Consequences of Prematurity on Cortisol Regulation and Adjustment Difficulties: A 9-Year Longitudinal Study
Abstract
:1. Introduction
1.1. Prematurity
1.2. Preterm Birth: A Stressful Event
1.2.1. Stress Regulation: Hypothalamic-Pituitary-Adrenal (HPA) Axis
1.2.2. Perinatal Stress
1.2.3. Parental Stress
1.2.4. Impact of Perinatal and Maternal Stress on Child Development
1.3. The Current Study
2. Materials and Methods
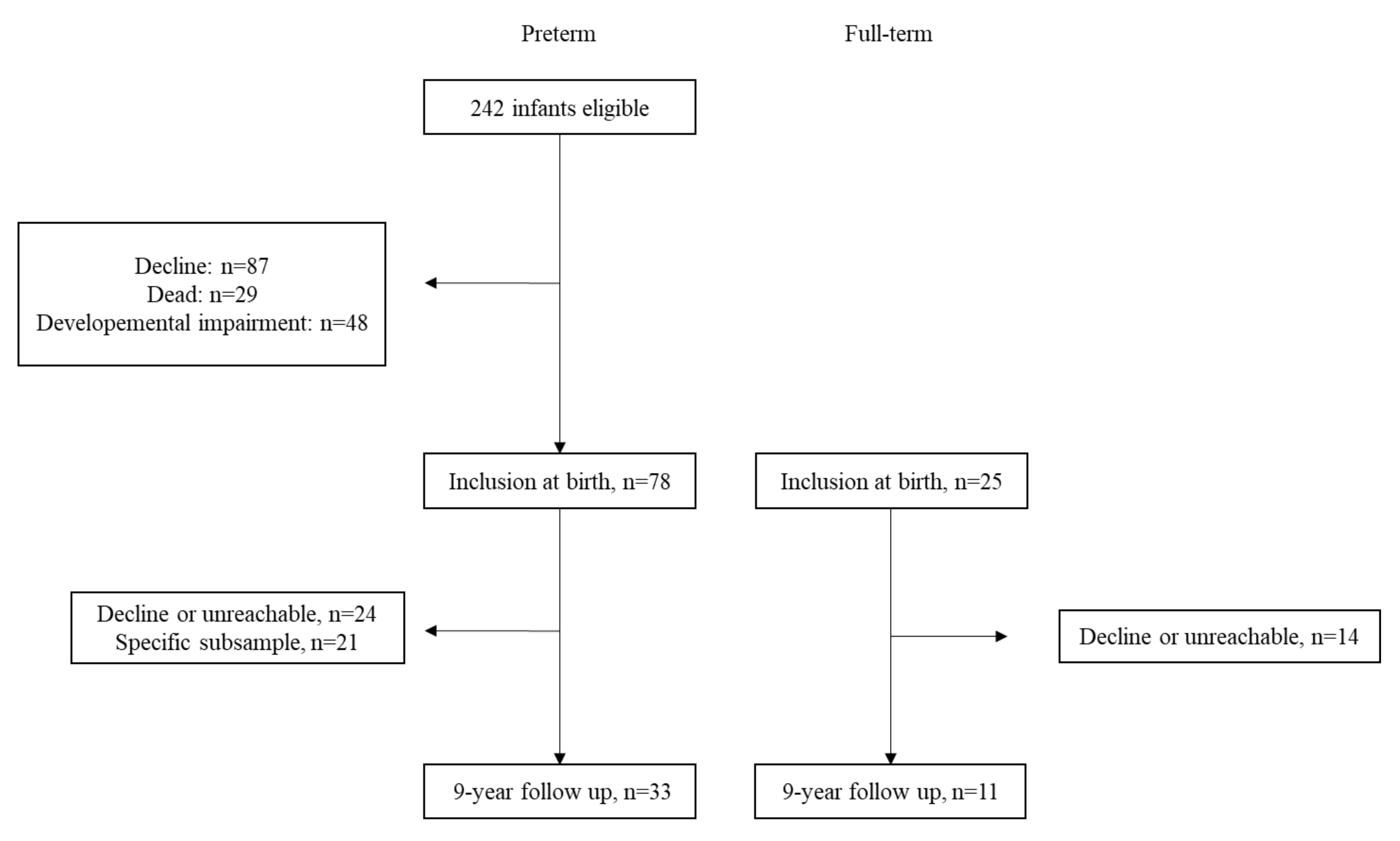
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Socio-Economic Status
2.3.2. Perinatal Risk Inventory: PERI
2.3.3. Cortisol Regulation
2.3.4. Maternal Post-Traumatic Stress Symptoms
2.3.5. Adjustment Problems
2.4. Data Analysis
3. Results
3.1. Between Group Differences on Neuroendocrine Regulation and Adjustment Problems
3.2. Association between Neuro-Endocrine Regulation, Adjustment Problems and Stress
4. Discussion
4.1. Cortisol Regulation
4.2. Adjustment Problems
4.3. Clinical Implications
4.4. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Preterm Birth: Fact Sheet; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Office Fédéral de la Statistique. Santé Des Nouveau-Nés; Office Fédéral de la Statistique: Neuchâtel, Switzerland, 2020. [Google Scholar]
- Cormack, B.E.; Harding, J.E.; Miller, S.P.; Bloomfield, F.H. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: A narrative review. Nutrients 2019, 11, 2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hack, M.; Fanaroff, A.A. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin. Neonatol. 2000, 5, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing Nutrition in Preterm Low Birth Weight Infants—Consensus Summary. Front. Nutr. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhutta, A.T.; Cleves, M.A.; Casey, P.H.; Cradock, M.M.; Anand, K.J.S. Cognitive and Behavioral Outcomes of School-Aged Children Who Were Born Preterm. JAMA 2002, 288, 728. [Google Scholar] [CrossRef]
- Cassiano, R.G.M.; Gaspardo, C.M.; Linhares, M.B.M. Prematurity, neonatal health status, and later child behavioral/emotional problems: A systematic review. Infant Ment. Health J. 2016, 37, 274–288. [Google Scholar] [CrossRef]
- Luu, T.M.; Katz, S.L.; Leeson, P.; Thébaud, B.; Nuyt, A.M. Preterm birth: Risk factor for early-onset chronic diseases. CMAJ 2016, 188, 736–740. [Google Scholar] [CrossRef] [Green Version]
- Woodward, L.J.; Moor, S.; Hood, K.M.; Champion, P.R.; Foster-Cohen, S.; Inder, T.E.; Austin, N.C. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, 339–344. [Google Scholar] [CrossRef]
- Blair, C. Stress and the Development of Self-Regulation in Context. Child Dev. Perspect. 2010, 4, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Chapieski, M.L.; Evankovich, K.D. Behavioral effects of prematurity. Semin. Perinatol. 1997, 21, 221–239. [Google Scholar] [CrossRef]
- Treyvaud, K.; Ure, A.; Doyle, L.W.; Lee, K.J.; Rogers, C.E.; Kidokoro, H.; Inder, T.E.; Anderson, P.J. Psychiatric outcomes at age seven for very preterm children: Rates and predictors. J. Child Psychol. Psychiatry Allied Discip. 2013, 54, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pereira, M.; Baños, L. Do healthy preterm children have behavior problems? An. Psicol. 2019, 35, 397–404. [Google Scholar] [CrossRef]
