Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways
Abstract
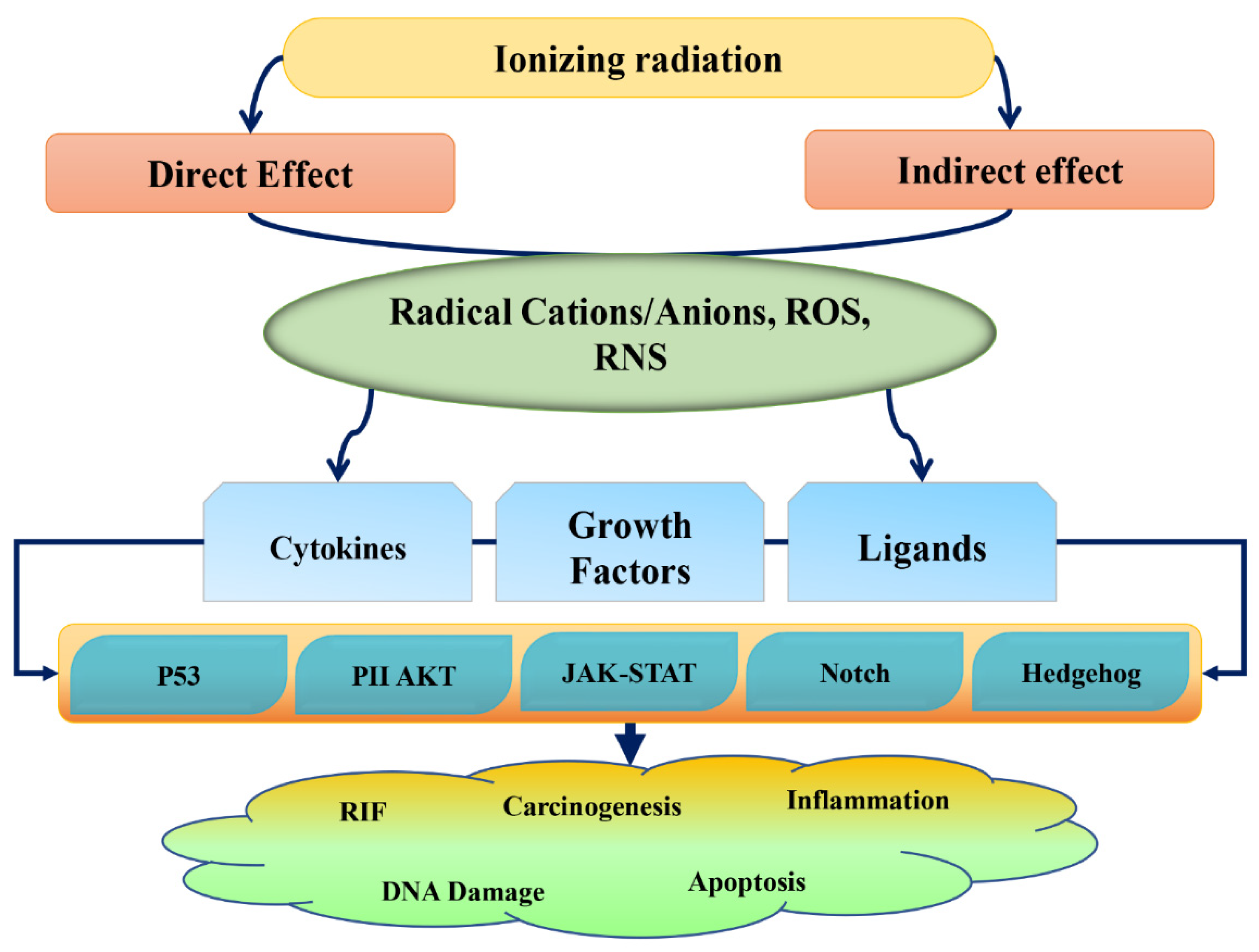
:1. Introduction
2. Radiation Damage and Phytochemical Action
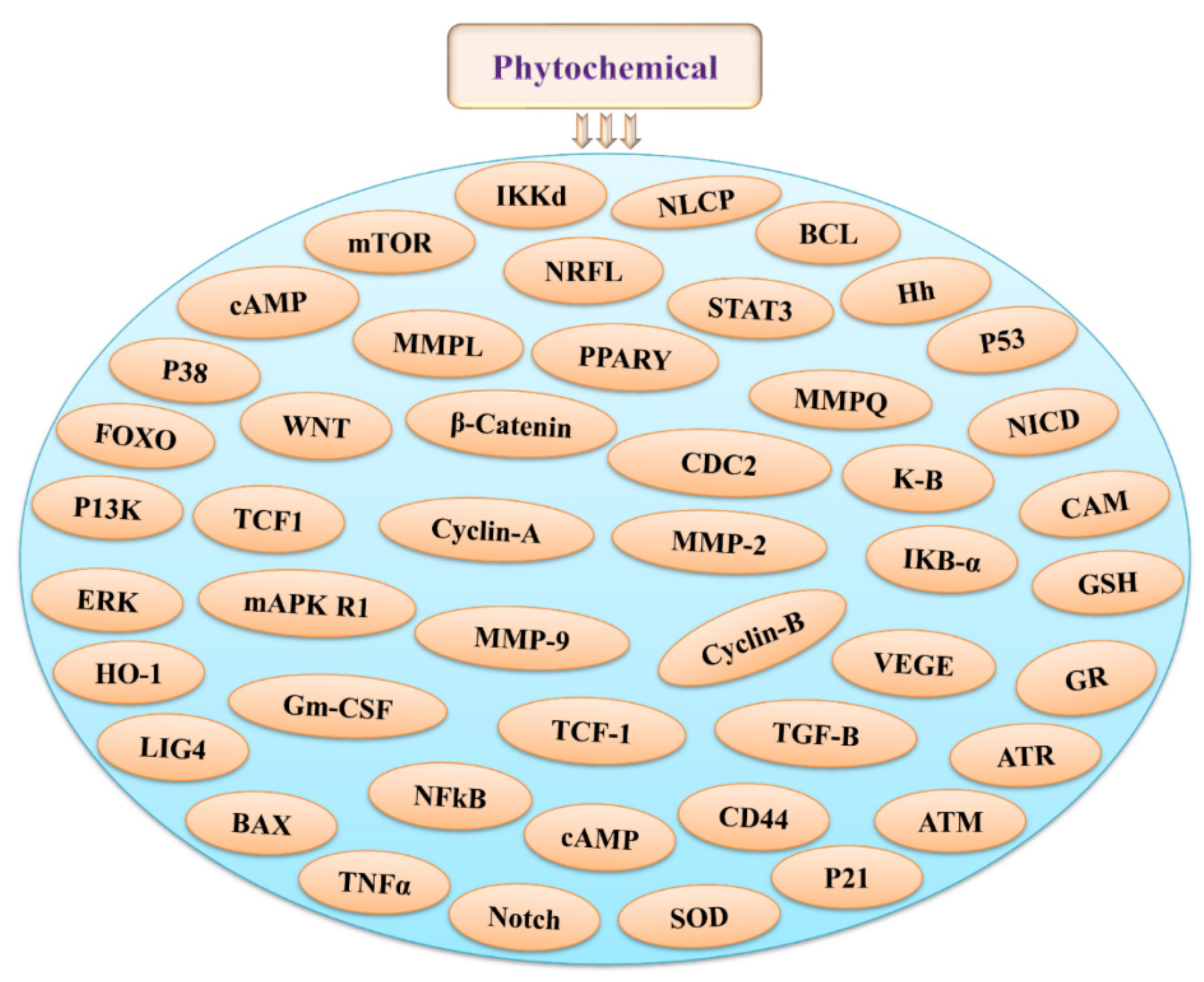
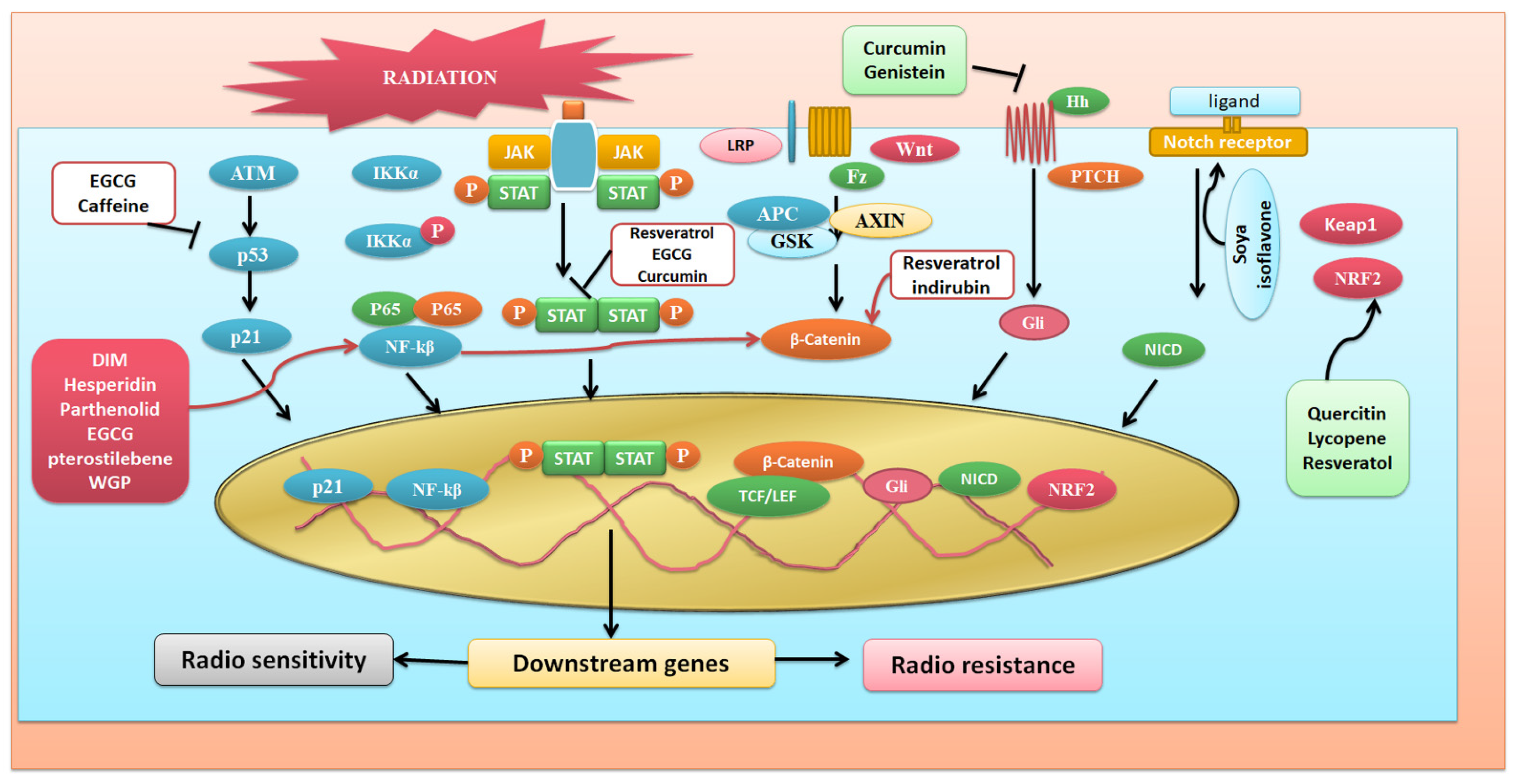
3. Phytochemicals and Their Possible Roles in Radioprotection via Different Signaling Pathways
3.1. NFκB Signaling Targetted Pathway
3.2. Targeting Wnt Signaling Pathway
3.3. Targeting Nrf2 Signaling Pathway
3.4. JAK/STAT Pathway
3.5. Agents Targeting P53 Signaling Pathway
3.6. Notch Signaling
3.7. Hedgehog Signaling
3.8. PI-Akt Signaling
| Compound Name | Signaling Target | Effect/Possible Role | Reference |
|---|---|---|---|
| Allicin | JNK pathway | Downregulate ICAM-1 expression | [162] |
| Apigenin | Nf-kβ pathway | Modulate p53, p21, Bax caspase3 & 9 | [163] |
| Arctiin | Wnt, MAPK pathway | [164] | |
| Baicalein | Nrf2 pathway | Stimulates ERK & Nrf2 activity | [94] |
| Betullinic acid | Nf-kβ pathway | Act as a radio sensitizer in cancer cell | [27] |
| Caffeine | p53 signaling | Increases ATM activity | [129] |
| Carvacrol | TNF α signaling | Decreases radiation induced oxidative stress | [165] |
| Chlorophyllin | Nrf 2 & Nf-kβ pathway | Possesses antioxidant, antiapoptotic activity | [166] |
| Curcumin | Notch pathway Nrf2 pathway | Decreases Notch 1 & 2 activity Induces PI3K, ERK, HO-1, P38-MAPK | [140,167] [83,168] |
| DIM | Nf-kβ pathway | ATM, DBR | [152] |
| Diosmin | Wnt/β-catenin pathway | Increases PPARγ expression & possess antioxidant, anti-inflammatory, anti-apoptotic property | [67] |
| Diospyrin (Diospyrin dimethylether) | P53 and Nf-kβ pathway | Downregulate COX-2, Bcl-2, Upregulates p53, p21 | [169] |
| EGCG | Nrf2 pathway | Induces PI3K, ERK, HO-1, P38-MAPK | [83,168] |
| Ferulic acid | c-JNK, ICAM-1, VCAM-1 mediated signaling | Antioxidant and Anti-inflammatory Activity | [170] |
| Fucoidan | TGF-β, Smad pathway | Inhibits TGF-β, Smad activity | [171] |
| Genistein | Hedghog pathway Notch pathway | Down regulate Hedgehog-GLI 1 Activity Decreases Notch 1 & 2 activity | [152] [140,167] |
| Hesperidin | Nf-kβ pathway | Increases COX2 & NO activity | [172] |
| Lycopene | Nf-kβ, JAK-STAT pathway | Possesses antioxidant, anti-inflammatory activity Inhibits NF-kB, p65, STAT3, IL-6, TNF-α, COX2, PGE2 | [173] |
| Mangiferin | Nrf2 pathway | Increases NOQ1 level | [174] |
| Melatonin | Nf-kβ, PI-Akt pathway | Decreases p-AKT, p-ERK, COX2, p65 | [175] |
| Parthenolide | Nf-kβ pathway | Inhibit NF-KB signaling | [176] |
| Piperine | Notch pathway | Decreases Notch 1 & 2 activity | [140,167] |
| Quercitin | Nf-kβ pathway | Inhibits ERK and p38 | [177] |
| Resveratol | Nf-kβ pathway Notch pathway | Decreases NF KB signalling of p65 & IKB kinase activity | [55] [140,167] |
| Rutin | PI3K/AKT/GSK-3β/NRF-2-pathway | Increases p-PI3K, p-AKT and p-GSK-3β activity | [178] |
| Saponin | Hedgehog pathway | Up regulate VEGF & Angiopoetin1 | [178] |
| Soya isoflavon | Notch pathway | Up regulate Notch 1 & HES 5 activities | [179] |
| Sulphora phane | Wnt/β-catenin pathway | Down regulate Wnt/B Catenine activity | [74] |
| Thymol | TNF α signaling | Decreases radiation induced oxidative stress | [165] |
| Ursolic acid | Nf-kB and JNK pathway | Decreases Nf-kB, IL-1β, TNF-α, IL-6 | [179] |
| Vanillin | P53-NOXA pathway | Decreases p53 activity | [180] |
| WGP | Nf-kβ, P38-MAPK pathway | Decreases level of ROS & RNS Production | [161] |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mu, H.; Sun, J.; Li, L.; Yin, J.; Hu, N.; Zhao, W.; Ding, D.; Yi, L. Ionizing radiation exposure: Hazards, prevention, and biomarker screening. Environ. Sci. Pollut. Res. 2018, 25, 15294–15306. [Google Scholar] [CrossRef] [PubMed]
- Sutou, S. Low-dose radiation from A-bombs elongated lifespan and reduced cancer mortality relative to un-irradiated individuals. Genes Environ. 2018, 40, 26. [Google Scholar] [CrossRef] [PubMed]
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef]
