Electrocochleography in Auditory Neuropathy Related to Mutations in the OTOF or OPA1 Gene
Abstract
:1. Introduction
2. Electrocochleography (ECochG)
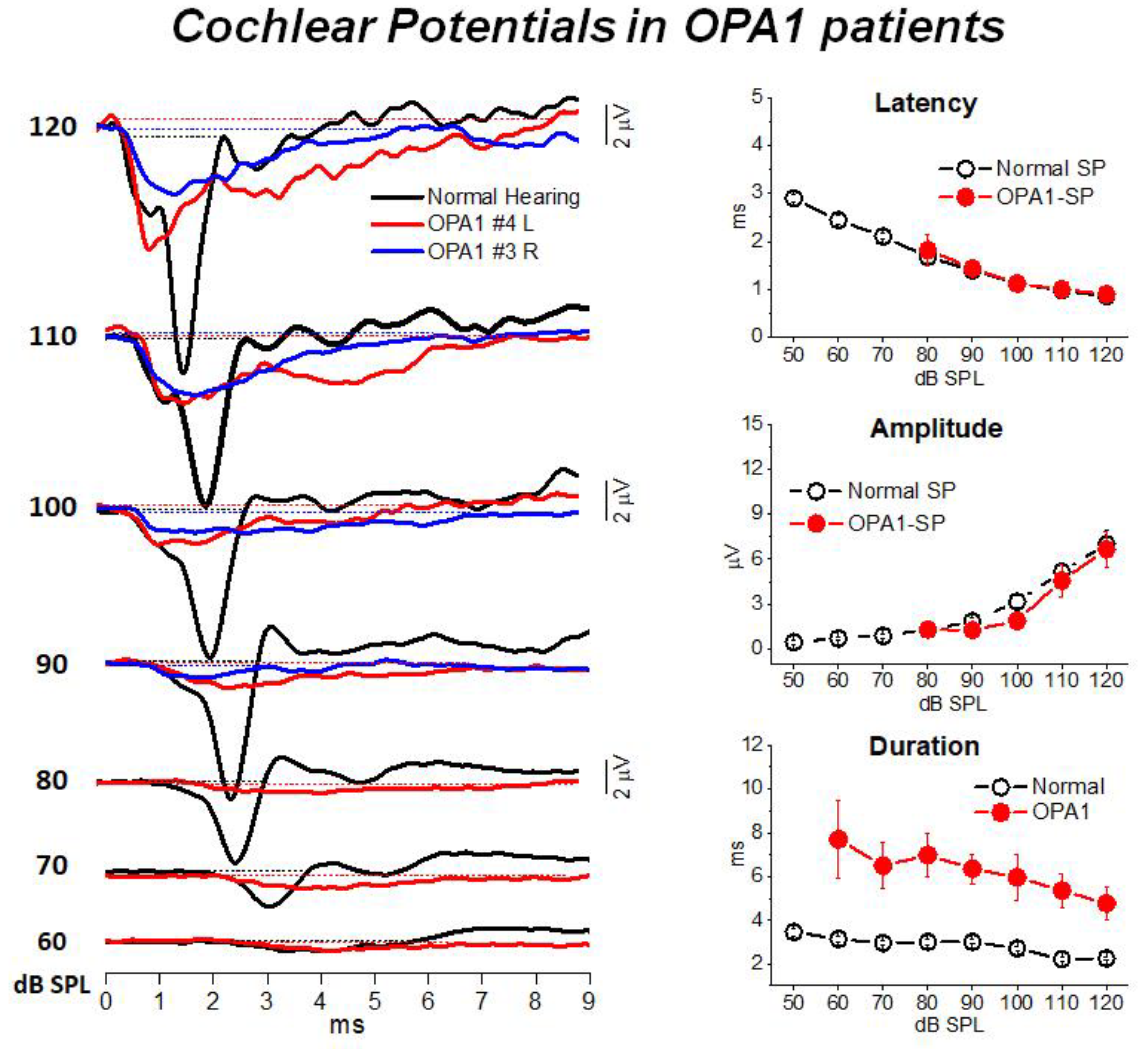
3. Mutations in the OPA1 Gene: A Model of Post-Synaptic AN
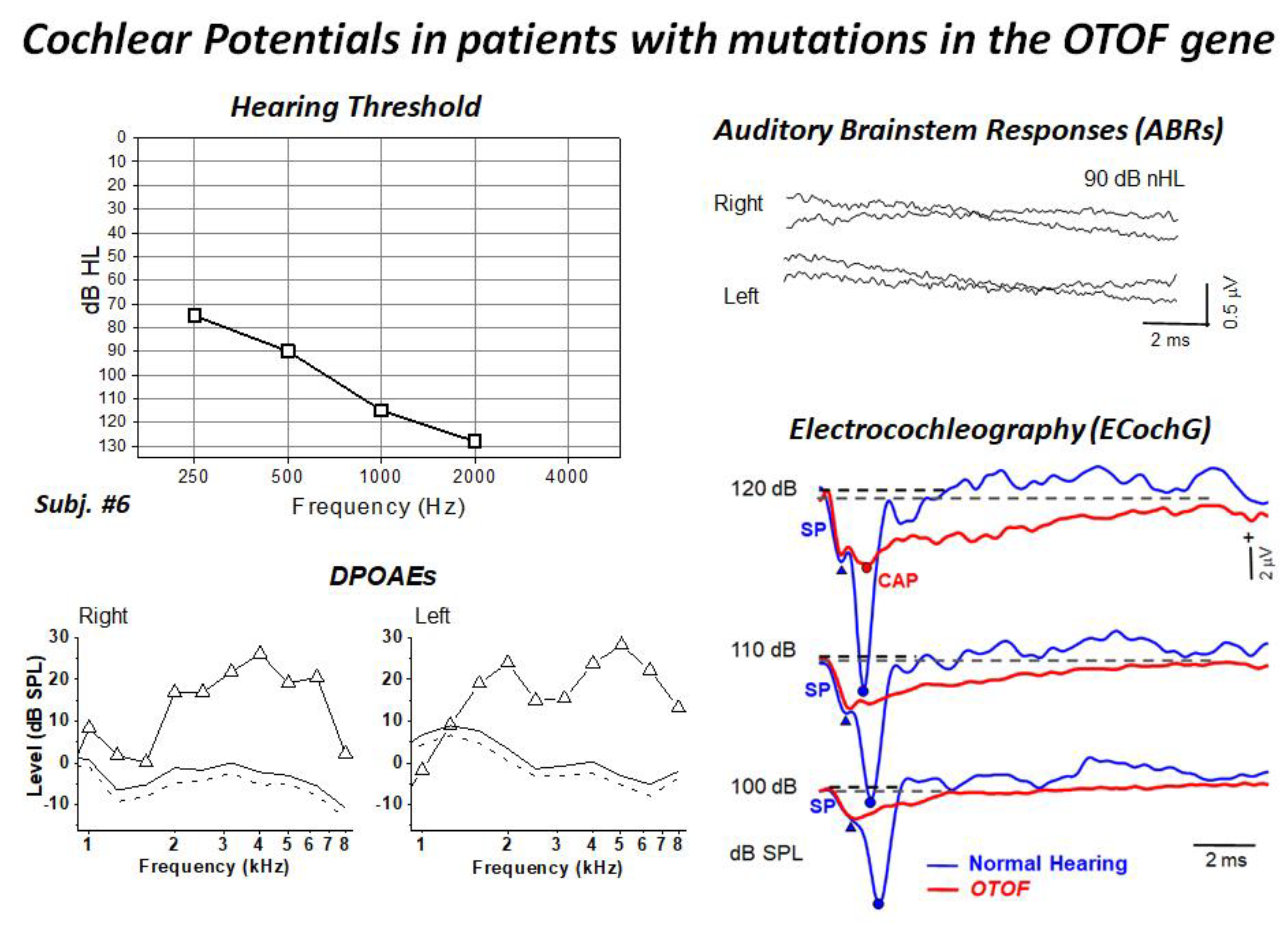
4. Mutations in the OTOF Gene: A Model of Synaptopathy
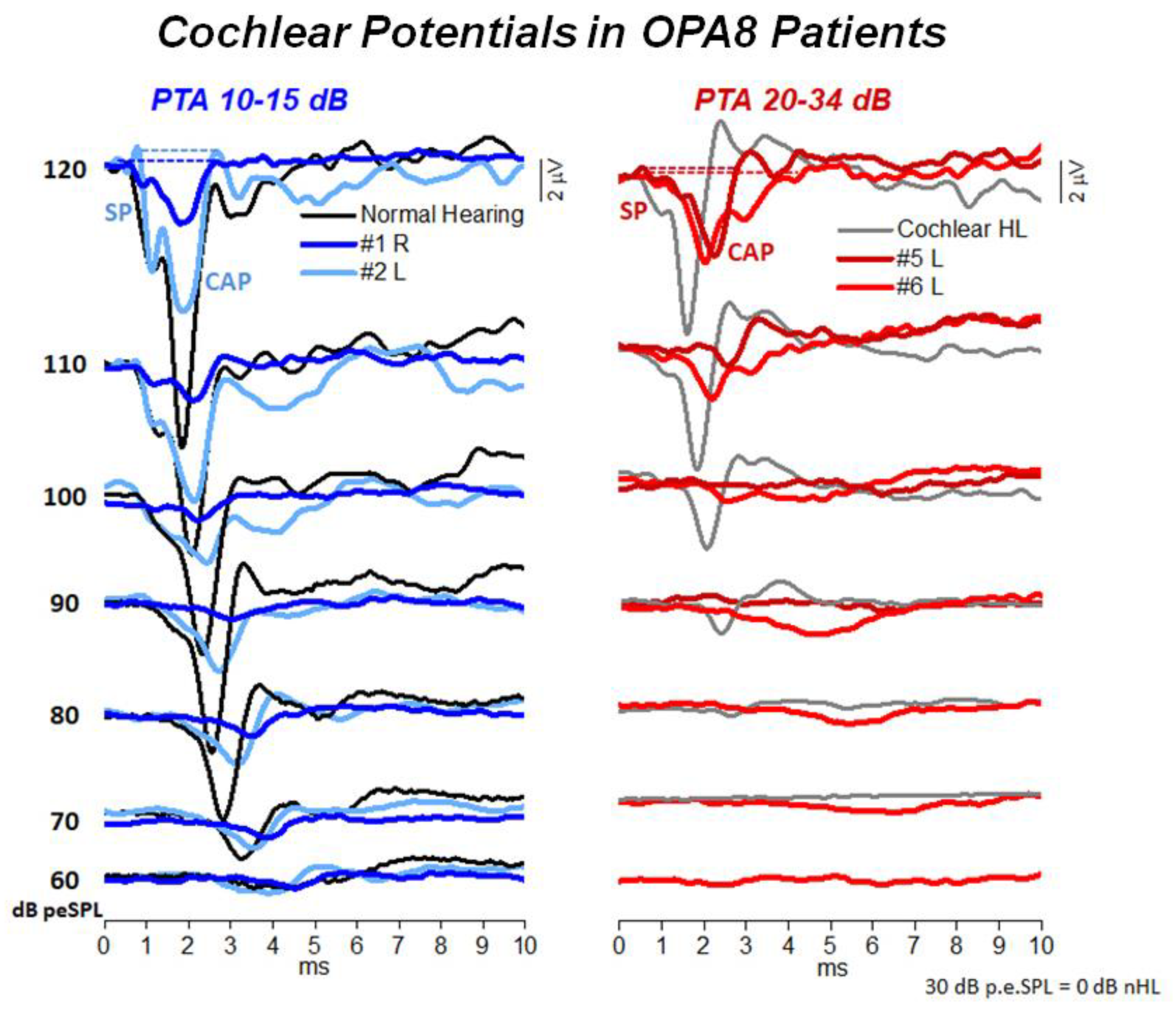
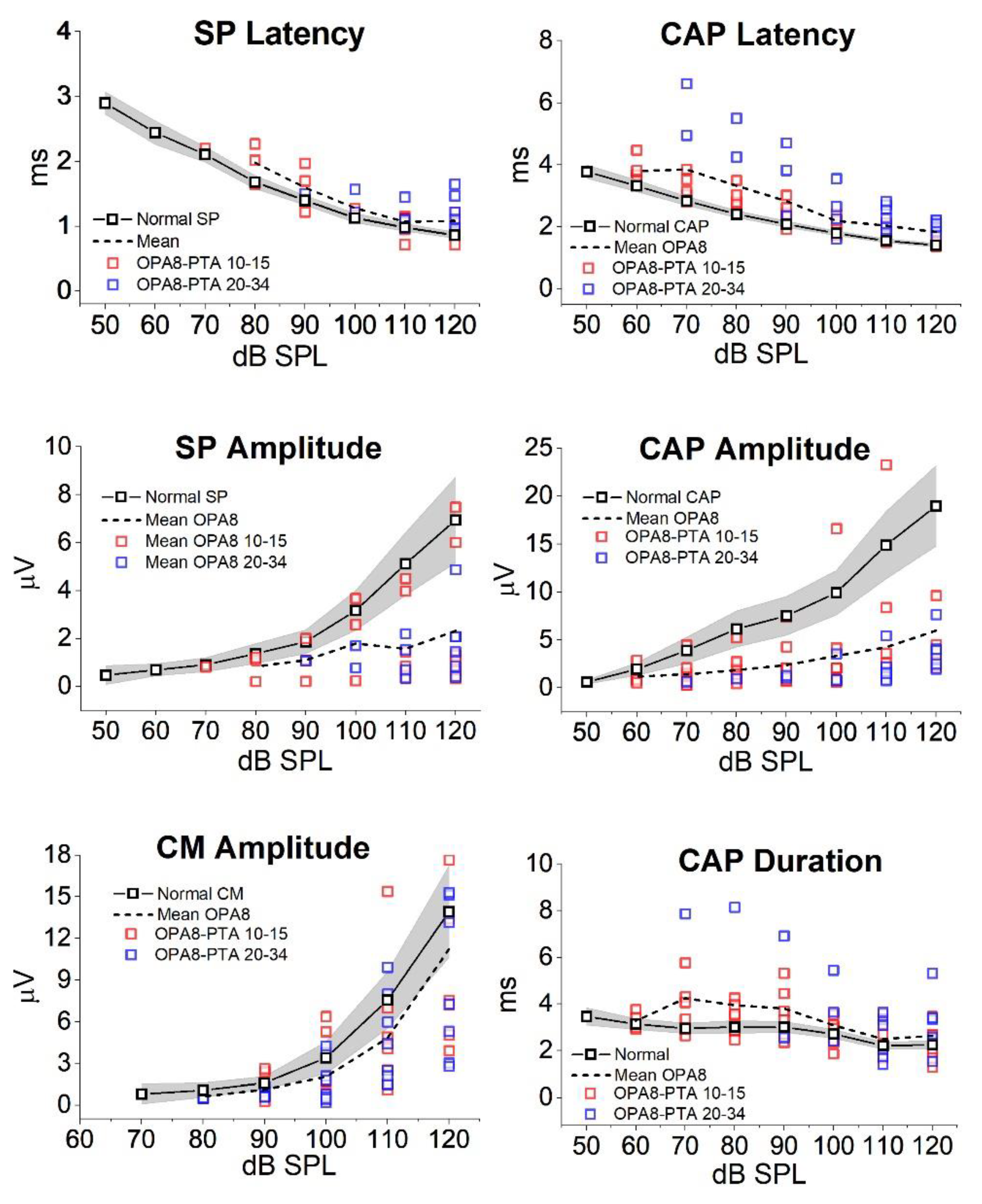
5. Hearing Dysfunction Related to the OPA8 Locus: A Model of Hidden AN
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Starr, A.; Picton, T.W.; Sininger, Y.; Hood, L.J.; Berlin, C.I. Auditory neuropathy. Brain 1996, 119, 741–753. [Google Scholar] [CrossRef]
- Starr, A.; Zeng, F.; Michalewski, H.; Moser, T. Perspectives on auditory neuropathy: Disorders of inner hair cell, auditory nerve, and their synapse. In The Senses: A Comprehensive Reference; Basbaum, A.I., Kaneko, A., Shepherd, G.M., Westheimer, G., Albright, T.D., Masland, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 3: Audition, pp. 397–412. [Google Scholar]
- Zeng, F.-G.; Kong, Y.-Y.; Michalewski, H.J.; Starr, A. Perceptual Consequences of Disrupted Auditory Nerve Activity. J. Neurophysiol. 2005, 93, 3050–3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, T.; Starr, A. Auditory neuropathy—Neural and synaptic mechanisms. Nat. Rev. Neurol. 2016, 12, 135–149. [Google Scholar] [CrossRef]
- Johnson, J.S.; Newport, E.L. Critical period effects on universal properties of language: The status of subjacency in the acquisition of a second language. Cognition 1991, 39, 215–258. [Google Scholar] [CrossRef]
- Butinar, D.; Zidar, J.; Leonardis, L.; Popovic, M.; Kalaydjieva, L.; Angelicheva, D.; Sininger, Y.; Keats, B.; Starr, A. Hereditary auditory, vestibular, motor, and sensory neuropathy in a Slovenian Roma (Gypsy) kindred. Ann. Neurol. 1999, 46, 36–44. [Google Scholar] [CrossRef]
- Santarelli, R. Information from cochlear potentials and genetic mutations helps localize the lesion site in auditory neuropathy. Genome Med. 2010, 2, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santarelli, R.; La Morgia, C.; Valentino, M.L.; Barboni, P.; Monteleone, A.; Scimemi, P.; Carelli, V. Hearing Dysfunction in a Large Family Affected by Dominant Optic Atrophy (OPA8-Related DOA): A Human Model of Hidden Auditory Neuropathy. Front. Neurosci. 