- Stene-Larsen, K.; Lang, A.M.; Landolt, M.A.; Latal, B.; Vollrath, M.E. Emotional and behavioral problems in late preterm and early term births: Outcomes at child age 36 months. BMC Pediatr. 2016, 16, 196. [Google Scholar] [CrossRef] [Green Version]
- Achenbach, T.M.; Howell, C.T.; Quay, H.C.; Conners, C.K. National survey of problems and competencies among four- to sixteen-year-olds: Parents’ reports for normative and clinical samples. Monogr. Soc. Res. Child Dev. 1991, 56, 1–131. [Google Scholar] [CrossRef]
- Lilienfeld, S.O. Comorbidity between and within childhood externalizing and internalizing disorders: Reflections and directions. J. Abnorm. Child Psychol. 2003, 31, 285–291. [Google Scholar] [CrossRef]
- White, B.A.; Jarrett, M.A.; Ollendick, T.H. Self-regulation deficits explain the link between reactive aggression and internalizing and externalizing behavior problems in children. J. Psychopathol. Behav. Assess. 2013, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Carragher, N.; Krueger, R.F.; Eaton, N.R.; Slade, T. Disorders without borders: Current and future directions in the meta-structure of mental disorders. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 339–350. [Google Scholar] [CrossRef]
- Willner, C.J.; Gatzke-Kopp, L.M.; Bray, B.C. The dynamics of internalizing and externalizing comorbidity across the early school years. Dev. Psychopathol. 2016, 28, 1033–1052. [Google Scholar] [CrossRef] [Green Version]
- Donado, C.; Friedrich, Y.; Kossowsky, J.; Locher, C.; Koechlin, H. Exposure to Parental Depressive Symptoms: A Longitudinal Analysis on the Association with Adolescents’ Depressive Symptoms and Adjustment Problems. J. Dev. Behav. Pediatr. 2020, 41, 522–533. [Google Scholar] [CrossRef]
- Koechlin, H.; Donado, C.; Berde, C.B.; Kossowsky, J. Effects of Childhood Life Events on Adjustment Problems in Adolescence: A Longitudinal Study. J. Dev. Behav. Pediatr. 2018, 39, 629–641. [Google Scholar] [CrossRef]
- Dimitrova, N.; Turpin, H.; Borghini, A.; Harari, M.M.; Urben, S.; Muller-Nix, C. Perinatal stress moderates the link between early and later emotional skills in very preterm-born children: An 11-year-long longitudinal study. Early Hum. Dev. 2018, 121, 8–14. [Google Scholar] [CrossRef]
- Cooke, R.W.I. Health, lifestyle, and quality of life for young adults born very preterm. Arch. Dis. Child. 2004, 89, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Hayes, B.; Sharif, F. Behavioural and emotional outcome of very low birth weight infants- literature review. J. Matern. Fetal Neonatal Med. 2009, 22, 849–856. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the Stress Response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar]
- Guilliams, T.G.; Edwards, L. Chronic stress and the HPA axis: Clinical assessment and therapeutic considerations. Standard 2010, 9, 1–12. [Google Scholar]
