Effect of Shading Nets on Yield, Leaf Biomass and Petiole Nutrients of a Muscat of Alexandria Vineyard Growing under Hyper-Arid Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. Experimental Design and Treatments
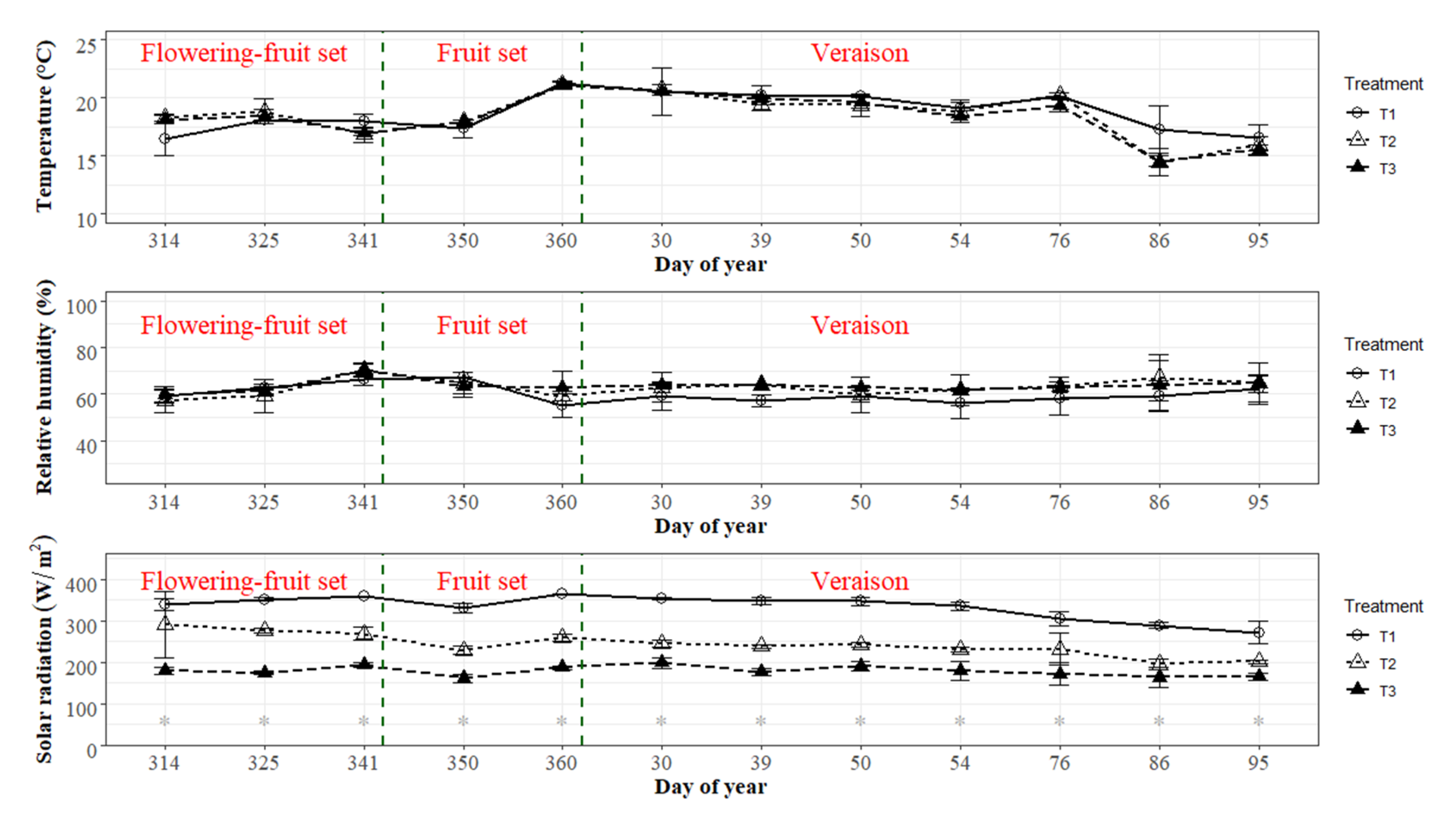
2.3. Microclimate Variables
2.4. Yield Determination, Yield Components and Bunch Traits
2.5. Determination of Berry Physicochemical Parameters
2.6. Determination of Vine Vigor Parameters
2.7. Determination of Leaf Dry Weight and Leaf Biomass
2.8. Determination of Petiole Nutrient Content
2.9. Statistical Analysis
3. Results
3.1. Climatic Conditions of the Study Site
3.2. Vine Microclimate Conditions
3.3. Yield, Yield Components and Bunch Characteristics
3.4. Yield, Yield Components and Bunch Characteristics
3.5. Vine Vigor Parameters
3.6. Leaf Dry Weight and Leaf Biomass
3.7. Petiole Nutrient Content
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Santillán, D.; Garrote, L.; Iglesias, A.; Sotes, V. Climate change risks and adaptation: New indicators for Mediterranean viticulture. Mitig. Adapt. Strat. Glob. Chang. 2019, 25, 881–899. [Google Scholar] [CrossRef]
- Neethling, E.; Barbeau, G.; Coulon-Leroy, C.; Quénol, H. Spatial complexity and temporal dynamics in viticulture: A review of climate-driven scales. Agric. For. Meteorol. 2019, 276–277. [Google Scholar] [CrossRef]
- Jones, G.V.; Davis, R.E. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar]
- de Cortázar-Atauri, I.G.; Duchêne, E.; Destrac-Irvine, A.; Barbeau, G.; De Rességuier, L.; Lacombe, T.; Parker, A.K.; Saurin, N.; Van Leeuwen, C. Grapevine phenology in France: From past observations to future evolutions in the context of climate change. OENO One 2017, 51, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; De Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Fraga, H.; Pinto, J.G.; Santos, J.A. Climate change projections for chilling and heat forcing conditions in European vineyards and olive orchards: A multi-model assessment. Clim. Chang. 2018, 152, 179–193. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Cardoso, R.M.; Soares, P.; Cancela, J.J.; Pinto, J.G.; dos Santos, J.C.A. Integrated Analysis of Climate, Soil, Topography and Vegetative Growth in Iberian Viticultural Regions. PLoS ONE 2014, 9, e108078. [Google Scholar] [CrossRef]
- Fraga, H.; Molitor, D.; Leolini, L.; Santos, J.A. What Is the Impact of Heatwaves on European Viticulture? A Modelling Assessment. Appl. Sci. 2020, 10, 3030. [Google Scholar] [CrossRef]