2019, 13, 501. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, R.; del Castillo, I.; Cama, E.; Scimemi, P.; Starr, A. Audibility, speech perception and processing of temporal cues in ribbon synaptic disorders due to OTOF mutations. Hear. Res. 2015, 330, 200–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santarelli, R.; Scimemi, P.; Costantini, M.; Domínguez-Ruiz, M.; Rodríguez-Ballesteros, M.; del Castillo, I. Cochlear Synaptopathy due to Mutations in OTOF Gene May Result in Stable Mild Hearing Loss and Severe Impairment of Speech Perception. Ear Hear. 2021, 42, 1627–1639. [Google Scholar] [CrossRef]
- Huang, T.; Santarelli, R.; Starr, A. Mutation of OPA1 gene causes deafness by affecting function of auditory nerve terminals. Brain Res. 2009, 1300, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santarelli, R.; Rossi, R.; Scimemi, P.; Cama, E.; Valentino, M.L.; La Morgia, C.; Caporali, L.; Liguori, R.; Magnavita, V.; Monteleone, A.; et al. OPA1-related auditory neuropathy: Site of lesion and outcome of cochlear implantation. Brain 2015, 138, 563–576. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ballesteros, M.; del Castillo, F.J.; Martín, Y.; Moreno-Pelayo, M.A.; Morera, C.; Prieto, F.; Marco, J.; Morant, A.; Gallo-Terán, J.; Morales-Angulo, C.; et al. Auditory neuropathy in patients carrying mutations in the otoferlin gene (OTOF). Hum. Mutat. 2003, 22, 451–456. [Google Scholar] [CrossRef]
- Delmaghani, S.; del Castillo, F.; Michel, V.; Leibovici, M.; Aghaie, A.; Ron, U.; Van Laer, L.; Ben-Tal, N.; Van Camp, G.; Weil, D.; et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 2006, 38, 770–778. [Google Scholar] [CrossRef]
- Schoen, C.J.; Emery, S.B.; Thorne, M.C.; Ammana, H.R.; Sliwerska, E.; Arnett, J.; Hortsch, M.; Hannan, F.; Burmeister, M.; Lesperance, M. Increased activity of Diaphanous homolog 3 (DIAPH3)/diaphanous causes hearing defects in humans with auditory neuropathy and in Drosophila. Proc. Natl. Acad. Sci. USA 2010, 107, 13396–13401. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.J.; Li, Q.Z.; Rao, S.Q.; Lee, K.; Huang, X.S.; Yang, W.Y.; Zhai, S.Q.; Guo, W.W.; Guo, Y.F.; Yu, N.; et al. AUNX1, a novel locus responsible for X linked recessive auditory and peripheral neuropathy, maps to Xq23-27.3. J. Med. Genet. 2005, 43, e33. [Google Scholar] [CrossRef] [Green Version]