- Van Bodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front. Cell. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Faravelli, C. Childhood stressful events, HPA axis and anxiety disorders. World J. Psychiatry 2012, 2, 13. [Google Scholar] [CrossRef]
- Ng, P.C. The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 82, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Lupien, S.J.; de Leon, M.; de Santi, S.; Convit, A.; Tarshish, C.; Nair, N.P.; Thakur, M.; McEwen, B.S.; Hauger, R.L.; Meaney, M.J. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998, 1, 69–73. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Maitre, N.L.; Key, A.P.; Chorna, O.D.; Slaughter, J.C.; Matusz, P.J.; Wallace, M.T.; Murray, M.M. The Dual Nature of Early-Life Experience on Somatosensory Processing in the Human Infant Brain. Curr. Biol. 2017, 27, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunau, R.E.; Holsti, L.; Peters, J.W.B. Long-term consequences of pain in human neonates. Semin. Fetal Neonatal Med. 2006, 11, 268–275. [Google Scholar] [CrossRef] [PubMed]
- El-Metwally, D.E.; Medina, A.E. The potential effects of NICU environment and multisensory stimulation in prematurity. Pediatric Res. 2020, 88, 161–162. [Google Scholar] [CrossRef]
- Graham, Y.P.; Heim, C.; Goodman, S.H.; Miller, A.H.; Nemeroff, C.B. The effects of neonatal stress on brain development: Implications for psychopathology. Dev. Psychopathol. 1999, 11, 545–565. [Google Scholar] [CrossRef]
- Lammertink, F.; Vinkers, C.H.; Tataranno, M.L.; Benders, M.J.N.L. Premature Birth and Developmental Programming: Mechanisms of Resilience and Vulnerability. Front. Psychiatry 2021, 11, 1515. [Google Scholar] [CrossRef]
- Sullivan, M.C.; Winchester, S.B.; Bryce, C.I.; Granger, D.A. Prematurity and perinatal adversity effects hypothalamic-pituitary-adrenal axis reactivity to social evaluative threat in adulthood. Dev. Psychobiol. 2017, 59, 976–983. [Google Scholar] [CrossRef]
- Ionio, C.; Mascheroni, E.; Colombo, C.; Castoldi, F.; Lista, G. Stress and feelings in mothers and fathers in NICU: Identifying risk factors for early interventions. Prim. Heal. Care Res. Dev. 2019, 20, e81. [Google Scholar] [CrossRef] [Green Version]
- Pierrehumbert, B.; Nicole, A.; Muller-Nix, C.; Forcada-Guex, M.; Ansermet, F. Parental post-traumatic reactions after premature birth: Implications for sleeping and eating problems in the infant. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F400–F404. [Google Scholar] [CrossRef]
- Barkmann, C.; Helle, N.; Bindt, C. Is very low infant birth weight a predictor for a five-year course of depression in parents? A latent growth curve model. J. Affect. Disord. 2018, 229, 415–420. [Google Scholar] [CrossRef]
- Treyvaud, K.; Lee, K.J.; Doyle, L.W.; Anderson, P.J. Very preterm birth influences parental mental health and family outcomes seven years after birth. J. Pediatr. 2014, 164, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Forcada-Guex, M.; Borghini, A.; Pierrehumbert, B.; Ansermet, F.; Muller-Nix, C. Prematurity, maternal posttraumatic stress and consequences on the mother-infant relationship. Early Hum. Dev. 2011, 87, 21–26. [Google Scholar] [CrossRef]