- Brücher, B.L.; Jamall, I.S. Cell-cell communication in the tumor microenvironment, carcinogenesis, and anticancer treatment. Cell. Physiol. Biochem. 2014, 34, 213–243. [Google Scholar] [CrossRef]
- Dörr, H.; Meineke, V. Acute radiation syndrome caused by accidental radiation exposure-therapeutic principles. BMC Med. 2011, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Salvador, J.A.; Carvalho, J.F.; Neves, M.A.; Silvestre, S.M.; Leitao, A.J.; Silva, M.M.C.; e Melo, M.L.S. Anticancer steroids: Linking natural and semi-synthetic compounds. Nat. Prod. Rep. 2013, 30, 324–374. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Baine, M.J.; Souchek, J.J.; Menning, M.; Kaur, S.; Yan, Y.; Ouellette, M.M.; Jain, M.; Lin, C.; Batra, S.K. Biological determinants of radioresistance and their remediation in pancreatic cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2017, 1868, 69–92. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Panda, K.C.; Das, S.; Jena, M. Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: Current evidences and future perspectives. Phytother. Res. 2021, 35, 4194–4214. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. 2021, 30, e00633. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomed. Int. J. Phytother. Phytopharm. 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.C.; Bosch, W.R.; Mutic, S.; Lewis, J.S.; Dehdashti, F.; Mintun, M.A.; Dempsey, J.F.; Perez, C.A.; Purdy, J.A.; Welch, M.J. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1171–1182. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.-L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, D.M.; Asaithamby, A.; Blattnig, S.R.; Costes, S.V.; Doetsch, P.W.; Dynan, W.S.; Hahnfeldt, P.; Hlatky, L.; Kidane, Y.; Kronenberg, A. Evaluating biomarkers to model cancer risk post cosmic ray exposure. Life Sci. Space Res. 2016, 9, 19–47. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, B.; Baral, S.; Patra, S.; Behera, C.; Nayak, R.; MubarakAli, D.; Jena, M. Delineation of gamma irradiation (60Co) induced oxidative stress by decrypting antioxidants and biochemical responses of microalga, Chlorella sp. Biocatal. Agric. Biotechnol. 2020, 25, 101595. [Google Scholar] [CrossRef]
- Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Pradhan, B.; Jena, M.; Bhutia, S.K. Gamma irradiation promotes chemo-sensitization potential of gallic acid through attenuation of autophagic flux to trigger apoptosis in an NRF2 inactivation signalling pathway. Free Radic. Biol. Med. 2020, 160, 111–124. [Google Scholar] [CrossRef]
- Prise, K.M.; O’sullivan, J.M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 2009, 9, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Ullrich, R.K.; Contessa, J.N.; Lammering, G.; Amorino, G.; Lin, P.-S. ERBB receptor tyrosine kinases and cellular radiation responses. Oncogene 2003, 22, 5855–5865. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.; Rayalam, S.; Wang, X. Cytoprotective effects of natural compounds against oxidative stress. Antioxidants 2018, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Nambiar, D.; Rajamani, P.; Singh, R.P. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat. Res./Rev. Mutat. Res. 2011, 728, 139–157. [Google Scholar] [CrossRef]
- Alcaraz, M.; Acevedo, C.; Castillo, J.; Benavente-Garcia, O.; Armero, D.; Vicente, V.; Canteras, M. Liposoluble antioxidants provide an effective radioprotective barrier. Br. J. Radiol. 2009, 82, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.; Armero, D.; Martínez-Beneyto, Y.; Castillo, J.; Benavente-GarcíaFernandez, O.; Alcaraz-Saura, M.; Canteras, M. Chemical genoprotection: Reducing biological damage to as low as reasonably achievable levels. Dentomaxillofac. Radiol. 2011, 40, 310–314. [Google Scholar] [CrossRef]