- Thirlwall, A.S.; Brown, D.J.; McMillan, P.M.; Barker, S.E.; Lesperance, M.M. Phenotypic Characterization of Hereditary Hearing Impairment Linked to DFNA25. Arch. Otolaryngol.-Head Neck Surg. 2003, 129, 830–835. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.W.; Oh, D.-Y.; Yi, E.; Liu, X.; Ling, J.; Kim, N.; Sharma, K.; Kim, T.Y.; Lee, S.; Kim, A.-R.; et al. A nonsense TMEM43 variant leads to disruption of connexin-linked function and autosomal dominant auditory neuropathy spectrum disorder. Proc. Natl. Acad. Sci. USA 2021, 118, e2019681118. [Google Scholar] [CrossRef]
- Kovach, M.; Campbell, K.; Herman, K.; Waggoner, B.; Gelber, D.; Hughes, L.; Kimonis, V. Anticipation in a unique family with Charcot-Marie-Tooth syndrome and deafness: Delineation of the clinical features and review of the literature. Am. J. Med. Genet. 2002, 108, 295–303. [Google Scholar] [CrossRef]
- Starr, A.; Michalewski, H.J.; Zeng, F.; Fujikawa-Brooks, S.; Linthicum, F.; Kim, C.S.; Winnier, D.; Keats, B. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser). Brain 2003, 126, 1604–1619. [Google Scholar] [CrossRef]
- Butinar, D.; Starr, A.; Zidar, J.; Koutsou, P.; Christodoulou, K. Auditory nerve is affected in one of two different point mutations of the neurofilament light gene. Clin. Neurophysiol. 2008, 119, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Kalaydjieva, L.; Gresham, D.; Gooding, R.; Heather, L.; Baas, F.; de Jonge, R.; Blechschmidt, K.; Angelicheva, D.; Chandler, D.; Worsley, P.; et al. N-myc Downstream-Regulated Gene 1 Is Mutated in Hereditary Motor and Sensory Neuropathy–Lom. Am. J. Hum. Genet. 2000, 67, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Bigas, N.; Olive, M.; Rabionet, R.; Ben-David, O.; Martínez-MatosJ, A.; Bravo, O.; Banchs, I.; Volpini, V.; Gasparini, P.; Avraham, K.B.; et al. Connexin 31 (GJB3) is expressed in the peripheral and auditory nerves and causes neuropathy and hearing impairment. Hum. Mol. Genet. 2001, 10, 947–952. [Google Scholar] [CrossRef] [Green Version]