- Hagen, I.H.; Iversen, V.C.; Svindseth, M.F. Differences and similarities between mothers and fathers of premature children: A qualitative study of parents’ coping experiences in a neonatal intensive care unit. BMC Pediatr. 2016, 16, 92. [Google Scholar] [CrossRef] [Green Version]
- Finken, M.J.J.; Van Der Voorn, B.; Hollanders, J.J.; Ruys, C.A.; De Waard, M.; Van Goudoever, J.B.; Rotteveel, J. Programming of the Hypothalamus-Pituitary-Adrenal Axis by Very Preterm Birth. Ann. Nutr. Metab. 2017, 70, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Habersaat, S.; Borghini, A.; Nessi, J.; Forcada-Guex, M.; Müller-Nix, C.; Pierrehumbert, B.; Ansermet, F. Effects of Perinatal Stress and Maternal Traumatic Stress on the Cortisol Regulation of Preterm Infants. J. Trauma. Stress 2014, 27, 488–491. [Google Scholar] [CrossRef]
- Matthews, S. Early programming of the hypothalamo–pituitary–adrenal axis. Trends Endocrinol. Metab. 2002, 13, 373–380. [Google Scholar] [CrossRef]
- Philbrook, L.E.; Hozella, A.C.; Kim, B.R.; Jian, N.; Shimizu, M.; Teti, D.M. Maternal emotional availability at bedtime and infant cortisol at 1 and 3months. Early Hum. Dev. 2014, 90, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Grunau, R.E.; Haley, D.W.; Whitfield, M.F.; Weinberg, J.; Yu, W.; Thiessen, P. Altered Basal Cortisol Levels at 3, 6, 8 and 18 Months in Infants Born at Extremely Low Gestational Age. J. Pediatr. 2007, 150, 151–156. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999, 22, 105–122. [Google Scholar] [CrossRef] [Green Version]
- Lengua, L.J.; Zalewski, M.; Fisher, P.; Moran, L. Does HPA-Axis Dysregulation Account for the Effects of Income on Effortful Control and Adjustment in Preschool Children? Infant Child Dev. 2013, 22, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, O.; Eras, Z.; Sari, F.N.; Dizdar, E.A.; Uras, N.; Canpolat, F.E.; Oguz, S.S. Does maternal psychological distress affect neurodevelopmental outcomes of preterm infants at a gestational age of ≤32 weeks. Early Hum. Dev. 2017, 104, 27–31. [Google Scholar] [CrossRef]
- Brumariu, L.E.; Kerns, K.A. Parent-child attachment and internalizing symptoms in childhood and adolescence: A review of empirical findings and future directions. Dev. Psychopathol. 2010, 22, 177–203. [Google Scholar] [CrossRef]
- Pisoni, C.; Garofoli, F.; Baiardini, I.; Tzialla, C.; Stronati, M. The development of parents-infant relationship in high-risk pregnancies and preterm birth. J. Pediatric Neonatal Individ. Med. 2014, 3, e030233. [Google Scholar] [CrossRef]
- Turpin, H.; Urben, S.; Ansermet, F.; Borghini, A.; Murray, M.M.; Muller-Nix, C. The interplay between prematurity, maternal stress and children’s intelligence quotient at age 11: A longitudinal study. Sci. Rep. 2019, 9, 450. [Google Scholar] [CrossRef]
