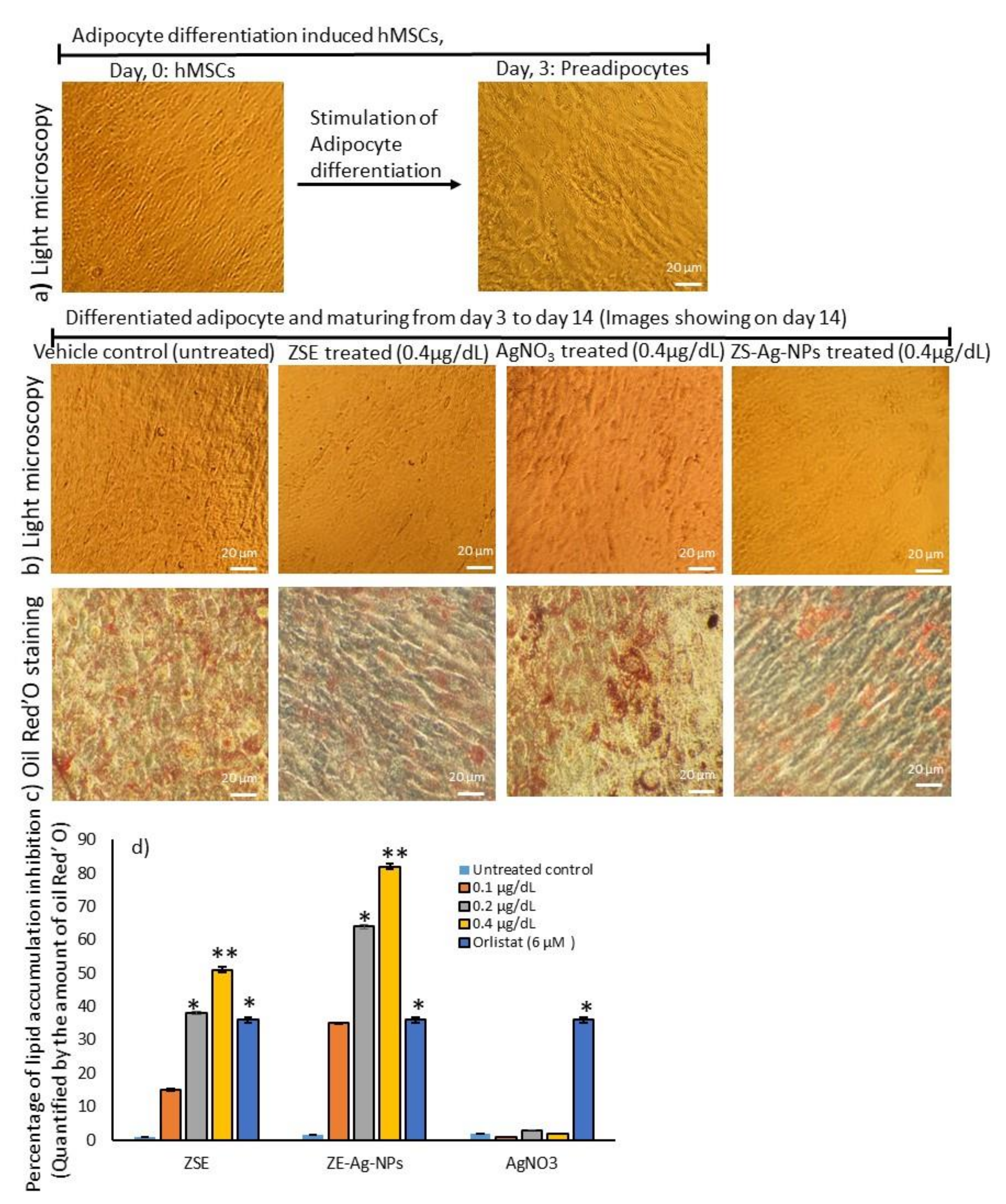
3.4. Dose Determination Based on Lipid Accumulation Inhibitory Potential Using Oil Red’O Staining Analysis
3.5. Determination of Hypertrophic Adipocytes Using Nile Red Fluorescence Staining Analysis
3.6. Mitochondrial Membrane Potential (JC-1) and Oxidative Capacity Analysis
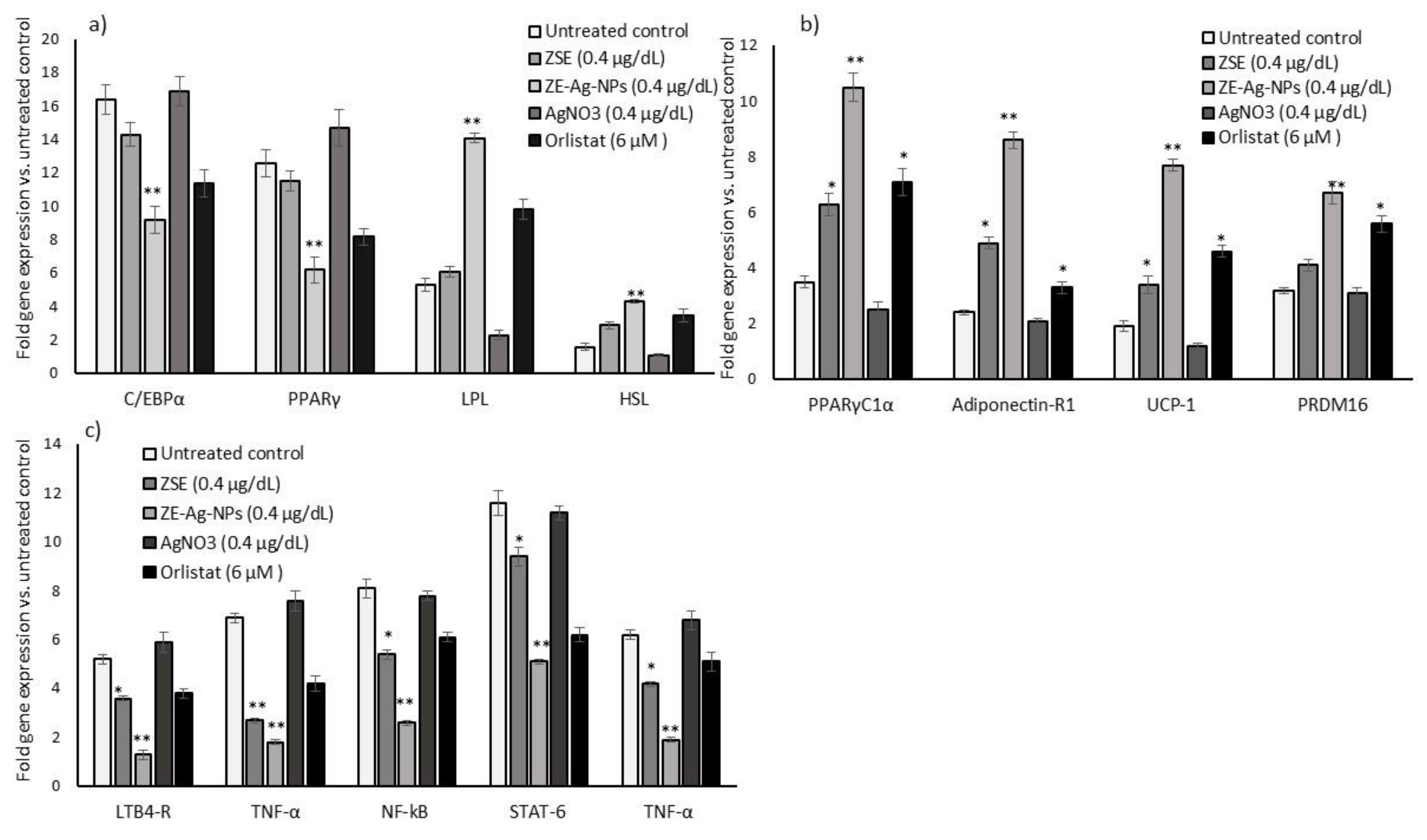
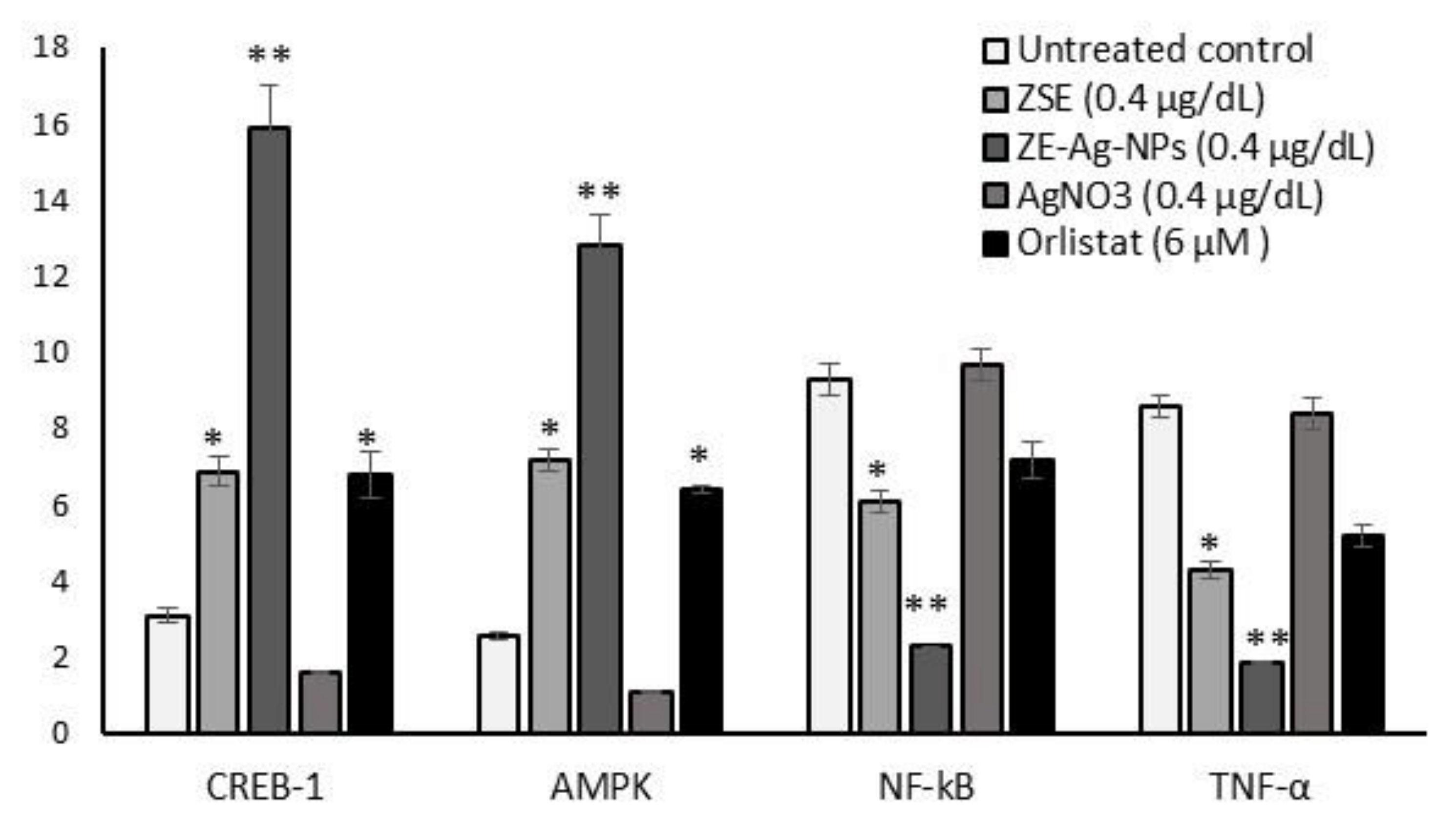
3.7. Adipogenesis, Mitochondrial Thermogenesis, and Inflammatory Gene Expression Analysis in ZSE and ZS-Ag-NPs Treated Adipocytes
3.8. Protein Levels in Adipocyte’s Stromal Vascular Fraction (SVF)
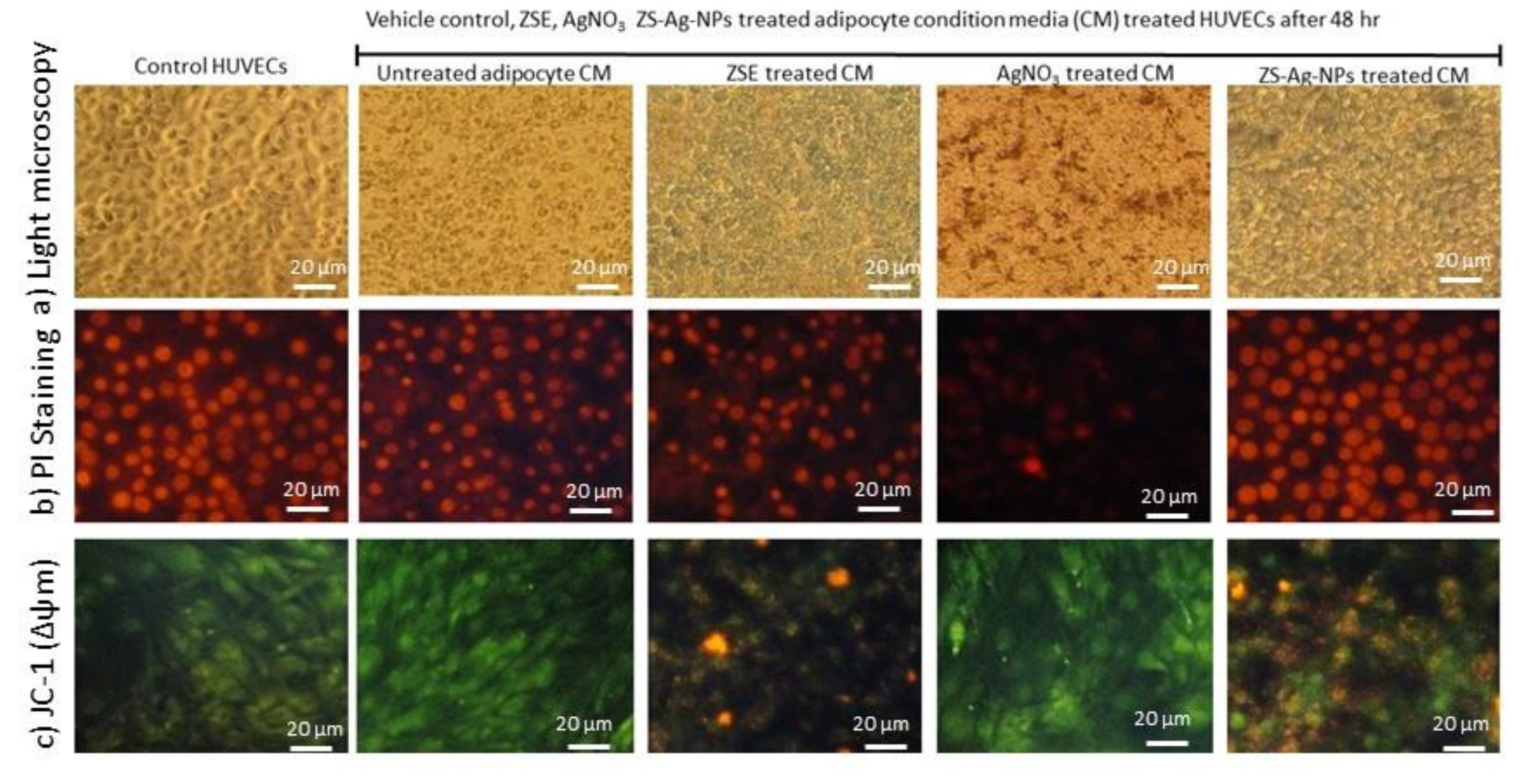
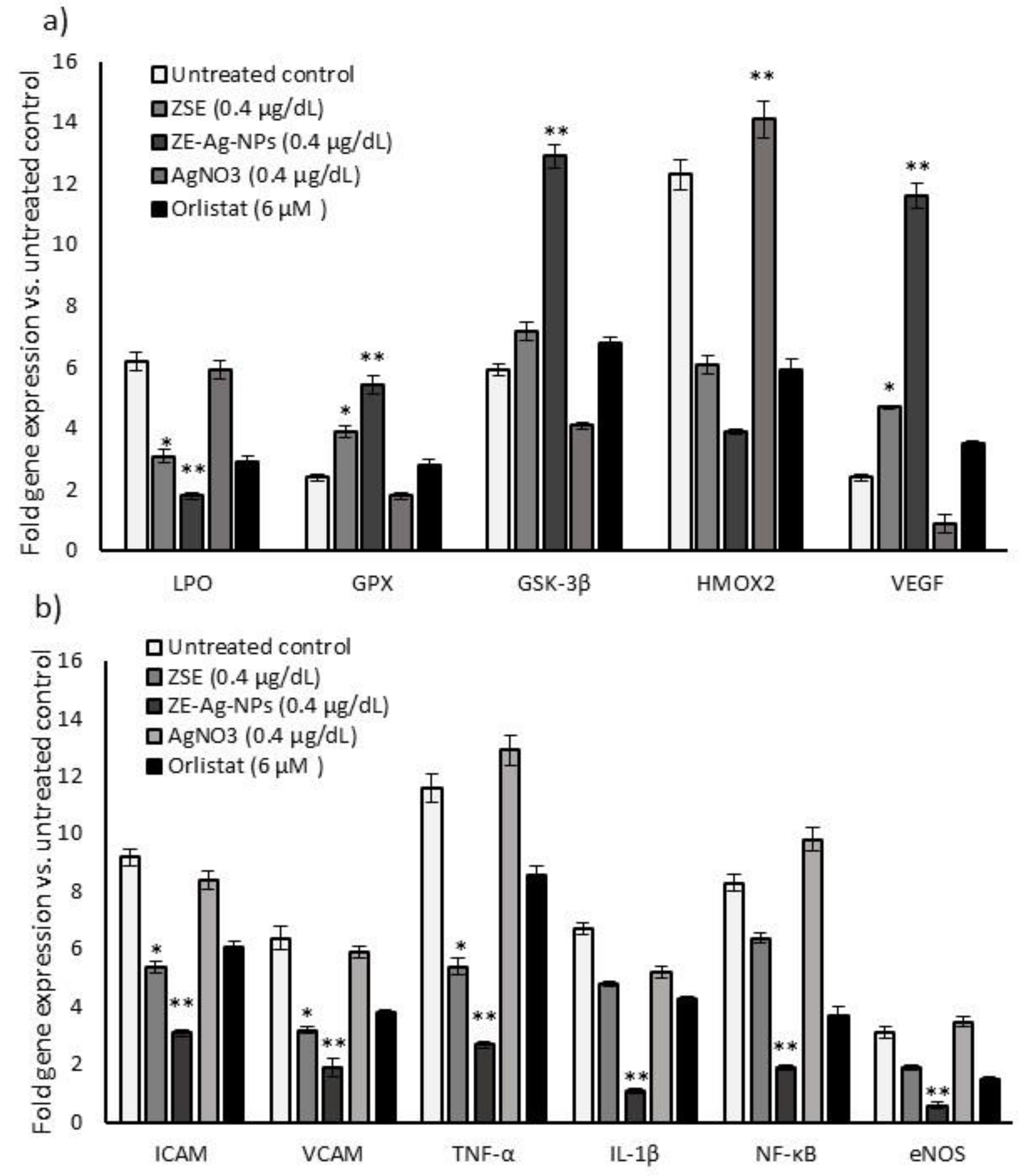
3.9. Effect of ZSE and ZS-Ag-NPs Treated Adipocyte Condition Media on HUVECs Nuclear Damage and Angiogenesis Potential
3.9.1. Light Microscopy, Propidium Iodide Staining for Morphology and JC-1 Staining for Mitochondrial Membrane Potential (Δψm) Analysis
3.9.2. Quantification of Gene Expression Levels in HUVECs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lau, W.B.; Ohashi, K.; Wang, Y.; Ogawa, H.; Murohara, T.; Ma, X.-L.; Ouchi, N. Role of adipokines in cardiovascular disease. Circ. J. 2017, 81, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.R.; Ansari, H.S. Anti-obesity effect of arq zeera and its main components thymol and cuminaldehyde in high fat diet induced obese rats. Drug Res. 2018, 68, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Bielecka-Dabrowa, A.; Bartlomiejczyk, M.A.; Sakowicz, A.; Maciejewski, M.; Banach, M. The role of adipokines in the development of arterial stiffness and hypertension. Angiology 2020, 71, 754–761. [Google Scholar] [CrossRef]
- Breyer, M.K.; Rutten, E.P.; Locantore, N.W.; Watkins, M.L.; Miller, B.E.; Wouters, E.F.; Investigators, E. Dysregulated adipokine metabolism in chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2012, 42, 983–991. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Deng, R.; Wang, N.; Zhang, Y.; Wang, Y.; Liu, Y.; Li, F.; Wang, X.; Zhou, L. Berberine suppresses adipocyte differentiation via decreasing CREB transcriptional activity. PLoS ONE 2015, 10, e0125667. [Google Scholar]
- Moon, Y.; Tong, T.; Kang, W.; Park, T. Filbertone ameliorates adiposity in mice fed a high-fat diet via activation of cAMP signaling. Nutrients 2019, 11, 1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.-T.; Wang, Y.-S.; Chou, H.-C.; Chang, C.-C.; Lee, C.-K.; Juan, S.-H. Kinsenoside-mediated lipolysis through an AMPK-dependent pathway in C3H10T1/2 adipocytes: Roles of AMPK and PPARα in the lipolytic effect of kinsenoside. Phytomedicine 2015, 22, 641–647. [Google Scholar] [CrossRef]