- Sharma, N.K. Modulation of radiation-induced and mitomycin C-induced chromosome damage by apigenin in human lymphocytes in vitro. J. Radiat. Res. 2013, 54, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Bache, M.; Zschornak, M.P.; Passin, S.; Keßler, J.; Wichmann, H.; Kappler, M.; Paschke, R.; Kaluđerović, G.N.; Kommera, H.; Taubert, H. Increased betulinic acid induced cytotoxicity and radiosensitivity in glioma cells under hypoxic conditions. Radiat. Oncol. 2011, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Kinoshita, M.; Yamamoto, T.; Ito, M.; Nishida, T.; Takeuchi, M.; Saitoh, D.; Seki, S.; Mukai, Y. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS ONE 2015, 10, e0117020. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Anjaria, K.; Bhat, N.; Shirsath, K.; Sreedevi, B. Effect of caffeine on radiation induced micronuclei in human lymphocytes. Radiat. Prot. Environ. 2010, 33, 195–198. [Google Scholar]
- Kukreja, M. Radioprotective Effects of 10 µM Curcumin on 2 Gy Gamma-Irradiated CHO Cells; San José State University: San José, CA, USA, 2012. [Google Scholar]
- Sebastia, N.; Montoro, A.; Hervas, D.; Pantelias, G.; Hatzi, V.; Soriano, J.; Villaescusa, J.; Terzoudi, G. Curcumin and trans-resveratrol exert cell cycle-dependent radioprotective or radiosensitizing effects as elucidated by the PCC and G2-assay. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2014, 766, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, K.; Yalcin, E. Radioprotective effect of lycopene on chromosomal aberrations (CAs) induced by gamma radiation in human lymphocytes. J. Environ. Biol. 2009, 30, 113–117. [Google Scholar]
- Khan, S.; Kumar, A.; Adhikari, J.; Rizvi, M.; Chaudhury, N. Protective effect of sesamol against 60Co γ-ray-induced hematopoietic and gastrointestinal injury in C57BL/6 male mice. Free Radic. Res. 2015, 49, 1344–1361. [Google Scholar] [CrossRef]
- Richi, B.; Kale, R.K.; Tiku, A.B. Radio-modulatory effects of green tea catechin EGCG on pBR322 plasmid DNA and murine splenocytes against gamma-radiation induced damage. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2012, 747, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.; Baliga, M. Radioprotection by mangiferin in DBAxC57BL mice: A preliminary study. Phytomed. Int. J. Phytother. Phytopharm. 2005, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Yáñez, J.; Vicente, V.; Alcaraz, M.; Benavente-García, O.; Castillo, J.; Lorente, J.; Lozano, J.A. Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: Relationship between structure and activity. Melanoma Res. 2002, 12, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Yàñez, J.; Vicente, V.; Alcaraz, M.; Benavente-García, O.; Castillo, J.; Lorente, J.; Lozano, J.A. Effects of several polyhydroxylated flavonoids on the growth of B16F10 melanoma and Melan-a melanocyte cell lines: Influence of the sequential oxidation state of the flavonoid skeleton. Melanoma Res. 2003, 13, 3–9. [Google Scholar] [CrossRef]
- Siebenlist, U.; Franzoso, G.; Brown, K. Structure, regulation and function of NF-kappaB. Annu. Rev. Cell Biol. 1994, 10, 405–455. [Google Scholar] [CrossRef]
- Guo, G.; Wang, T.; Gao, Q.; Tamae, D.; Wong, P.; Chen, T.; Chen, W.-C.; Shively, J.E.; Wong, J.Y.; Li, J.J. Expression of erbb2 enhances radiation-induced nf-κ b activation. Oncogene 2004, 23, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Nabel, G.J.; Verma, I.M. Proposed NF-κB/IκB family nomenclature. Genes Dev. 1993, 7, 2063. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, R.Z.; Baldwin, A.S., Jr. NF-κB as a therapeutic target in cancer. Trends Mol. Med. 2002, 8, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Häcker, H.; Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, 17, re13. [Google Scholar]