- Largo, R.H.; Pfister, D.; Molinari, L.; Kundu, S.; Lipp, A.; Due, G. Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev. Med. Child Neurol. 2008, 31, 440–456. [Google Scholar] [CrossRef]
- McGauhey, P.J.; Starfield, B.; Alexander, C.; Ensminger, M.E. Social environment and vulnerability of low birth weight children: A social-epidemiological perspective. Pediatrics 1991, 88, 943–953. [Google Scholar] [CrossRef]
- Habersaat, S.; Pierrehumbert, B.; Forcada-Guex, M.; Nessi, J.; Ansermet, F.; Müller-Nix, C.; Borghini, A. Early stress exposure and later cortisol regulation: Impact of early intervention on mother–infant relationship in preterm infants. Psychol. Trauma: Theory Res. Pract. Policy 2014, 6, 457–464. [Google Scholar] [CrossRef]
- Scheiner, A.P.; Sexton, M.E. Prediction of developmental outcome using a perinatal risk inventory. Pediatrics 1991, 88, 1135–1143. [Google Scholar] [CrossRef]
- Pierrehumbert, B.; Borghini, A.; Forcada-Guex, M.; Jaunin, L.; Müller-Nix, C.; Ansermet, F. Validation française d’un questionnaire de stress post-traumatique destiné aux parents d’enfants présentant un risque périnatal élevé. Ann. Médico-Psychol. Rev. Psychiatr. 2004, 162, 711–721. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Edelbrock, C. Child behavior checklist. Burlington 1991, 7, 371–392. [Google Scholar]
- Pierrehumbert, B.; Ramstein, T.; Karmaniola, A.; Halfon, O. Child care in the preschool years: Attachment, behaviour problems and cognitive development. Eur. J. Psychol. Educ. 1996, 11, 201–214. [Google Scholar] [CrossRef]
- Dressendörfer, R.A.; Kirschbaum, C.; Rohde, W.; Stahl, F.; Strasburger, C.J. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. Mol. Biol. 1992, 43, 683–692. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Braaten, E.B. Child Behavior Checklist. In The SAGE Encyclopedia of Intellectual and Developmental Disorders; SAGE Publications, Inc.: Los Angeles, CA, USA, 2018. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.; Stalder, T.; Jarczok, M.; Almeida, D.M.; Badrick, E.; Bartels, M.; Boomsma, D.I.; Coe, C.L.; Dekker, M.C.J.; Donzella, B.; et al. The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies. Psychoneuroendocrinology 2016, 73, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Granger, D.A.; Serbin, L.A.; Schwartzman, A.; Lehoux, P.; Cooperman, J.; Ikeda, S. Children’s Salivary Cortisol, Internalising Behaviour Problems, and Family Environment: Results from the Concordia Longitudinal Risk Project. Int. J. Behav. Dev. 1998, 22, 707–728. [Google Scholar] [CrossRef]
- Oosterlaan, J.; Geurts, H.M.; Knol, D.L.; Sergeant, J.A. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Res. 2005, 134, 1–10. [Google Scholar] [CrossRef]
- Shirtcliff, E.A.; Granger, D.A.; Booth, A.; Johnson, D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev. Psychopathol. 2005, 17, 167–184. [Google Scholar] [CrossRef]
- De Graaf, J.; Van Den Akker, E.L.T.; Van Lingen, R.A.; Groot Jebbink, L.J.M.; De Jong, F.H.; Grunau, R.E.; Van Dijk, M.; Tibboel, D. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. J. Pediatr. 2014, 165, 459–463. [Google Scholar] [CrossRef]