- Allison, D.B.; Gadde, K.M.; Garvey, W.T.; Peterson, C.A.; Schwiers, M.L.; Najarian, T.; Tam, P.Y.; Troupin, B.; Day, W.W. Controlled-release phentermine/topiramate in severely obese adults: A randomized controlled trial (EQUIP). Obesity 2012, 20, 330–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincer, Y.; Yuksel, S. Anti-OBESITY Effects of Phytochemicals from an Epigenetic Perspective. Nutrition 2020, 84, 111119. [Google Scholar] [CrossRef]
- Cheng, Y.-A.; Chen, I.-J.; Su, Y.-C.; Cheng, K.-W.; Lu, Y.-C.; Lin, W.-W.; Hsieh, Y.-C.; Kao, C.-H.; Chen, F.-M.; Roffler, S.R. Enhanced drug internalization and therapeutic efficacy of PEGylated nanoparticles by one-step formulation with anti-mPEG bispecific antibody in intrinsic drug-resistant breast cancer. Biomater. Sci. 2019, 7, 3404–3417. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Alvi, A.M.; Shah, F.A. Potential and applications of nanocarriers for efficient delivery of biopharmaceuticals. Pharmaceutics 2020, 12, 1184. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, L.; Deng, H.; Zhang, Z. Structural characterization and stability study of green synthesized starch stabilized silver nanoparticles loaded with isoorientin. Food Chem. 2021, 338, 127807. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.A.; Raafat, D.; El-Gowelli, H.M.; El-Kamel, A.H. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: Characterization and antimicrobial properties. Int. J. Nanomed. 2015, 10, 7207. [Google Scholar]
- Sati, S.C.; Kour, G.; Bartwal, A.S.; Sati, M.D. Biosynthesis of Metal Nanoparticles from Leaves of Ficus palmata and Evaluation of Their Anti-inflammatory and Anti-diabetic Activities. Biochemistry 2020, 59, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Shin, H.-S. Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. Int. J. Nanomed. 2019, 14, 6679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subash-Babu, P.; Alshatwi, A.A. Ononitol monohydrate enhances PRDM16 & UCP-1 expression, mitochondrial biogenesis and insulin sensitivity via STAT6 and LTB4R in maturing adipocytes. Biomed. Pharmacother. 2018, 99, 375–383. [Google Scholar] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara-Tsujii, N.; Yamagata, N.; Takeda, T.; Mizunami, M.; Yamaoka, R. Behavioral responses to the alarm pheromone of the ant Camponotus obscuripes (Hymenoptera: Formicidae). Zool. Sci. 2006, 23, 353–358. [Google Scholar] [CrossRef]