- Nasuhara, Y.; Adcock, I.M.; Catley, M.; Barnes, P.J.; Newton, R. Differential IκB kinase activation and IκBα degradation by interleukin-1β and tumor necrosis factor-α in human U937 monocytic cells: Evidence for additional regulatory steps in κB-dependent transcription. J. Biol. Chem. 1999, 274, 19965–19972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.-C. Activation by IKKα of a second, evolutionary conserved, NF-κB pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.M.; Li, J.J. NF-κB-mediated adaptive resistance to ionizing radiation. Free Radic. Biol. Med. 2008, 44, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, L.J.; Eckmann, L.; Greten, F.R.; Chae, S.; Li, Z.-W.; Myhre, G.M.; Robine, S.; Karin, M.; Kagnoff, M.F. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc. Natl. Acad. Sci. USA 2004, 101, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Carpio, D.F.; Zheng, Y.; Bruzzo, P.; Singh, V.; Ouaaz, F.; Medzhitov, R.M.; Beg, A.A. An essential role of the NF-κB/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 2001, 166, 7128–7135. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; SuYang, H.; Erdjument-Bromage, H.; Tempst, P.; Ghosh, S. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP–independent mechanism. Cell 1997, 89, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Stehlik, C.; De Martin, R.; Kumabashiri, I.; Schmid, J.A.; Binder, B.R.; Lipp, J. Nuclear factor (NF)-κB–regulated X-chromosome–linked iap gene expression protects endothelial cells from tumor necrosis factor α–induced apoptosis. J. Exp. Med. 1998, 188, 211–216. [Google Scholar] [CrossRef]
- Heckman, C.A.; Mehew, J.W.; Boxer, L.M. NF-κB activates Bcl-2 expression in t (14; 18) lymphoma cells. Oncogene 2002, 21, 3898–3908. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Penthala, N.R.; Bommagani, S.; Janganati, V.; MacNicol, K.B.; Cragle, C.E.; Madadi, N.R.; Hardy, L.L.; MacNicol, A.M.; Crooks, P.A. Heck products of parthenolide and melampomagnolide-B as anticancer modulators that modify cell cycle progression. Eur. J. Med. Chem. 2014, 85, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Jiao, Y.; Xue, J.; Zhang, Q.; Yang, H.; Xing, L.; Chen, G.; Wu, J.; Zhang, S.; Zhu, W. Metformin sensitizes non-small cell lung cancer cells to an epigallocatechin-3-gallate (EGCG) treatment by suppressing the Nrf2/HO-1 pathway. Int. J. Biol. Sci. 2017, 13, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-κB signaling through suppression of p65 and IB kinase activities. Die Pharm.-Int. J. Pharm. Sci. 2013, 68, 689–694. [Google Scholar]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget 2017, 8, 90579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blauwkamp, T.A.; Chang, M.V.; Cadigan, K.M. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008, 27, 1436–1446. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.R.; Nam, S.; Kook, M.-C.; Kim, K.-T.; Liu, X.; Yao, H.; Jung, H.R.; Lemos, R.; Seo, H.H.; Park, H.S. HNF4α is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer. Gut 2016, 65, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Shen, A.H.; Borrelli, M.R.; Adem, S.; Deleon, N.M.D.; Patel, R.A.; Mascharak, S.; Yen, S.J.; Sun, B.Y.; Taylor, W.L.; Januszyk, M. Prophylactic treatment with transdermal deferoxamine mitigates radiation-induced skin fibrosis. Sci. Rep. 2020, 10, 12346. [Google Scholar] [CrossRef]
- Dreesen, O.; Brivanlou, A.H. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007, 3, 7–17. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.-M.; Ng, Y.-Y.; Ma, F.Y.; Zhou, S.; Zhang, Y.; Yang, C.; Huang, X.-R.; Xiao, J.; Wang, Y.-Y. TGF-β/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 2016, 7, 8809–8822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H.; Abdollah, S.; Qiu, Y.; Cai, J.; Xu, Y.-Y.; Grinnell, B.W.; Richardson, M.A.; Topper, J.N.; Gimbrone, M.A., Jr.; Wrana, J.L. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ. Cell 1997, 89, 1165–1173. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Rui, H.; Wang, J.; Lin, S.; He, Y.; Chen, M.; Li, Q.; Ye, Z.; Zhang, S.; Chan, S.C. Axin is a scaffold protein in TGF-β that promotes degradation of Smad7 by Arkadia. EMBO J. 2006, 25, 1646–1658. [Google Scholar] [CrossRef] [Green Version]
- Vallee, A.; Vallee, J.-N.; Lecarpentier, Y. PPARγ agonists: Potential treatment for autism spectrum disorder by inhibiting the canonical WNT/β-catenin pathway. Mol. Psychiatry 2019, 24, 643–652. [Google Scholar] [CrossRef]