- Ladd, C.O.; Huot, R.L.; Thrivikraman, K.V.; Nemeroff, C.B.; Meaney, M.J.; Plotsky, P.M. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 2000, 122, 81–103. [Google Scholar] [CrossRef]
- Pryce, C.R.; Feldon, J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci. Biobehav. Rev. 2003, 27, 57–71. [Google Scholar] [CrossRef]
- Andersen, S.L. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Brummelte, S.; Grunau, R.E.; Chau, V.; Poskitt, K.J.; Brant, R.; Vinall, J.; Gover, A.; Synnes, A.R.; Miller, S.P. Procedural pain and brain development in premature newborns. Ann. Neurol. 2012, 71, 385–396. [Google Scholar] [CrossRef]
- Duerden, E.G.; Grunau, R.E.; Guo, T.; Foong, J.; Pearson, A.; Au-Young, S.; Lavoie, R.; Chakravarty, M.M.; Chau, V.; Synnes, A.; et al. Early Procedural Pain Is Associated with Regionally-Specific Alterations in Thalamic Development in Preterm Neonates. J. Neurosci. 2018, 38, 878–886. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.A.C.; Woodward, L.J.; Horwood, L.J.; Moor, S. Development of Emotional and Behavioral Regulation in Children Born Extremely Preterm and Very Preterm: Biological and Social Influences. Child Dev. 2008, 79, 1444–1462. [Google Scholar] [CrossRef]
- Wadsby, M.; Nelson, N.; Ingemansson, F.; Samuelsson, S.; Leijon, I. Behaviour problems and cortisol levels in very-low-birth-weight children. Nord. J. Psychiatry 2014, 68, 626–632. [Google Scholar] [CrossRef]
- Loe, I.M.; Lee, E.S.; Luna, B.; Feldman, H.M. Behavior problems of 9–16year old preterm children: Biological, sociodemographic, and intellectual contributions. Early Hum. Dev. 2011, 87, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Elgen, I.; Sommerfelt, K.; Markestad, T. Population based, controlled study of behavioural problems and psychiatric disorders in low birthweight children at 11 years of age. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, 128–132. [Google Scholar] [CrossRef]
- Bartal, T.; Adams, M.; Natalucci, G.; Borradori-Tolsa, C.; Latal, B. Behavioral problems in very preterm children at five years of age using the Strengths and Difficulties Questionnaire: A multicenter cohort study. Early Hum. Dev. 2020, 151, 105200. [Google Scholar] [CrossRef]
- Schechter, D.S.; Suardi, F.; Manini, A.; Cordero, M.I.; Rossignol, A.S.; Merminod, G.; Gex-Fabry, M.; Moser, D.A.; Serpa, S.R. How do maternal PTSD and alexithymia interact to impact maternal behavior? Child Psychiatry Hum. Dev. 2015, 46, 406–417. [Google Scholar] [CrossRef]
- Muller-Nix, C.; Forcada-Guex, M.; Pierrehumbert, B.; Jaunin, L.; Borghini, A.; Ansermet, F. Prematurity, maternal stress and mother-child interactions. Early Hum. Dev. 2004, 79, 145–158. [Google Scholar] [CrossRef]
- Giddens, A.; Bowlby, J. Attachment and Loss, Volume I: Attachment. Br. J. Sociol. 1970, 21, 111. [Google Scholar] [CrossRef]