- Saniewski, M.; Horbowicz, M.; Kanlayanarat, S. The biological activities of troponoids and their use in agriculture. A review. J. Hortic. Res. 2014, 22. [Google Scholar] [CrossRef] [Green Version]
- Bhowmik, A.; Das, S.; Sarkar, W.; Saidalavi, K.; Mishra, A.; Roy, A.; Deb, I. Diastereoselective Spirocyclization via Intramolecular C (sp3)− H Bond Functionalization Triggered by Sequential [1,5]-Hydride Shift/Cyclization Process: Approach to Spiro-tetrahydroquinolines. Adv. Synth. Catal. 2021, 363, 826–832. [Google Scholar] [CrossRef]
- Schoepfer, H.; Nestelberger, T.; Boeddinghaus, J.; Twerenbold, R.; Lopez-Ayala, P.; Koechlin, L.; Wussler, D.; Zimmermann, T.; Miro, O.; Martín-Sánchez, J.F. Effect of a Proposed Modification of the Type 1 and Type 2 Myocardial Infarction Definition on Incidence and Prognosis. Circulation 2020, 142, 2083–2085. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Rawal, R.K.; Malhotra, D.; Dhingra, R.; Deep, A.; Sharma, P.C. Synthesis, characterization and pharmacological evaluation of (Z)-2-(5-(biphenyl-4-yl)-3-(1-(imino) ethyl)-2, 3-dihydro-1, 3, 4-oxadiazol-2-yl) phenol derivatives as potent antimicrobial and antioxidant agents. Arab. J. Chem. 2017, 10, S1022–S1031. [Google Scholar] [CrossRef] [Green Version]
- Karjalainen, A.; Doan, P.; Chandraseelan, J.G.; Sandberg, O.; Yli-Harja, O.; Candeias, N.R.; Kandhavelu, M. Synthesis of phenol-derivatives and biological screening for anticancer activity. Anti-Cancer Agents Med. Chem. 2017, 17, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P. Phytoalexins: Current progress and future prospects. Molecules 2015, 20, 2770. [Google Scholar] [CrossRef]
- Mahadevan, A.; Siegel, C.; Martin, B.R.; Abood, M.E.; Beletskaya, I.; Razdan, R.K. Novel cannabinol probes for CB1 and CB2 cannabinoid receptors. J. Med. Chem. 2000, 43, 3778–3785. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147, S163–S171. [Google Scholar] [CrossRef] [Green Version]
- Hofer, S.; Geisler, S.; Lisandrelli, R.; Nguyen Ngoc, H.; Ganzera, M.; Schennach, H.; Fuchs, D.; Fuchs, J.E.; Gostner, J.M.; Kurz, K. Pharmacological Targets of Kaempferol Within Inflammatory Pathways—A Hint Towards the Central Role of Tryptophan Metabolism. Antioxidants 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aziz, M.; A Metwally, K.; Gamal-Eldeen, A.M.; M Aly, O. 1,3,4-oxadiazole-2-thione derivatives; novel approach for anticancer and tubulin polymerization inhibitory activities. Anti-Cancer Agents Med. Chem. 2016, 16, 269–277. [Google Scholar] [CrossRef]