- Wu, M.; Melichian, D.S.; Chang, E.; Warner-Blankenship, M.; Ghosh, A.K.; Varga, J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am. J. Pathol. 2009, 174, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Chen, A. Disruption of transforming growth factor-β by curcumin induces gene expression of peroxisome proliferator-activated receptor-γ in rat hepatic stellate cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G113–G123. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Lateef, A.; Hamiza, O.O.; Ali, F.; Sultana, S. Diosmin protects against trichloroethylene-induced renal injury in Wistar rats: Plausible role of p53, Bax and caspases. Br. J. Nutr. 2013, 110, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Zhou, L.; Han, N.; Zhang, M.; Lyu, X. Wnt/β-catenin pathway involvement in ionizing radiation-induced invasion of U87 glioblastoma cells. Strahlenther. Und Onkol. 2015, 191, 672–680. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.-T. ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid. Med. Cell. Longev. 2020, 2020, 5047987. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.-H.; Gaascht, F.; Cronauer, M.; Henry, E.; Dicato, M.; Diederich, M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless pathway. Int. J. Oncol. 2011, 38, 603–611. [Google Scholar]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010, 633, 78–84. [Google Scholar] [CrossRef]
- Hope, C.; Planutis, K.; Planutiene, M.; Moyer, M.P.; Johal, K.S.; Woo, J.; Santoso, C.; Hanson, J.A.; Holcombe, R.F. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: Implications for colon cancer prevention. Mol. Nutr. Food Res. 2008, 52, S52–S61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, S.; Zwetsloot, K.; Lawrence, M.; Nieman, D.; Lila, M.; Grace, M.; Howden, R.; Cooley, I.; Tkach, J.; Keith, M. Ajuga turkestanica increases Notch and Wnt in aged skeletal muscle. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2584–2592. [Google Scholar]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.-F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Cao, C.; Su, Y.; Wang, J.; Sun, J.; Chen, H.; Xu, A. Ginkgo biloba exocarp extracts inhibits angiogenesis and its effects on Wnt/β-catenin-VEGF pathway in Lewis lung cancer. J. Ethnopharmacol. 2016, 192, 406–412. [Google Scholar] [CrossRef]
- Park, M.-G.; Kim, J.-S.; Park, S.-Y.; Lee, S.A.; Kim, H.-J.; Kim, C.S.; Kim, J.-S.; Chun, H.S.; Park, J.-C.; Kim, D.K. MicroRNA-27 promotes the differentiation of odontoblastic cell by targeting APC and activating Wnt/β-catenin. Gene 2014, 538, 266–272. [Google Scholar] [CrossRef]
- Park, P.J.; Moon, B.-S.; Lee, S.-H.; Kim, S.-N.; Kim, A.-R.; Kim, H.-J.; Park, W.-S.; Choi, K.-Y.; Cho, E.-G.; Lee, T.R. Hair growth-promoting effect of Aconiti Ciliare Tuber extract mediated by the activation of Wnt/β-catenin. Life Sci. 2012, 91, 935–943. [Google Scholar] [CrossRef]
- Zahoor, M.; Cha, P.-H.; Choi, K.-Y. Indirubin-3′-oxime, an activator of Wnt/β-catenin, enhances osteogenic commitment of ST2 cells and restores bone loss in high-fat diet-induced obese male mice. Bone 2014, 65, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, Z.; Xu, Y.; Chen, S.; Han, Y.; Li, L.; Wang, M.; Jin, X. Roles of reactive oxygen species in biological behaviors of prostate cancer. BioMed Res. Int. 2020, 2020, 1269624. [Google Scholar] [CrossRef]
- Kundu, J.; Kim, D.-H.; Kundu, J.K.; Chun, K.-S. Thymoquinone induces heme oxygenase-1 expression in HaCaT cells via Nrf2/ARE activation: Akt and AMPKα as upstream targets. Food Chem. Toxicol. 2014, 65, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Khor, T.O.; Shu, L.; Su, Z.-Y.; Fuentes, F.; Kong, A.-N.T. Dietary phytochemicals and cancer prevention: Nrf2, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Romeo, L.; Intrieri, M.; D’Agata, V.; Mangano, N.G.; Oriani, G.; Ontario, M.L.; Scapagnini, G. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J. Am. Coll. Nutr. 2009, 28, 492S–499S. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Na, H.-K.; Surh, Y.