1
The Vascular Biology Unit, Queensland Research Centre for Peripheral Vascular Disease, College of Medicine & Dentistry, James Cook University, Townsville, QLD 4811, Australia
2
Department of Vascular and Endovascular Surgery, the Townsville Hospital, Townsville, QLD 4810, Australia
Int. J. Mol. Sci. 2015, 16(5), 11294-11322; https://doi.org/10.3390/ijms160511294 - 18 May 2015
Cited by 130 | Viewed by 25278
Abstract
Peripheral artery disease (PAD) is due to the blockage of the arteries supplying blood to the lower limbs usually secondary to atherosclerosis. The most severe clinical manifestation of PAD is critical limb ischemia (CLI), which is associated with a risk of limb loss
[...] Read more.
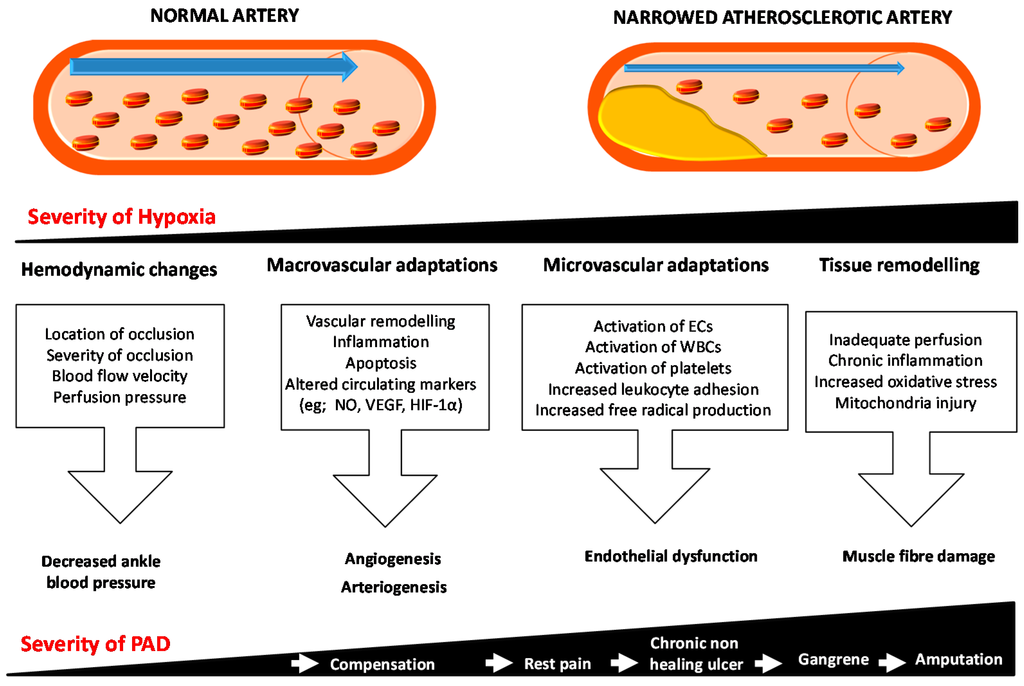
Peripheral artery disease (PAD) is due to the blockage of the arteries supplying blood to the lower limbs usually secondary to atherosclerosis. The most severe clinical manifestation of PAD is critical limb ischemia (CLI), which is associated with a risk of limb loss and mortality due to cardiovascular events. Currently CLI is mainly treated by surgical or endovascular revascularization, with few other treatments in routine clinical practice. There are a number of problems with current PAD management strategies, such as the difficulty in selecting the appropriate treatments for individual patients. Many patients undergo repeated attempts at revascularization surgery, but ultimately require an amputation. There is great interest in developing new methods to identify patients who are unlikely to benefit from revascularization and to improve management of patients unsuitable for surgery. Circulating biomarkers that predict the progression of PAD and the response to therapies could assist in the management of patients. This review provides an overview of the pathophysiology of PAD and examines the association between circulating biomarkers and PAD presence, severity and prognosis. While some currently identified circulating markers show promise, further larger studies focused on the clinical value of the biomarkers over existing risk predictors are needed.
Full article
(This article belongs to the Special Issue Advances in Peripheral Artery Disease)
▼
Show Figures