- Khan, K.M.; Fatima, N.; Rasheed, M.; Jalil, S.; Ambreen, N.; Perveen, S.; Choudhary, M.I. 1,3,4-Oxadiazole-2 (3H)-thione and its analogues: A new class of non-competitive nucleotide pyrophosphatases/phosphodiesterases 1 inhibitors. Bioorganic Med. Chem. 2009, 17, 7816–7822. [Google Scholar] [CrossRef]
- Kumar, S.; Ritika. A brief review of the biological potential of indole derivatives. Future J. Pharm. Sci. 2020, 6, 121. [Google Scholar] [CrossRef]
- Gamir, J.; Pastor, V.; Cerezo, M.; Flors, V. Identification of indole-3-carboxylic acid as mediator of priming against Plectosphaerella cucumerina. Plant Physiol. Biochem. 2012, 61, 169–179. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Patil, S.A. Recent developments in biological activities of indanones. Eur. J. Med. Chem. 2017, 138, 182–198. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.; Chen, H.; Fang, B.; Qiu, Y.; Chen, X.; Chen, L.; Shu, S.; Zhang, Y.; Zhao, Y. Design, synthesis, and structure–activity relationships of 2-benzylidene-1-indanone derivatives as anti-inflammatory agents for treatment of acute lung injury. Drug Des. Dev. Ther. 2018, 12, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munín, J.; Quezada, E.; Cuiñas, A.; Campos-Toimil, M.; Uriarte, E.; Santana, L.; Viña, D. Synthesis, biological evaluation and structure–activity relationships of new phthalazinedione derivatives with vasorelaxant activity. Eur. J. Med. Chem. 2014, 82, 407–417. [Google Scholar] [CrossRef]
- Khalil, E.-G.A.M.; Berghot, M.A.; Gouda, M.A. Design, synthesis and antibacterial activity of new phthalazinedione derivatives. J. Serb. Chem. Soc. 2011, 76, 329–339. [Google Scholar] [CrossRef]
- Madriz, L.; Vargas, R. Key aspects of surface plasmon resonance spectroscopy for analytical chemistry applications. J. Anal. Pharm. Res. 2018, 7, 412–415. [Google Scholar] [CrossRef]
- Shehzad, A.; Qureshi, M.; Jabeen, S.; Ahmad, R.; Alabdalall, A.H.; Aljafary, M.A.; Al-Suhaimi, E. Synthesis, characterization and antibacterial activity of silver nanoparticles using Rhazya stricta. PeerJ 2018, 6, e6086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, M.F.; Eisa, W.H.; Abdel-Moneam, Y.K.; El-Kousy, S.M.; Atia, A. Ziziphus spina-christi based bio-synthesis of Ag nanoparticles. J. Ind. Eng. Chem. 2015, 23, 50–56. [Google Scholar] [CrossRef]