- Bähr, M.; Andres, F.; Timmerman, V.; E Nelis, M.; Van Broeckhoven, C.; Dichgans, J. Central visual, acoustic, and motor pathway involvement in a Charcot-Marie-Tooth family with an Asn205Ser mutation in the connexin 32 gene. J. Neurol. Neurosurg. Psychiatry 1999, 66, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Amati-Bonneau, P.; Guichet, A.; Olichon, A.; Chevrollier, A.; Viala, F.; Miot, S.; Ayuso, C.; Odent, S.; Arrouet, C.; Verny, C.; et al. OPA1 R445H mutation in optic atrophy associated with sensorineural deafness. Ann. Neurol. 2005, 58, 958–963. [Google Scholar] [CrossRef]
- Carelli, V.; Schimpf, S.; Fuhrmann, N.; Valentino, M.L.; Zanna, C.; Iommarini, L.; Papke, M.; Schaich, S.; Tippmann, S.; Baumann, B.; et al. A clinically complex form of dominant optic atrophy (OPA8) maps on chromosome 16. Hum. Mol. Genet. 2011, 20, 1893–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, E.; Michaelides, M.; Tee, L.J.; Robson, A.; Rahman, F.; Pasha, S.; Luxon, L.M.; Moore, A.T.; Maher, E.R. Nonsense mutation in TMEM126A causing autosomal recessive optic atrophy and auditory neuropathy. Mol. Vis. 2010, 16, 650–664. [Google Scholar] [PubMed]
- Rance, G.; Fava, R.; Baldock, H.; Chong, A.; Barker, E.; Corben, L.; Delatycki, M.B. Speech perception ability in individuals with Friedreich ataxia. Brain 2008, 131, 2002–2012. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, R.; Zhao, H.; Peters, J.L.; Liu, Q.; Yang, L.; Han, D.; Greinwald, J.H., Jr.; Young, W.-Y.; Guan, M.-X. Clinical and molecular characterization of a Chinese patient with auditory neuropathy associated with mitochondrial 12S rRNA T1095C mutation. Am. J. Med. Genet. Part A 2005, 133A, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Bahmad, F.; Merchant, S.N.; Nadol, J.B.; Tranebjaerg, L. Otopathology in Mohr-Tranebjaerg syndrome. Laryngoscope 2007, 117, 1202–1208. [Google Scholar] [CrossRef] [Green Version]
- Ceranić, B.; Luxon, L.M. Progressive auditory neuropathy in patients with Leber’s hereditary optic neuropathy. J. Neurol. Neurosurg. Psychiatr. 2004, 75, 626–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ołdak, M.; Oziębło, D.; Pollak, A.; Stępniak, I.; Lazniewski, M.; Lechowicz, U.; Kochanek, K.; Furmanek, M.; Tacikowska, G.; Plewczynski, D.; et al. Novel neuro-audiological findings and further evidence for TWNK involvement in Perrault syndrome. J. Transl. Med. 2017, 15, 25. [Google Scholar] [CrossRef]
- Dulon, D.; Papal, S.; Patni, P.; Cortese, M.; Vincent, M.; Tertrais, M.; Emptoz, A.; Tlili, A.; Bouleau, Y.; Michel, V.; et al. Clarin-1 gene transfer rescues auditory synaptopathy in model of Usher syndrome. J. Clin. Investig. 2018, 128, 3382–3401. [Google Scholar] [CrossRef]
