Province-Wide Stool Color Card Screening for Biliary Atresia in Lower-Saxony: Experiences with Passive Distribution Strategies and Results
Abstract
:1. Introduction
2. Patients and Methods
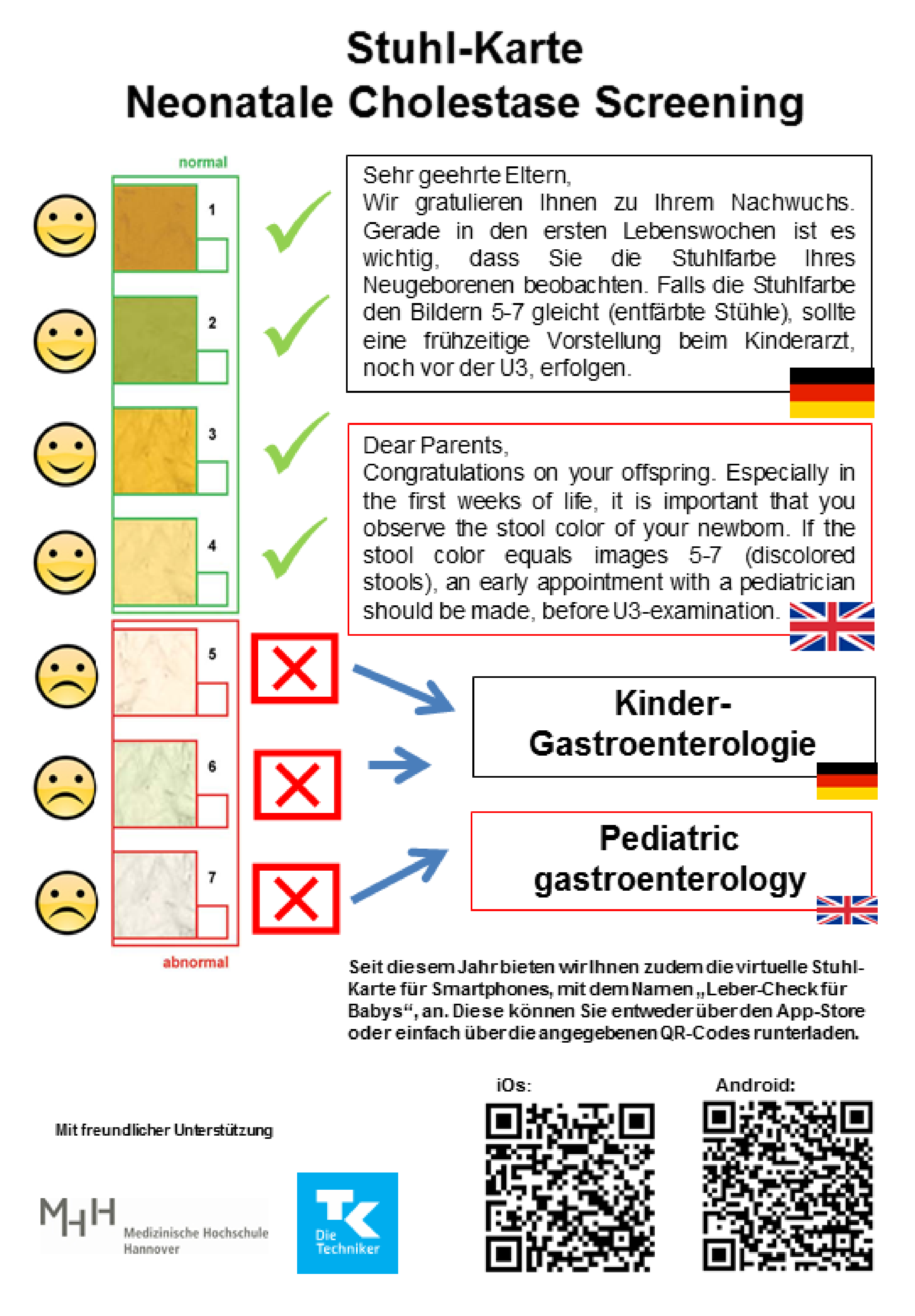
2.1. Screening Strategy in Lower Saxony
2.2. Questionnaire for Attending Pediatricians in Lower Saxony
2.3. Questionnaire for Parents of Infants with Biliary Atresia from Lower Saxony
2.4. Patients and Public Involvement
3. Results
3.1. Survey of Attending Pediatricians in Lower Saxony
3.1.1. Experiences with the Stool Color Cards in Out-Patient Clinics
3.1.2. Diagnostic Algorithms for Abnormal Results
3.2. Results of the SCC Screening in Out-Patient Clinics
3.3. Evaluation of the SCC Screening by Attending Pediatricians
3.4. Survey of Parents of BA Patients Born between 2017 and 2020 in Lower Saxony
3.5. SCC Supply and Education at the Maternity Hospitals
3.6. Timing of Referral to a Children’s Hospital
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nio, M.; Wada, M.; Sasaki, H.; Tanaka, H. Effects of age at Kasai portoenterostomy on the surgical outcome: A review of the literature. Surg. Today 2015, 45, 813–818. [Google Scholar] [CrossRef]
- Lopez, R.N.; Ooi, C.Y.; Krishnan, U. Early and Peri-operative Prognostic Indicators in Infants Undergoing Hepatic Portoenterostomy for Biliary Atresia: A Review. Curr. Gastroenterol. Rep. 2017, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-H.; Yokoyama, K.; Mizuta, K.; Tsuchioka, T.; Kudo, T.; Sasaki, H.; Nio, M.; Tang, J.; Ohkubo, T.; Matsui, A. Stool Color Card Screening for Early Detection of Biliary Atresia and Long-Term Native Liver Survival: A 19-Year Cohort Study in Japan. J. Pediatr. 2015, 166, 897–902.e1. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Miethke, A.; Bezerra, J.A. Pathogenesis of biliary atresia: Defining biology to understand clinical phenotypes. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.-H.; Zhao, J.-Q.; Kong, Y.-Y.; Yang, H.-H.; Diao, M.; Li, L.; Nomachi, S.; Tezuka, M.; Hanai, J.; Matsui, A. Repeatability and Reliability of Home-Based Stool Color Card Screening for Biliary Atresia Based on Results in China and Japan. Tohoku J. Exp. Med. 2020, 252, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.