- Naulleau, A.; Gary, C.; Prévot, L.; Hossard, L. Evaluating Strategies for Adaptation to Climate Change in Grapevine Production–A Systematic Review. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; de Toda, F.M. Current viticultural techniques to mitigate the effects of global warming on grape and wine quality: A comprehensive review. Food Res. Int. 2020, 139, 109946. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; De Toda, F.M. Strategies in vineyard establishment to face global warming in viticulture: A mini review. J. Sci. Food Agric. 2020, 101, 1261–1269. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; DiBari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Duchene, E. How can grapevine genetics contribute to the adaptation to climate change? OENO One 2016, 50, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, T.; Lebon, E.; Coupel-Ledru, A.; Marguerit, E.; Rossdeutsch, L.; Ollat, N. Adapting plant material to face water stress in vineyards: Which physiological targets for an optimal control of plant water status? OENO One 2017, 51, 167–179. [Google Scholar] [CrossRef]
- Santo, S.D.; Fasoli, M.; Negri, S.; D’Incà, E.; Vicenzi, N.; Guzzo, F.; Tornielli, G.B.; Pezzotti, M.; Zenoni, S. Plasticity of the Berry Ripening Program in a White Grape Variety. Front. Plant Sci. 2016, 7, 970. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, C.; Pieri, P.; Gowdy, M.; Ollat, N.; Roby, J.P. Reduced density is an environmental friendly and cost effective solution to increase resilience to drought in vineyards in a context of climate change. OENO One 2019, 3, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Partial Solar Radiation Exclusion with Color Shade Nets Reduces the Degradation of Organic Acids and Flavonoids of Grape Berry (Vitis vinifera L.). J. Agric. Food Chem. 2017, 65, 10693–10702. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Teles, J.; Barbosa, P.; Olazabal, F.; Queiroz, J. Shading of the fruit zone to reduce grape yield and quality losses caused by sunburn. OENO One 2014, 48, 179–187. [Google Scholar] [CrossRef]
- Ghiglieno, I.; Mattivi, F.; Cola, G.; Trionfini, D.; Perenzoni, D.; Simonetto, A.; Gilioli, G.; Valenti, L. The effects of leaf removal and artificial shading on the composition of Chardonnay and Pinot noir grapes. OENO One 2020, 54, 761–777. [Google Scholar] [CrossRef]
- Lobos, G.A.; Acevedo-Opazo, C.; Guajardo-Moreno, A.; Valdés-Gómez, H.; Taylor, J.A.; Laurie, V.F. Effects of kaolin-based particle film and fruit zone netting on Cabernet Sauvignon grapevine physiology and fruit quality. OENO One 2015, 49, 137. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Cameron, W.; Petrie, P.; Barlow, E.; Patrick, C.; Howell, K.; Fuentes, S. Advancement of grape maturity: Comparison between contrasting cultivars and regions. Aust. J. Grape Wine Res. 2019, 26, 53–67. [Google Scholar] [CrossRef]