- Tranebjærg, L.; Strenzke, N.; Lindholm, S.; Rendtorff, N.D.; Poulsen, H.; Khandelia, H.; Kopec, W.; Lyngbye, T.J.B.; Hamel, C.; Delettre, C.; et al. The CAPOS mutation in ATP1A3 alters Na/K-ATPase function and results in auditory neuropathy which has implications for management. Qual. Life Res. 2018, 137, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, R.; Starr, A.; Michalewski, H.J.; Arslan, E. Neural and receptor cochlear potentials obtained by transtympanic electrocochleography in auditory neuropathy. Clin. Neurophysiol. 2008, 119, 1028–1041. [Google Scholar] [CrossRef] [Green Version]
- Santarelli, R.; Arslan, E. Electrocochleography. In Handbook of Clinical Neurophysiology; Celesia, G.G., Ed.; Disorders of Peripheral and Central Auditory Processing; Elsevier: Amsterdam, The Netherlands, 2013; pp. 83–113. [Google Scholar]
- Dallos, P.; Cheatham, M.A. Production of cochlear potentials by inner and outer hair cells. J. Acoust. Soc. Am. 1976, 60, 510–512. [Google Scholar] [CrossRef]
- Durrant, J.D.; Wang, J.; Ding, D.L.; Salvi, R.J. Are inner or outer hair cells the source of summating potentials recorded from the round wi‘ndow? J. Acoust. Soc. Am. 1998, 104, 370–377. [Google Scholar] [CrossRef]
- Pappa, A.K.; Hutson, K.A.; Scott, W.C.; Wilson, J.D.; Fox, K.E.; Masood, M.M.; Giardina, C.K.; Pulver, S.H.; Grana, G.D.; Askew, C.; et al. Hair cell and neural contributions to the cochlear summating potential. J. Neurophysiol. 2019, 121, 2163–2180. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Kiang, N.Y. Synchrony of Neural Activity in Electric Responses Evoked by Transient Acoustic Stimuli. J. Acoust. Soc. Am. 1958, 30, 107–114. [Google Scholar] [CrossRef]
- Bourien, J.; Tang, Y.; Batrel, C.; Huet, A.; Lenoir, M.; Ladrech, S.; Desmadryl, G.; Nouvian, R.; Puel, J.-L.; Wang, J. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J. Neurophysiol. 2014, 112, 1025–1039. [Google Scholar] [CrossRef] [Green Version]
- Carelli, V.; Ross-Cisneros, F.N.; Sadun, A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004, 23, 53–89. [Google Scholar] [CrossRef]