- Gondwe, K.W.; Holditch-Davis, D. Posttraumatic stress symptoms in mothers of preterm infants. Int. J. Afr. Nurs. Sci. 2015, 3, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.; Perry, R.; Sloan, A.; Kleinhaus, K.; Burtchen, N. Infant Bonding and Attachment to the Caregiver: Insights from Basic and Clinical Science. Clin. Perinatol. 2011, 38, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015, 2015, CD005495. [Google Scholar] [CrossRef]
- Landry, S.H.; Smith, K.E.; Swank, P.R. Responsive parenting: Establishing early foundations for social, communication, and independent problem-solving skills. Dev. Psychol. 2006, 42, 627–642. [Google Scholar] [CrossRef] [Green Version]
- Landsem, I.P.; Handegård, B.H.; Ulvund, S.E.; Kaaresen, P.I.; Rønning, J.A. Early intervention influences positively quality of life as reported by prematurely born children at age nine and their parents: A randomized clinical trial. Health Qual. Life Outcomes 2015, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- McKenzie-McHarg, K.; Ayers, S.; Ford, E.; Horsch, A.; Jomeen, J.; Sawyer, A.; Stramrood, C.; Thomson, G.; Slade, P. Post-traumatic stress disorder following childbirth: An update of current issues and recommendations for future research. J. Reprod. Infant Psychol. 2015, 33, 219–237. [Google Scholar] [CrossRef] [Green Version]
- Als, H. A Synactive Model of Neonatal Behavioral Organization. Phys. Occup. Ther. Pediatr. 1986, 6, 3–53. [Google Scholar] [CrossRef]
- Cho, E.-S.; Kim, S.-J.; Kwon, M.S.; Cho, H.; Kim, E.H.; Jun, E.M.; Lee, S. The Effects of Kangaroo Care in the Neonatal Intensive Care Unit on the Physiological Functions of Preterm Infants, Maternal–Infant Attachment, and Maternal Stress. J. Pediatric Nurs. 2016, 31, 430–438. [Google Scholar] [CrossRef]
- Lee, J.; Bang, K.-S. The Effects of Kangaroo Care on Maternal Self-esteem and Premature Infants’ Physiological Stability. Korean J. Women Health Nurs. 2011, 17, 454. [Google Scholar] [CrossRef] [Green Version]
- Gitau, R.; Modi, N.; Gianakoulopoulos, X.; Bond, C.; Glover, V.; Stevenson, J. Acute effects of maternal skin-to-skin contact and massage on saliva cortisol in preterm babies. J. Reprod. Infant Psychol. 2002, 20, 83–88. [Google Scholar] [CrossRef]
- Mörelius, E.; Örtenstrand, A.; Theodorsson, E.; Frostell, A. A randomised trial of continuous skin-to-skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Hum. Dev. 2015, 91, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milgrom, J.; Newnham, C.; Anderson, P.J.; Doyle, L.W.; Gemmill, A.W.; Lee, K.; Hunt, R.W.; Bear, M.; Inder, T. Early Sensitivity Training for Parents of Preterm Infants: Impact on the Developing Brain. Pediatric Res. 2010, 67, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulou, E.; Fox, J.R.E. Effect of kangaroo mother care on maternal mood and interaction patterns between parents and their preterm, low birth weight infants: A systematic review. Infant Ment. Health J. 2014, 35, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Bullinger, A.; Goubet, N. Le bébé prématuré, acteur de son développement. Enfance 1999, 52, 27–32. [Google Scholar] [CrossRef]