- Vršič, S.; Šuštar, V.; Pulko, B.; Šumenjak, T. Trends in climate parameters affecting winegrape ripening in northeastern Slovenia. Clim. Res. 2014, 58, 257–266. [Google Scholar] [CrossRef]
- Conde, A.; Pimentel, D.; Neves, A.; Dinis, L.-T.; Bernardo, S.; Correia, C.M.; Gerós, H.; Moutinho-Pereira, J. Kaolin Foliar Application Has a Stimulatory Effect on Phenylpropanoid and Flavonoid Pathways in Grape Berries. Front. Plant Sci. 2016, 7, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing Kaolin and Pinolene to Improve Sustainable Grapevine Production during Drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Bernardo, S.; Matos, C.; Malheiro, A.; Flores, R.; Alves, S.; Costa, C.; Rocha, S.; Correia, C.; Luzio, A.; et al. Overview of Kaolin Outcomes from Vine to Wine: Cerceal White Variety Case Study. Agronomy 2020, 10, 1422. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.; Machado, N.; Barros, A.; Pitarch-Bielsa, M.; Malheiro, A.C.; Gómez-Cadenas, A.; Moutinho-Pereira, J. Uncovering the effects of kaolin on balancing berry phytohormones and quality attributes of Vitis vinifera grown in warm-temperate climate regions. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Singh, R.K.; Afonso, J.; Nogueira, M.; Oliveira, A.A.; Cosme, F.; Falco, V. Silicates of Potassium and Aluminium (Kaolin); Comparative Foliar Mitigation Treatments and Biochemical Insight on Grape Berry Quality in Vitis vinifera L. (cv. Touriga National and Touriga Franca). Biology 2020, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening and anthocyanin accumulation in Nebbiolo grapes. Am. J. Enol. Vitic. 2010, 61, 23–30. [Google Scholar]
- Novello, V.; de Palma, L. Viticultural strategy to reduce alcohol levels in wine. In Alcohol Level Reduction in Wine; Oenoviti International Network: Bordeaux, France, 2013; pp. 3–8. [Google Scholar]
- Caravia, L.; Collins, C.; Petrie, P.; Tyerman, S. Application of shade treatments during Shiraz berry ripening to reduce the impact of high temperature. Aust. J. Grape Wine Res. 2016, 22, 422–437. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Hirzel, J.; Moya-Elizondo, E.; Hernández, M.; Guzmán, P.; González, D. Effect of shade cloth on fruit and leaf nutritional concentration and bitter pit incidence in ‘Fuji’ apples. Chil. J. Agric. Res. 2020, 80, 535–545. [Google Scholar] [CrossRef]
- Balbontín, C.; Campos, I.; Odi-Lara, M.; Ibacache, A.; Calera, A. Irrigation Performance Assessment in Table Grape Using the Reflectance-Based Crop Coefficient. Remote. Sens. 2017, 9, 1276. [Google Scholar] [CrossRef] [Green Version]
- Verdugo-Vásquez, N.; Gutiérrez-Gamboa, G.; Díaz-Gálvez, I.; Ibacache, A.; Zurita-Silva, A. Modifications Induced by Rootstocks on Yield, Vigor and Nutritional Status on Vitis vinifera Cv Syrah under Hyper-Arid Conditions in Northern Chile. Agronomy 2021, 11, 979. [Google Scholar] [CrossRef]