- Olichon, A.; Guillou, E.; Delettre, C.; Landes, T.; Arnauné-Pelloquin, L.; Emorine, L.J.; Mils, V.; Daloyau, M.; Hamel, C.; Amati-Bonneau, P.; et al. Mitochondrial dynamics and disease, OPA1. Biochim. Biophys. Acta 2006, 1763, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frezza, C.; Cipolat, S.; De Brito, O.M.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Danial, N.N.; De Strooper, B.; et al. OPA1 Controls Apoptotic Cristae Remodeling Independently from Mitochondrial Fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Lodi, R.; Tonon, C.; Valentino, M.L.; Iotti, S.; Clementi, V.; Malucelli, E.; Barboni, P.; Longanesi, L.; Schimpf, S.; Wissinger, B.; et al. Deficit of in vivo mitochondrial ATP production in OPA1-related dominant optic atrophy. Ann. Neurol. 2004, 56, 719–723. [Google Scholar] [CrossRef]
- Amati-Bonneau, P.; Valentino, M.L.; Reynier, P.; Gallardo, M.E.; Bornstein, B.; Boissière, A.; Campos, Y.; Rivera, H.; de la Aleja, J.G.; Carroccia, R.; et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ’plus’ phenotypes. Brain 2008, 131, 338–351. [Google Scholar] [CrossRef] [Green Version]
- Yu-Wai-Man, P.; Griffiths, P.G.; Gorman, G.S.; Lourenco, C.M.; Wright, A.F.; Auer-Grumbach, M.; Toscano, A.; Musumeci, O.; Valentino, M.L.; Caporali, L.; et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain A J. Neurol. 2010, 133, 771–786. [Google Scholar] [CrossRef]
- Miyamoto, R.T.; Kirk, K.H.; Renshaw, J.; Hussain, D. Cochlear Implantation in Auditory Neuropathy. Laryngoscope 1999, 109, 181–185. [Google Scholar] [CrossRef]
- Frewin, B.; Chung, M.; Donnelly, N. Bilateral cochlear implantation in Friedreich’s ataxia: A case study. Cochlear Implants Int. 2013, 14, 287–290. [Google Scholar] [CrossRef]
- Rodríguez-Ballesteros, M.; Reynoso, R.; Olarte, M.; Villamar, M.; Morera, C.; Santarelli, R.; Arslan, E.; Medá, C.; Curet, C.; Völter, C.; et al. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum. Mutat. 2008, 29, 823–831. [Google Scholar] [CrossRef]
- Roux, I.; Safieddine, S.; Nouvian, R.; Grati, M.; Simmler, M.-C.; Bahloul, A.; Perfettini, I.; Le Gall, M.; Rostaing, P.; Hamard, G.; et al. Otoferlin, Defective in a Human Deafness Form, Is Essential for Exocytosis at the Auditory Ribbon Synapse. Cell 2006, 127, 277–289. [Google Scholar] [CrossRef]