- De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Revealing the importance of aging, environment, size and stabilization mechanisms on the stability of metal nanoparticles: A case study for silver nanoparticles in a minimally defined and complex undefined bacterial growth medium. Nanomaterials 2019, 9, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamri, B.; Aabed, K. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, B.; Tafvizi, F.; Zaker Bostanabad, S. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif. CellsNanomed. Biotechnol. 2018, 46, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatachalam, P.; Sangeetha, P.; Geetha, N.; Sahi, S.V. Phytofabrication of bioactive molecules encapsulated metallic silver nanoparticles from Cucumis sativus L. and its enhanced wound healing potential in rat model. J. Nanomater. 2015, 2015, 753193. [Google Scholar] [CrossRef] [Green Version]
- Zayed, M.F.; Eisa, W.H.; Shabaka, A. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 98, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ni, X.; Zheng, H.; Li, Y.; Zhang, X.; Yang, Z. Synthesis of needle-like nickel nanoparticles in water-in-oil microemulsion. Mater. Lett. 2005, 59, 2011–2014. [Google Scholar] [CrossRef]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Kokati Venkata, B.R. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016, 11, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater. Nanotechnol. 2012, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.; Shim, Y.Y.; Reaney, M.J.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, H.; Li, Y.; Mo, F.; Li, Z.; Chai, R.; Wang, H. Dispersibility and Size Control of Silver Nanoparticles with Anti-Algal Potential Based on Coupling Effects of Polyvinylpyrrolidone and Sodium Tripolyphosphate. Nanomaterials 2020, 10, 1042. [Google Scholar] [CrossRef]
- Sangeetha, J.; Sandhya, J.; Philip, J. Biosynthesis and functionalization of silver nanoparticles using Nigellasativa, Dioscorea alata and Ferula asafoetida. Sci. Adv. Mater. 2014, 6, 1681–1690. [Google Scholar] [CrossRef]
- Jini, D.; Sharmila, S. Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater. Today Proc. 2020, 22, 432–438. [Google Scholar] [CrossRef]
- Alkhalaf, M.I.; Hussein, R.H.; Hamza, A. Green synthesis of silver nanoparticles by Nigella sativa extract alleviates diabetic neuropathy through anti-inflammatory and antioxidant effects. Saudi J. Biol. Sci. 2020, 27, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green synthesis of silver nanoparticles: A review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef] [Green Version]