- Verdugo-Vásquez, N.; Gutiérrez-Gamboa, G.; Villalobos-Soublett, E.; Zurita-Silva, A. Effects of Rootstocks on Blade Nutritional Content of Two Minority Grapevine Varieties Cultivated under Hyper-Arid Conditions in Northern Chile. Agronomy 2021, 11, 327. [Google Scholar] [CrossRef]
- Jones, G.V. Climate and terroir: Impacts of climate variability and change on wine. GeoSci. Can. 2006, 9, 1–14. [Google Scholar]
- Tonietto, J.; Carbonneau, A. A multicriteria climatic classification system for grape-growing regions worldwide. Agric. For. Meteorol. 2004, 124, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Rose, N.K.; Messou, M.; Bertrand, K.J.; Patrick, Y.J.; Franck, L.G. Management of Ovarian Hernia in Children, in Teaching Hospital of Bouaké, Côte d’Ivoire Prise en Charge des Hernies de l’ovaire de l’enfant au CHU de Bouaké, Côte d’Ivoire. Open J. Obstet. Gynecol. 2018, 08, 1438–1444. [Google Scholar] [CrossRef] [Green Version]
- Amerine, M.; Winkler, A. Composition and Quality of Musts and Wines of California Grapes. Hilgardia 1944, 15, 493–675. [Google Scholar] [CrossRef] [Green Version]
- Gladstones, J. Viticulture and Environment; Winetitles: Adelaide, Australia, 1992. [Google Scholar]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis. Physical and Chemical Analysis; OIV: Paris, France, 2003. [Google Scholar]
- Muñoz-Robredo, P.; Robledo, P.; Manríquez, D.; Molina, R.; Defilippi, B.G. Characterization of Sugars and Organic Acids in Commercial Varieties of Table Grapes. Chil. J. Agric. Res. 2011, 71, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Nikolaou, N.; Koukourikou, M.A.; Karagiannidis, N. Effects of various rootstocks on xylem exudates cytokinin content, nutrient uptake and growth patterns of grapevine Vitis vinifera L. cv. Thompson seedless. Agronomie 2000, 20, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.; Gallego, P.; Daverède, C.; Ibrahim, H. Effect of Three Roots tocks on Grapevine (Vitis vinifera L.) CV. Négrette, Grown Hydroponically. I. Potassium, Calcium and Magnesium Nutrition. S. Afr. J. Enol. Vitic. 2017, 22, 101–103. [Google Scholar] [CrossRef] [Green Version]
- Moratiel, R.; Martínez-Cob, A. Evapotranspiration of grapevine trained to a gable trellis system under netting and black plastic mulching. Irrig. Sci. 2011, 30, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, M.; Gommes, R.; Baier, W. Agrometeorology and sustainable agriculture. Agric. For. Meteorol. 2000, 103, 11–26. [Google Scholar] [CrossRef]
- Buesa, I.; Pérez, D.; Castel, J.; Intrigliolo, D. Effect of deficit irrigation on vine performance and grape composition of Vitis vinifera L. cv. Muscat of Alexandria. Aust. J. Grape Wine Res. 2017, 23, 251–259. [Google Scholar] [CrossRef]