- Pangršič, T.; Lasarow, L.; Reuter, K.; Takago, H.; Schwander, M.; Riedel, D.; Frank, T.; Tarantino, L.M.; Bailey, J.S.; Strenzke, N.; et al. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nat. Neurosci. 2010, 13, 869–876. [Google Scholar] [CrossRef]
- Santarelli, R.; del Castillo, I.; Rodríguez-Ballesteros, M.; Scimemi, P.; Cama, E.; Arslan, E.; Starr, A. Abnormal Cochlear Potentials from Deaf Patients with Mutations in the Otoferlin Gene. J. Assoc. Res. Otolaryngol. 2009, 10, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Akil, O.; Dyka, F.; Calvet, C.; Emptoz, A.; Lahlou, G.; Nouaille, S.; de Monvel, J.B.; Hardelin, J.-P.; Hauswirth, W.W.; Avan, P.; et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl. Acad. Sci. USA 2019, 116, 4496–4501. [Google Scholar] [CrossRef] [Green Version]
- Rankovic, V.; Vogl, C.; Dörje, N.M.; Bahader, I.; Duque-Afonso, C.J.; Thirumalai, A.; Weber, T.; Kusch, K.; Strenzke, N.; Moser, T. Overloaded Adeno-Associated Virus as a Novel Gene Therapeutic Tool for Otoferlin-Related Deafness. Front. Mol. Neurosci. 2021, 13, 253. [Google Scholar] [CrossRef]
- Schaette, R.; McAlpine, D. Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. J. Neurosci. 2011, 31, 13452–13457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Hancock, K.E.; O’Brien, B.; Santarelli, R.; Liberman, M.C.; Maison, S.F. The summating potential in human electrocochleography: Gaussian models and Fourier analysis. J. Acoust. Soc. Am. 2021, 150, 2492–2502. [Google Scholar] [CrossRef]

| Locus | Gene | Transmission | Phenotype | Reference | |
|---|---|---|---|---|---|
| Isolated AN | |||||
| 2p23–p22 | OTOF | Recessive | Congenital profound deafness | [13] | |
| 2q31.1–q31.3 | PJVK | Recessive | Congenital profound deafness | [14] | |
| 13q21–q24 | DIAPH3 | Dominant | Moderate to profound deafness | [15] | |
| mtDNA | 12S rRNA (T1095C) | Moderate deafness | [16] | ||
| 12q23.1 | SLC17A8 | Dominant | Progressive | [17] | |
| 3P25.1 | TMEM43 | Dominant | Post-lingual moderate to profound deafness | [18] | |
| Non-isolated AN | |||||
| CMT 1A | 17p11.2–p12 | PMP22 | Dominant | Mild to severe deafness; demyelinating neuropathy | [19] |
| CMT 1B | 1q22 | MPZ | Dominant | Mild to severe deafness; demyelinating neuropathy | [20] |
| CMT 2E | 8p21 | NF-L | Dominant | Normal hearing; axonal neuropathy | [21] |
| CMT 4D | 8q24.3 | NDRG1 | Recessive | Mild to severe deafness; axonal/demyelinating neuropathy | [6,22] |