-H.; Chang, M.-H.; Wu, J.-F.; Chen, H.-L.; Lee, H.-C.; Chen, A.-C.; Tiao, M.-M.; Wu, T.-C.; Yang, Y.-J.; Lin, C.-C.; et al. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in taiwan. Hepatology 2011, 53, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, J.P.; Schreiber, R.A.; Butler, A.E.; MacFarlane, J.; Kaczorowski, J.; Masucci, L.; Bryan, S.; Collet, J.P. Province-wide Biliary Atresia Home Screening Program in British Columbia: Evaluation of First 2 Years. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, J.; Tavares, M.; Silva, E.S.; Lopes, A.I. The Stool Color Card as a Screening Tool for Biliary Atresia in the Digital Version of the Portuguese Child and Youth Health Booklet. Acta Méd. Port. 2021, 34, 632. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Liccardo, D.; Paoletti, M.; Pietrobattista, A.; Basso, M.S.; Mosca, A.; Safarikia, S.; Grimaldi, C.; Saffioti, M.C.; Candusso, M.; et al. A novel mobile phone application for infant stool color recognition: An easy and effective tool to identify acholic stools in newborns. J. Med. Screen. 2020, 28, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wildhaber, B.E. Screening for biliary atresia: Swiss stool color card. Hepatology 2011, 54, 368. [Google Scholar] [CrossRef] [PubMed]
- Masucci, L.; A Schreiber, R.; Kaczorowski, J.; Collet, J.; Bryan, S. Universal screening of newborns for biliary atresia: Cost-effectiveness of alternative strategies. J. Med. Screen. 2019, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mogul, D.; Zhou, M.; Intihar, P.; Schwarz, K.; Frick, K. Cost-Effective Analysis of Screening for Biliary Atresia with the Stool Color Card. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 91–98. [Google Scholar] [CrossRef]
- Madadi-Sanjani, O.; Blaser, J.; Voigt, G.; Kuebler, J.F.; Petersen, C. Home-based color card screening for biliary atresia: The first steps for implementation of a nationwide newborn screening in Germany. Pediatr. Surg. Int. 2019, 35, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Geburten in Niedersachsen bis 2020. Statista. Available online: https://de.statista.com/statistik/daten/studie/588918/umfrage/anzahl-der-geburten-in-niedersachsen/ (accessed on 16 September 2021).