- Gamboa, G.G.; Díaz-Galvéz, I.; Verdugo-Vásquez, N.; Moreno-Simunovic, Y. Leaf-to-Fruit Ratios in Vitis vinifera L. cv. “Sauvignon Blanc”, “Carmenère”, “Cabernet Sauvignon”, and “Syrah” Growing in Maule Valley (Chile): Influence on Yield and Fruit Composition. Agriculture 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Verdenal, T.; Spangenberg, J.E.; Zufferey, V.; Lorenzini, F.; Dienes-Nagy, A.; Gindro, K.; Spring, J.-L.; Viret, O. Leaf-to-fruit ratio affects the impact of foliar-applied nitrogen on N accumulation in the grape must. OENO One 2016, 50, 23. [Google Scholar] [CrossRef]
- Verdenal, T.; Spangenberg, J.; Zufferey, V.; Lorenzini, F.; Spring, J.-L.; Viret, O. Effect of fertilisation timing on the partitioning of foliar-applied nitrogen inVitis viniferacv. Chasselas: a15N labelling approach. Aust. J. Grape Wine Res. 2015, 21, 110–117. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Verdugo-Vásquez, N.; Acevedo-Opazo, C.; Valdés-Gómez, H.; la Fuente, C.P.-D.; Ingram, B.; de Cortázar-Atauri, I.G.; Tisseyre, B. Identification of main factors affecting the within-field spatial variability of grapevine phenology and total soluble solids accumulation: Towards the vineyard zoning using auxiliary information. Precis. Agric. 2021, 1–25. [Google Scholar] [CrossRef]
- Ebadi, A.; Coombe, B.; May, P. Fruit-set on small Chardonnay and Shiraz vines grown under varying temperature regimes between budburst and flowering. Aust. J. Grape Wine Res. 1995, 1, 3–10. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.T.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar]
- Acimovic, D.; Tozzini, L.; Green, A.; Sivilotti, P.; Sabbatini, P. Identification of a defoliation severity threshold for changing fruitset, bunch morphology and fruit composition in Pinot Noir. Aust. J. Grape Wine Res. 2016, 22, 399–408. [Google Scholar] [CrossRef]
- Domingos, S.; Fino, J.; Cardoso, V.; Sánchez, C.; Ramalho, J.C.; Larcher, R.; Paulo, O.S.; Oliveira, C.M.; Goulao, L.F. Shared and divergent pathways for flower abscission are triggered by gibberellic acid and carbon starvation in seedless Vitis vinifera L. BMC Plant Biol. 2016, 16, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, M. The Science of Grapevines. Anatomy and Physiology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128167021. [Google Scholar]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Baeza, P.; Sánchez-De-Miguel, P.; Lissarrague, J.R. Radiation Balance in Vineyards. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 21–29. [Google Scholar]