- 5-substituted Indole-3-Carboxylic Acid Derivatives, Having Antiviral Activity, Synthesis Method Thereof and Use. RU2387642C2, P.N. Available online: https://patents.google.com/patent/RU2387642C2/en (accessed on 3 May 2021).
- Enna, S.J.; Bylund, D.B. xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2008. [Google Scholar]
- Mezencev, R.; Galizzi, M.; Kutschy, P.; Docampo, R. Trypanosoma cruzi: Antiproliferative effect of indole phytoalexins on intracellular amastigotes in vitro. Exp. Parasitol. 2009, 122, 66–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.-P.; Wang, M.-L.; Chan, M.-H.; Chiu, Y.-S.; Chen, Y.-H. Antiobesity activities of indole-3-carbinol in high-fat-diet–induced obese mice. Nutrition 2011, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mishra, A.K. Biological importance of phenol derivatives as potent bioactive compound: A review. Lett. Org. Chem. 2018, 15, 251–264. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624. [Google Scholar] [CrossRef]
- Rao, A.; Mahajan, K.; Bankar, A.; Srikanth, R.; Kumar, A.R.; Gosavi, S.; Zinjarde, S. Facile synthesis of size-tunable gold nanoparticles by pomegranate (Punica granatum) leaf extract: Applications in arsenate sensing. Mater. Res. Bull. 2013, 48, 1166–1173. [Google Scholar] [CrossRef]
- Parashar, U.K.; Kumar, V.; Bera, T.; Saxena, P.S.; Nath, G.; Srivastava, S.K.; Giri, R.; Srivastava, A. Study of mechanism of enhanced antibacterial activity by green synthesis of silver nanoparticles. Nanotechnology 2011, 22, 415104. [Google Scholar] [CrossRef]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S.-I.; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crop. Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Park, Y.-J.; Seo, D.-W.; Gil, T.-Y.; Cominguez, D.C.; Lee, H.; Lee, D.-S.; Han, I.; An, H.-J. Pharmacological Properties of a Traditional Korean Formula Bojungchiseup-tang on 3T3-L1 Preadipocytes and High-Fat Diet-Induced Obesity Mouse Model. Biomed. Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Kim, D.H.; Vijayakumar, M.; Srigopalram, S.; Roh, S.G.; Arasu, M.V.; Lee, J.S.; Choi, K.C. Potential role of marine algae extract on 3T3-L1 cell proliferation and differentiation: An in vitro approach. Biol. Res. 2016, 49, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, J.; Cook, C.; Blough, E.; Santanam, N. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis 2010, 212, 488–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.Y.; Li, Z.Y. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br. J. Pharmacol. 2012, 165, 643–658. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Schlich, R.; Willems, M.; Greulich, S.; Ruppe, F.; Knoefel, W.T.; Ouwens, D.M.; Maxhera, B.; Lichtenberg, A.; Eckel, J.; Sell, H. VEGF in the crosstalk between human adipocytes and smooth muscle cells: Depot-specific release from visceral and perivascular adipose tissue. Mediat. Inflamm. 2013, 2013, 982458. [Google Scholar] [CrossRef] [PubMed]
- Oreopoulos, A.; Padwal, R.; McAlister, F.; Ezekowitz, J.; Sharma, A.; Kalantar-Zadeh, K.; Fonarow, G.; Norris, C. Association between obesity and health-related quality of life in patients with coronary artery disease. Int. J. Obes. 2010, 34, 1434–1441. [Google Scholar] [CrossRef] [Green Version]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012, 686, 116–123. [Google Scholar] [CrossRef] [PubMed]

| No | RT (min) | Peak Area (%) | Compound Name | Molecular Formula | Molecular Weight (g/mol) | Compound Nature | Bioactivity |
|---|---|---|---|---|---|---|---|
| 1 | 9.707 | 5.15 | Undecane | C11H24 | 156.31 | Alkane | Alarm pheromone of the ant Componotus obscuripes [18]. |
| 2 | 18.054 | 9.28 | 2,4,6-Cycloheptatrien-1-one | C7H6O | 106.12 | Cyclic aliphatic ketone | Troponoid derivatives have antibacterial, antifungal, insecticidal, antimalarial, antitumor, anti-ischemic, iron chelating, and the inhibitory activity against polyphenol oxidase activity [19]. |
| 2-Coumaranone | C8H6O2 | 134.13 | Benzofurn ketone | Coumaranone derivatives have pharmaceutical activities against different biological targets [20,21]. | |||
| 3 | 18.348 | 18.04 | Benzeneacetonitrile, 4-hydroxy- | C8H7NO | 133.15 | Aromatic cyanide | Not reported |
| 4 | 25.654 | 8.85 | 2-Propenal, 3-(2-furanyl)- | C7H6O2 | 122.12 | Organoheterocyclic compound | Not reported |
| 5 | 40.460 | 5.22 | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl | C23H32O2 | 340.50 | Aromatic organic compound | Derivatives are potent antimicrobial, antioxidant and anti-cancer agents [22,23]. |
| 6 | 41.626 | 2.73 | Benzene, 1,2,4-trichloro-5-nitro- | C6H2Cl3NO2 | 226.40 | Aromatic compound | Not reported |
| 1H-Inden-1-one, 2,3-diphenyl- | C21H14O | 282.30 | Heterocyclic aromatic ketone | Derivatives (Phytoalexins) are antimicrobial agents [24]. | |||
| 7 | 43.614 | 3.41 | Cannabinol | C21H26O2 | 310.40 | Dibenzo(b,d)pyran derivative | Immunosuppressive and anti-inflammatory activities, agonist at the CB1 and CB2 receptors [25,26]. |