- Leonhardt, J.; Kuebler, J.F.; Leute, P.J.; Turowski, C.; Becker, T.; Pfister, E.-D.; Ure, B.; Petersen, C. Biliary Atresia: Lessons Learned from the Voluntary German Registry. Eur. J. Pediatr. Surg. 2010, 21, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Morinville, V.; Ahmed, N.; Ibberson, C.; Kovacs, L.; Kaczorowski, J.; Bryan, S.; Collet, J.-P.; Schreiber, R. Home-Based Screening for Biliary Atresia Using Infant Stool Color Cards in Canada: Quebec Feasibility Study. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-H.; Chang, M.H.; Chen, H.-L.; Lee, H.-C.; Wu, T.-C.; Lin, C.-C.; Yang, Y.-J.; Chen, A.-C.; Tiao, M.-M.; Lau, B.-H.; et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology 2007, 47, 1233–1240. [Google Scholar] [CrossRef]
- Chen, S.-M.; Chang, M.-H.; Du, J.-C.; Lin, C.-C.; Lee, H.-C.; Lau, B.-H.; Yang, Y.-J.; Wu, T.-C.; Chu, C.-H.; Lai, M.-W. Screening for Biliary Atresia by Infant Stool Color Card in Taiwan. Pediatrics 2006, 117, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Y.-Y.; Zhao, J.-Q.; Wang, J.; Qiu, L.; Yang, H.-H.; Diao, M.; Li, L.; Gu, Y.-H.; Matsui, A. Modified stool color card with digital images was efficient and feasible for early detection of biliary atresia—A pilot study in Beijing, China. World J. Pediatr. 2016, 12, 415–420. [Google Scholar] [CrossRef]
- Borgeat, M.; Korff, S.; E Wildhaber, B. Newborn biliary atresia screening with the stool colour card: A questionnaire survey of parents. BMJ Paediatr. Open 2018, 2, e000269. [Google Scholar] [CrossRef] [Green Version]
| Country/Region | SCC Type | Distribution | Assessment |
|---|---|---|---|
| Taiwan [17] | 6 photographs stool samples (3 pathological images) | Postnatally at maternity hospitals |
|
| Japan [3] | 7 photographs of stool samples (3 pathological images) | Antenatally, in the Maternal and Child Health Handbook |
|
| Canada/Quebec [16] | 6 photographs of stool samples (3 pathological images) | Postnatally, at maternity hospitals |
|
| China/Bejing [19] | 7 photographs of stool samples + 7 digitally created images (3 pathological images/photographs) | Postnatally, by trained nurses in maternal facilities |
|
| Switzerland [10,20] | 7 photographs of stool samples (3 pathological images) | Postnatally, by the attending pediatrician or midwife |
|
| Germany/Lower Saxony [13] | 7 photographs of stool samples (3 pathological images) | Postnatally, at maternity hospitals |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madadi-Sanjani, O.; Kuebler, J.F.; Uecker, M.; Pfister, E.-D.; Baumann, U.; Kunze-Hullmann, B.; Blaser, J.; Buck, T.; Petersen, C. Province-Wide Stool Color Card Screening for Biliary Atresia in Lower-Saxony: Experiences with Passive Distribution Strategies and Results. Int. J. Neonatal Screen. 2021, 7, 75. https://doi.org/10.3390/ijns7040075
Madadi-Sanjani O, Kuebler JF, Uecker M, Pfister E-D, Baumann U, Kunze-Hullmann B, Blaser J, Buck T, Petersen C. Province-Wide Stool Color Card Screening for Biliary Atresia in Lower-Saxony: Experiences with Passive Distribution Strategies and Results. International Journal of Neonatal Screening. 2021; 7(4):75. https://doi.org/10.3390/ijns7040075
Chicago/Turabian StyleMadadi-Sanjani, Omid, Joachim F. Kuebler, Marie Uecker, Eva-Doreen Pfister, Ulrich Baumann, Berit Kunze-Hullmann, Jochen Blaser, Thomas Buck, and Claus Petersen. 2021. "Province-Wide Stool Color Card Screening for Biliary Atresia in Lower-Saxony: Experiences with Passive Distribution Strategies and Results" International Journal of Neonatal Screening 7, no. 4: 75. https://doi.org/10.3390/ijns7040075
APA StyleMadadi-Sanjani, O., Kuebler, J. F., Uecker, M., Pfister, E. -D., Baumann, U., Kunze-Hullmann, B., Blaser, J., Buck, T., & Petersen, C. (2021). Province-Wide Stool Color Card Screening for Biliary Atresia in Lower-Saxony: Experiences with Passive Distribution Strategies and Results. International Journal of Neonatal Screening, 7(4), 75. https://doi.org/10.3390/ijns7040075