- Cartechini, A.; Palliotti, A. Effect of shading on vine morphology and productivity and leaf gas exchange characteristics in grapevines in the field. Am. J. Enol. Vitic. 1995, 46, 227–235. [Google Scholar]
- Bathellier, C.; Yu, L.-J.; Farquhar, G.D.; Coote, M.L.; Lorimer, G.H.; Tcherkez, G. Ribulose 1,5-bisphosphate carboxylase/oxygenase activates O2 by electron transfer. Proc. Natl. Acad. Sci. USA 2020, 117, 24234–24242. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tazoe, Y.; Vyas, P.; Yano, S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 2005, 57, 343–354. [Google Scholar] [CrossRef] [Green Version]
| Season | GST (°C) | CI (°C) | HI (Heat Units) | GDD (Heat Units) | BEDD (Heat Units) |
|---|---|---|---|---|---|
| 2006–2007 | 18.28 | 9.0 | 2389 | 1756 | 1543 |
| 2007–2008 | 17.89 | 8.8 | 2311 | 1682 | 1473 |
| 2008–2009 | 18.45 | 9.1 | 2425 | 1798 | 1538 |
| 30-years (mean) a | 18.5 | 10.0 | 2409.7 | 1808.2 | 1528.7 |
| Factor | Yield (kg Vine−1) | N° of Bunches Per Vine | Bunch Weight (g) | N° of Berries Per Bunch | Berry Diameter (mm) | Rachis Length (cm) | Rachis Weight (g) |
|---|---|---|---|---|---|---|---|
| Treatments (T) | |||||||
| Control | 6.3 | 22.1 | 218.6b | 75.8ab | 17.4b | 17.5b | 10.5 |
| White shading net | 6.8 | 21.6 | 277.6a | 84.6a | 18.3a | 19.0ab | 10.8 |
| Black shading net | 6.8 | 20.4 | 211.9b | 66.6b | 18.1a | 20.4a | 9.2 |
| Season (S) | |||||||
| 2006–2007 | 5.1b | 16.3b | 204.7b | 70.9b | 17.9 | 20.7a | 9.3 |
| 2007–2008 | 8.2a | 30.9a | 203.4b | 69.8b | 17.8 | 20.7a | 10.6 |
| 2008–2009 | 6.5ab | 16.8b | 300.0a | 86.4a | 18.1 | 15.6b | 10.5 |
| Significance (p-value) | |||||||
| T | 0.751 | 0.835 | 0.002 a | 0.003 | 0.003 | 0.001 | 0.343 |
| S | 0.004 | 0.000 | 0.000 | 0.002 | 0.497 | 0.000 | 0.463 |
| T × S | 0.233 | 0.256 | 0.578 | 0.439 | 0.183 | 0.479 | 0.631 |
| Factor | TSS (°brix) | TA (%) | pH | Maturity Index |
|---|---|---|---|---|
| Treatments (T) | ||||
| Control | 21.8b | 3.2 | 3.4 | 7.5 |
| White shading net | 21.9b | 2.9 | 3.4 | 7.9 |
| Black shading net | 24.2a | 3.0 | 3.5 | 8.7 |
| Season (S) | ||||
| 2006–2007 | 21.9b | 3.3a | 3.3b | 7.0b |
| 2007–2008 | 22.2b | 3.6a | 3.3b | 6.1b |
| 2008–2009 | 23.8a | 2.2b | 3.8a | 11.0a |
| Significance (p-value) | ||||
| T | 0.000a | 0.247 | 0.771 | 0.110 |
| S | 0.005 | 0.000 | 0.001 | 0.000 |
| T × S | 0.514 | 0.287 | 0.776 | 0.305 |
| Factor | Pruning Weight (kg vine−1) | Ravaz Index |
|---|---|---|
| Treatments (T) | ||
| Control | 0.16b | 46.6a |
| White shading net | 0.17b | 43.5ab |
| Black shading net | 0.27a | 25.6b |
| Season (S) | ||
| 2006–2007 | 0.19 | 30.2 |
| 2007–2008 | 0.21 | 48.3 |
| 2008–2009 | 0.20 | 37.2 |
| Significance (p-value) | ||
| T | 0.003 a | 0.040 |
| S | 0.750 | 0.110 |
| T × S | 0.478 | 0.634 |