| CMT | 1p34 | GJB3 (Cx31) | Dominant | Mild deafness | [23] |
| CMT 1X | Xp13 | GJB1 (Cx32) | X-linked Dominant | Demyelinating neuropathy | [24] |
| ADOA | 3q28–q29 | OPA1 (R445H) | Dominant | Optic neuropathy; moderate deafness | [25] |
| ADOA | 16q21–q22 | Dominant | Optic neuropathy, cardiac abnormalities | [26] | |
| AROA | 11q14.1–11q22.3 | TMEM126A | Recessive | Optic neuropathy; mild hearing loss | [27] |
| Friedreich | 9q13 | FXN | Recessive | Ataxia; axonal neuropathy; optic neuropathy; cardiomyopathy; normal hearing threshold-mild deafness | [28] |
| AUNX1 | Xq23–q27.3 | X-linked Recessive | Sensory axonal neuropathy; mild-to-severe deafness | [29] | |
| DDON (Mohr-Tranebjaerg) | Xq22.1 | TIMM8A | X-linked Recessive | Progressive deafness; dystonia, optic neuropathy; dementia | [30] |
| LHON (Leber) | mtDNA | MTND4 (11778mtDNA) | Optic neuropathy; mild-to-moderate deafness | [31] | |
| Perrault | 10q24.31 | TWNK | Recessive | Hypogonadism, cerebellar atrophy, cochlear nerve thinning | [32] |
| USH3A | 3q25.1 | CLRN1 | Recessive | Retinitis pigmentosa | [33] |
| CAPOS | 19q13.2 | ATP1A3 | Dominant | Cerebellar ataxia, areflexia, pes cavus, optic atrophy | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santarelli, R.; Scimemi, P.; La Morgia, C.; Cama, E.; del Castillo, I.; Carelli, V. Electrocochleography in Auditory Neuropathy Related to Mutations in the OTOF or OPA1 Gene. Audiol. Res. 2021, 11, 639-652. https://doi.org/10.3390/audiolres11040059
Santarelli R, Scimemi P, La Morgia C, Cama E, del Castillo I, Carelli V. Electrocochleography in Auditory Neuropathy Related to Mutations in the OTOF or OPA1 Gene. Audiology Research. 2021; 11(4):639-652. https://doi.org/10.3390/audiolres11040059
Chicago/Turabian StyleSantarelli, Rosamaria, Pietro Scimemi, Chiara La Morgia, Elona Cama, Ignacio del Castillo, and Valerio Carelli. 2021. "Electrocochleography in Auditory Neuropathy Related to Mutations in the OTOF or OPA1 Gene" Audiology Research 11, no. 4: 639-652. https://doi.org/10.3390/audiolres11040059
APA StyleSantarelli, R., Scimemi, P., La Morgia, C., Cama, E., del Castillo, I., & Carelli, V. (2021). Electrocochleography in Auditory Neuropathy Related to Mutations in the OTOF or OPA1 Gene. Audiology Research, 11(4), 639-652. https://doi.org/10.3390/audiolres11040059