-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hsu, M.; Hsieh, C.; Lin, J.; Lai, P.; Wung, B. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006, 78, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.J.; Wong, H.-K.; Oddos, T.; Southall, M.; Frei, B.; Kaur, S. A purified feverfew extract protects from oxidative damage by inducing DNA repair in skin cells via a PI3-kinase-dependent Nrf2/ARE pathway. J. Dermatol. Sci. 2013, 72, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Kode, A.; Rajendrasozhan, S.; Caito, S.; Yang, S.-R.; Megson, I.L.; Rahman, I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L478–L488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-Y.; Jang, J.-H.; Li, M.-H.; Surh, Y.-J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 2005, 331, 993–1000. [Google Scholar] [CrossRef]
- Lian, F.; Wang, X.D. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer 2008, 123, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Z.-Y.; Khor, T.O.; Shu, L.; Kong, A.-N.T. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem. Pharmacol. 2013, 85, 1398–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanneken, A.; Lin, F.-F.; Johnson, J.; Maher, P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress–induced death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3164–3177. [Google Scholar] [CrossRef]
- Patwardhan, M.R. Amelioration of Ionizing Radiation Induced Cell Death in Lymphocytes by Baicalein; Homi Bhabha National Institute: Mumbai, India, 2015. [Google Scholar]
- Thomas, S.; Snowden, J.; Zeidler, M.; Danson, S. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [Green Version]
- Horvath, C.M. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem. Sci. 2000, 25, 496–502. [Google Scholar] [CrossRef]
- Murray, P.J. The JAK-STAT pathway: Input and output integration. J. Immunol. 2007, 178, 2623–2629. [Google Scholar] [CrossRef] [Green Version]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in radiobiological responses: A review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [Green Version]
- Shan, N.-N.; Zhu, X.-J.; Wang, Q.; Wang, C.-Y.; Qin, P.; Peng, J.; Hou, M. High-dose dexamethasone regulates interleukin-18 and interleukin-18 binding protein in idiopathic thrombocytopenic purpura. Haematologica 2009, 94, 1603–1607. [Google Scholar] [CrossRef] [Green Version]
- Dancea, H.C.; Shareef, M.M.; Ahmed, M.M. Role of radiation-induced TGF-beta in cancer therapy. Mol. Cell. Pharmacol. 2009, 1, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Yang, G.; Jiang, T.; Huang, K.; Cao, J.; Qiu, Z. Effects of IL-6 and AG490 on regulation of Stat3 pathway and invasion of human pancreatic cancer cells in vitro. J. Exp. Clin. Cancer Res. 2010, 29, 51. [Google Scholar] [CrossRef] [Green Version]
- Bonner, J.A.; Trummell, H.Q.; Willey, C.D.; Plants, B.A.; Raisch, K.P. Inhibition of STAT-3 results in radiosensitization of human squamous cell carcinoma. Radiother. Oncol. 2009, 92, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.S.; Vadgama, J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3–NFκB. Anticancer Res. 2015, 35, 39–46. [Google Scholar]
- Blaskovich, M.A.; Sun, J.; Cantor, A.; Turkson, J.; Jove, R.; Sebti, S.M. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003, 63, 1270–1279. [Google Scholar]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Investig. Drugs 2007, 16, 1753–1773. [Google Scholar] [CrossRef]
- Madan, E.; Prasad, S.; Roy, P.; George, J.; Shukla, Y. Regulation of apoptosis by resveratrol through JAK/STAT and mitochondria mediated pathway in human epidermoid carcinoma A431 cells. Biochem. Biophys. Res. Commun. 2008, 377, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Molavi, O.; Haddadi, A.; Lai, R.; Gossage, R.A.; Lavasanifar, A. Resveratrol analog trans 3, 4, 5, 4′-tetramethoxystilbene (DMU-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother. Pharmacol. 2008, 63, 27–35. [Google Scholar] [CrossRef]