| 8 | 43.706 | 3.04 | Kaempferol | C15H10O6 | 286.24 | Flavonoid | Antioxidant, anti-inflammatory, antimicrobial, cardio-, and neuroprotective effects [27]. |
| 9 | 46.223 | 4.44 | 3H-[1,3,4]Oxadiazole-2-thione, 5-(4,6-dimethylpyrimidin-2-ylsulfanylmethyl)- | C9H10N4OS2 | 254.30 | An oxadiazole-2-thione derivative | Derivatives showed anticancer and tubulin polymerization inhibitor [28] and nucleotide pyrophosphatases/phosphodiesterases 1 inhibitors [29]. |
| 10 | 47.011 | 19.02 | Indole-3-carboxylic acid, 5-methoxy-2-methyl-1-(3-methylphenyl)-, ethyl ester | C20H25N3O | 323.40 | Heterocyclic benzopyrrole | Anticonvulsant, anticancer, antibacterial, and anti-inflammatory, antitubercular, antimalarial activities [30]. Plant resistance mediator against necrotrophic pathogens [31]. |
| 11 | 47.943 | 9.16 | 3,3-Diphenyl-1-indanone | C21H16O | 284.30 | Heterocyclic aromatic ketone | 1-indanone derivatives have anti-inflammatory effects [32,33]. |
| 1,4- Phthalazinedione, 2,3-dihydro-6-nitro- | C8H5N3O4 | 207.14 | Heterocyclic organic compound | Derivatives have vasorelaxant activity [34] and antibacterial activity [35]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagoub, A.E.A.; Alshammari, G.M.; Subash-Babu, P.; Mohammed, M.A.a.; Yahya, M.A.; Alhosain, A.I. Synthesis of Ziziphus spina-christi (Jujube) Root Methanol Extract Loaded Functionalized Silver Nanoparticle (ZS-Ag-NPs); Physiochemical Characterization and Effect of ZS-Ag-NPs on Adipocyte Maturation, Adipokine and Vascular Smooth Muscle Cell Interaction. Nanomaterials 2021, 11, 2563. https://doi.org/10.3390/nano11102563
Yagoub AEA, Alshammari GM, Subash-Babu P, Mohammed MAa, Yahya MA, Alhosain AI. Synthesis of Ziziphus spina-christi (Jujube) Root Methanol Extract Loaded Functionalized Silver Nanoparticle (ZS-Ag-NPs); Physiochemical Characterization and Effect of ZS-Ag-NPs on Adipocyte Maturation, Adipokine and Vascular Smooth Muscle Cell Interaction. Nanomaterials. 2021; 11(10):2563. https://doi.org/10.3390/nano11102563
Chicago/Turabian StyleYagoub, Abu ElGasim Ahmed, Ghedeir Muslem Alshammari, Pandurangan Subash-Babu, Mohammed Awad alkareem Mohammed, Mohammed Abdo Yahya, and Aesha Ibrahim Alhosain. 2021. "Synthesis of Ziziphus spina-christi (Jujube) Root Methanol Extract Loaded Functionalized Silver Nanoparticle (ZS-Ag-NPs); Physiochemical Characterization and Effect of ZS-Ag-NPs on Adipocyte Maturation, Adipokine and Vascular Smooth Muscle Cell Interaction" Nanomaterials 11, no. 10: 2563. https://doi.org/10.3390/nano11102563