- White-Traut, R.C.; Schwertz, D.; McFarlin, B.; Kogan, J. Salivary cortisol and behavioral state responses of healthy newborn infants to tactile-only and multisensory interventions. J. Obstet. Gynecol. Neonatal Nurs. JOGNN 2009, 38, 22–34. [Google Scholar] [CrossRef] [PubMed]


| Included (N = 44) | Dropout (N = 38) | p1 | p2 | p3 | |||
|---|---|---|---|---|---|---|---|
| 1. Preterm | 2. Full-Term | 3. Preterm | 4. Full-Term | ||||
| n = 33 | n = 11 | n = 24 | n = 14 | ||||
| GA | 30.11 (1.84) | 40.18 (0.90) | 30.15 (2.00) | 39.34 (1.49) | 0.001 | ns | ns |
| Gender (F/M) | 54.5/45.5 | 36.4/63.6 | 37.5/62.5 | 35.7/64.3 | ns | ns | ns |
| SES | 2.71 (0.61) | 2.55 (0.47) | 2.54 (0.88) | 2.56 (0.93) | ns | ns | ns |
| Mat. Age (yrs) | 32.73 (4.30) | 31.64 (5.08) | 33.75 (4.19) | 30.86 (5.17) | ns | ns | ns |
| PERI | 7.73 (2.38) | 0.00 (0.00) | 8.08 (3.45) | 0.00 (0.00) | <0.001 | ns | ns |
| BW (gr) | 1369.24 (373.63) | 3383.00 (425.02) | 1406.46 (418.73) | 3256.43 (365.36) | <0.001 | ns | ns |
| PPQ | 2.73 (2.67) | 1.22 (1.64) | 3.56 (3.31) | 0.90 (1.45) | ns | ns | ns |
| Preterm (n = 33) | Full-Term (n = 11) | p 1 | ɳ2p | |
|---|---|---|---|---|
| AUCG | 106.51 (42.14) | 35.39 (17.94) | <0.001 | 0.34 |
| Adjustment problems | 52.63 (7.04) | 53.09 (6.93) | ns | - |
| PERI | Maternal PPQ | AUCG at 9y | AUCG at 6m | |
|---|---|---|---|---|
| Maternal PTSD | 0.29 | |||
| AUCG at 9y | 0.53 ** | 0.22 | ||
| AUCG at 6m | −0.26 | −0.37 * | −0.17 | |
| Adjustment problems | 0.05 | 0.41 ** | −0.25 | 0.04 |
| Criterion | Variables | β | p |
|---|---|---|---|
| AUCG at 9y | Age | −0.364 | 0.022 |
| PERI | 0.418 | 0.012 | |
| Maternal PPQ | 0.271 | 0.137 | |
| Adjustment problems | −0.414 | 0.014 | |
| AUCG at 6m | 0.136 | 0.403 | |
| Adjustment problems | Age | −0.222 | 0.245 |
| PERI | 0.236 | 0.243 | |
| Maternal PPQ | 0.522 | 0.009 | |
| AUCG 9y | −0.549 | 0.014 | |
| AUCG at 6m | 0.232 | 0.212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urfer, A.; Turpin, H.; Dimitrova, N.; Borghini, A.; Plessen, K.J.; Morisod Harari, M.; Urben, S. Consequences of Prematurity on Cortisol Regulation and Adjustment Difficulties: A 9-Year Longitudinal Study. Children 2022, 9, 9. https://doi.org/10.3390/children9010009
Urfer A, Turpin H, Dimitrova N, Borghini A, Plessen KJ, Morisod Harari M, Urben S. Consequences of Prematurity on Cortisol Regulation and Adjustment Difficulties: A 9-Year Longitudinal Study. Children. 2022; 9(1):9. https://doi.org/10.3390/children9010009
Chicago/Turabian StyleUrfer, Auriana, Hélène Turpin, Nevena Dimitrova, Ayala Borghini, Kerstin Jessica Plessen, Mathilde Morisod Harari, and Sébastien Urben. 2022. "Consequences of Prematurity on Cortisol Regulation and Adjustment Difficulties: A 9-Year Longitudinal Study" Children 9, no. 1: 9. https://doi.org/10.3390/children9010009
APA StyleUrfer, A., Turpin, H., Dimitrova, N., Borghini, A., Plessen, K. J., Morisod Harari, M., & Urben, S. (2022). Consequences of Prematurity on Cortisol Regulation and Adjustment Difficulties: A 9-Year Longitudinal Study. Children, 9(1), 9. https://doi.org/10.3390/children9010009