| Factor | Leaf Dry Weight (g) | Leaf Biomass (g cm2−1) | ||||
|---|---|---|---|---|---|---|
| Flowering | Fruit Set | Veraison | Flowering | Fruit Set | Veraison | |
| Treatments (T) | ||||||
| Control | 0.125a | 0.126 | 0.197 | 0.076a | 0.077 | 0.126a |
| White shading net | 0.092b | 0.122 | 0.190 | 0.056b | 0.075 | 0.116ab |
| Black shading net | 0.086b | 0.112 | 0.175 | 0.052b | 0.068 | 0.107b |
| Season (S) | ||||||
| 2006–2007 | 0.095b | 0.083b | 0.203a | 0.058b | 0.051b | 0.124a |
| 2007–2008 | 0.107a | 0.157a | 0.172b | 0.065a | 0.096a | 0.109b |
| Significance (p-value) | ||||||
| T | 0.000 a | 0.193 | 0.074 | 0.000 | 0.193 | 0.002 |
| S | 0.030 | 0.000 | 0.001 | 0.030 | 0.000 | 0.001 |
| T × S | 0.003 | 0.529 | 0.027 | 0.003 | 0.529 | 0.040 |
| Factor | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | Zn (ppm) | Mn (ppm) | Cu (ppm) |
|---|---|---|---|---|---|---|---|---|
| Treatments (T) | ||||||||
| Control | 0.68 | 0.12 | 3.13 | 1.38a | 0.34a | 86.7a | 53.4 | 6.6 |
| White shading net | 0.61 | 0.11 | 2.81 | 1.26b | 0.30ab | 75.8ab | 53.4 | 6.0 |
| Black shading net | 0.61 | 0.09 | 2.98 | 1.17b | 0.26b | 69.4b | 46 | 6.2 |
| Season (S) | ||||||||
| 2006–2007 | 0.67 | 0.13a | 3.47a | 1.49a | 0.38a | 79.3b | 50.2 | 8.3a |
| 2007–2008 | 0.60 | 0.12a | 2.98b | 1.05c | 0.23b | 58.2c | 50.3 | 5.4b |
| 2008–2009 | 0.63 | 0.07b | 2.47c | 1.27b | 0.28b | 94.3a | 52.3 | 5.0b |
| Significance (p-value) | ||||||||
| T | 0.057 | 0.110 | 0.191 | 0.001 a | 0.002 | 0.021 | 0.077 | 0.590 |
| S | 0.139 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.799 | 0.000 |
| T × S | 0.625 | 0.791 | 0.807 | 0.364 | 0.278 | 0.485 | 0.704 | 0.883 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Soublett, E.; Gutiérrez-Gamboa, G.; Balbontín, C.; Zurita-Silva, A.; Ibacache, A.; Verdugo-Vásquez, N. Effect of Shading Nets on Yield, Leaf Biomass and Petiole Nutrients of a Muscat of Alexandria Vineyard Growing under Hyper-Arid Conditions. Horticulturae 2021, 7, 445. https://doi.org/10.3390/horticulturae7110445
Villalobos-Soublett E, Gutiérrez-Gamboa G, Balbontín C, Zurita-Silva A, Ibacache A, Verdugo-Vásquez N. Effect of Shading Nets on Yield, Leaf Biomass and Petiole Nutrients of a Muscat of Alexandria Vineyard Growing under Hyper-Arid Conditions. Horticulturae. 2021; 7(11):445. https://doi.org/10.3390/horticulturae7110445
Chicago/Turabian StyleVillalobos-Soublett, Emilio, Gastón Gutiérrez-Gamboa, Claudio Balbontín, Andrés Zurita-Silva, Antonio Ibacache, and Nicolás Verdugo-Vásquez. 2021. "Effect of Shading Nets on Yield, Leaf Biomass and Petiole Nutrients of a Muscat of Alexandria Vineyard Growing under Hyper-Arid Conditions" Horticulturae 7, no. 11: 445. https://doi.org/10.3390/horticulturae7110445