- Kotha, A.; Sekharam, M.; Cilenti, L.; Siddiquee, K.; Khaled, A.; Zervos, A.S.; Carter, B.; Turkson, J.; Jove, R. Resveratrol inhibits Src and Stat3 and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 2006, 5, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Bharti, A.C.; Donato, N.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J. Immunol. 2003, 171, 3863–3871. [Google Scholar] [CrossRef]
- Yang, C.-L.; Liu, Y.-Y.; Ma, Y.-G.; Xue, Y.-X.; Liu, D.-G.; Ren, Y.; Liu, X.-B.; Li, Y.; Li, Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS ONE 2012, 7, e37960. [Google Scholar] [CrossRef]
- Tang, S.-N.; Fu, J.; Shankar, S.; Srivastava, R.K. EGCG enhances the therapeutic potential of gemcitabine and CP690550 by inhibiting STAT3 pathway in human pancreatic cancer. PLoS ONE 2012, 7, e31067. [Google Scholar] [CrossRef]
- Masuda, M.; Suzui, M.; Lim, J.T.; Weinstein, I.B. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream pathways in human head and neck and breast carcinoma cells. Clin. Cancer Res. 2003, 9, 3486–3491. [Google Scholar] [PubMed]
- Jung, J.E.; Kim, H.S.; Lee, C.S.; Park, D.-H.; Kim, Y.-N.; Lee, M.-J.; Lee, J.W.; Park, J.-W.; Kim, M.-S.; Ye, S.K. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis 2007, 28, 1780–1787. [Google Scholar] [CrossRef]
- Balint, E.; Vousden, K. Activation and activities of the p53 tumour suppressor protein. Br. J. Cancer 2001, 85, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Kurz, E.U.; Lees-Miller, S.P. DNA damage-induced activation of ATM and ATM-dependent pathways. DNA Repair 2004, 3, 889–900. [Google Scholar] [CrossRef]
- Chong, M.J.; Murray, M.R.; Gosink, E.C.; Russell, H.R.; Srinivasan, A.; Kapsetaki, M.; Korsmeyer, S.J.; McKinnon, P.J. Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system. Proc. Natl. Acad. Sci. USA 2000, 97, 889–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selivanova, G. Therapeutic targeting of p53 by small molecules. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2010; Volume 20, pp. 46–56. [Google Scholar]
- Canman, C.E.; Lim, D.-S.; Cimprich, K.A.; Taya, Y.; Tamai, K.; Sakaguchi, K.; Appella, E.; Kastan, M.B.; Siliciano, J.D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998, 281, 1677–1679. [Google Scholar] [CrossRef]
- Harris, S.L.; Levine, A.J. The p53 pathway: Positive and negative feedback loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef] [Green Version]
- Maurya, D.K.; Salvi, V.P.; Nair, C.K.K. Radiation protection of DNA by ferulic acid under in vitro and in vivo conditions. Mol. Cell. Biochem. 2005, 280, 209–217. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, L.-T.; Lin, C.-C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Pharmacol. Exp. Ther. 2005, 313, 333–344. [Google Scholar] [CrossRef]
- Gao, N.; Cheng, S.; Budhraja, A.; Gao, Z.; Chen, J.; Liu, E.H.; Huang, C.; Chen, D.; Yang, Z.; Liu, Q. Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br. J. Pharmacol. 2012, 165, 1813–1826. [Google Scholar] [CrossRef] [Green Version]
- Chtourou, Y.; Aouey, B.; Aroui, S.; Kebieche, M.; Fetoui, H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem.-Biol. Interact. 2016, 243, 1–9. [Google Scholar] [CrossRef]
- Jamal, J.; Mustafa, M.R.; Wong, P.-F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anticancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Lee, H.J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.O.; Kim, S.H.; Lü, J. Penta-1,2,3,4,6-O-galloyl-beta-D-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tichy, A.; Durisova, K.; Salovska, B.; Pejchal, J.; Zarybnicka, L.; Vavrova, J.; Dye, N.A.; Sinkorova, Z. Radio-sensitization of human leukaemic MOLT-4 cells by DNA-dependent protein kinase inhibitor, NU7441. Radiat. Environ. Biophys. 2014, 53, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Z.; Ma, F.; Yang, X.; Cheng, C.; Yao, L. Protective effect of anthocyanin from Lonicera caerulea var. edulis on radiation-induced damage in mice. Int. J. Mol. Sci. 2012, 13, 11773–11782. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X.G. The canonical Notch pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-C.; He, F.; Feng, F.; Liu, X.-W.; Dong, G.-Y.; Qin, H.-Y.; Hu, X.-B.; Zheng, M.-H.; Liang, L.; Feng, L. Notch determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010, 70, 4840–4849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagadec, C.; Vlashi, E.; Alhiyari, Y.; Phillips, T.M.; Dratver, M.B.; Pajonk, F. Radiation-induced Notch in breast cancer stem cells. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theys, J.; Yahyanejad, S.; Habets, R.; Span, P.; Dubois, L.; Paesmans, K.; Kattenbeld, B.; Cleutjens, J.; Groot, A.J.; Schuurbiers, O.C. High NOTCH activity induces radiation resistance in non-small cell lung cancer. Radiother. Oncol. 2013, 108, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Szymczyk, K.; Shapiro, I.; Adams, C.S. Ionizing radiation sensitizes bone cells to apoptosis. Bone 2004, 34, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Sawada, Y.; Yoshimoto, M.; Kawai, M.; Miyakoshi, J. Radiation-induced reduction of osteoblast differentiation in C2C12 cells. J. Radiat. Res. 2007, 48, 515–521. [Google Scholar] [CrossRef]
- Zanotti, S.; Canalis, E. Notch1 and Notch2 expression in osteoblast precursors regulates femoral microarchitecture. Bone 2014, 62, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.; Petrella, M.; Shateri, H. Effects of administering tocopherol after irradiation on survival and proliferation of murine lymphocytes. Pharmacol. Ther. 1988, 39, 393–395. [Google Scholar] [CrossRef]
- Kim, Y.S.; Farrar, W.; Colburn, N.H.; Milner, J.A. Cancer stem cells: Potential target for bioactive food components. J. Nutr. Biochem. 2012, 23, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roma, J.; Almazán-Moga, A.; Sánchez de Toledo, J.; Gallego, S. Notch, wnt, and hedgehog pathways in rhabdomyosarcoma: From single pathways to an integrated network. Sarcoma 2012, 2012, 695603. [Google Scholar] [CrossRef] [Green Version]
- Tutel’ian, V.; Lashneva, N. Biologically active substances of plant origin. Flavonols and flavones: Prevalence, dietary sourses and consumption. Vopr. Pitan. 2013, 82, 4–22. [Google Scholar]
- Huang, G.; Cao, X.; Zhang, X.; Chang, H.; Yang, Y.; Du, W.; Wilson, J.X. Effects of soybean isoflavone on the notch signal pathway of the brain in rats with cerebral ischemia. J. Nutr. Sci. Vitaminol. 2009, 55, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Takabe, K.; Spiegel, S. Export of sphingosine-1-phosphate and cancer progression. J. Lipid Res. 2014, 55, 1839–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingham, P.W.; McMahon, A.P. Hedgehog in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [Green Version]
- Aszterbaum, M.; Epstein, J.; Oro, A.; Douglas, V.; LeBoit, P.E.; Scott, M.P.; Epstein, E.H. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat. Med. 1999, 5, 1285–1291. [Google Scholar] [CrossRef]
- Mancuso, M.; Pazzaglia, S.; Tanori, M.; Hahn, H.; Merola, P.; Rebessi, S.; Atkinson, M.J.; Di Majo, V.; Covelli, V.; Saran, A. Basal cell carcinoma and its development: Insights from radiation-induced tumors in Ptch1-deficient mice. Cancer Res. 2004, 64, 934–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Chen, Y.; Yang, N.; Zhu, X.; Sun, L.; Li, G. Interaction between curcumin and mimetic biomembrane. Sci. China Life Sci. 2012, 55, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Gan, G.N.; Eagles, J.; Keysar, S.B.; Wang, G.; Glogowska, M.J.; Altunbas, C.; Anderson, R.T.; Le, P.N.; Morton, J.J.; Frederick, B. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res. 2014, 74, 7024–7036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabarriti, R.; Guha, C. Radiobiology of the Liver. In Radiotherapy of Liver Cancer; Springer: Singapore, 2021; pp. 15–30. [Google Scholar]
- Wang, H.; Zhang, Y.; Bai, R.; Wang, M.; Du, S. Baicalin attenuates alcoholic liver injury through modulation of hepatic oxidative stress, inflammation and sonic hedgehog pathway in rats. Cell. Physiol. Biochem. 2016, 39, 1129–1140. [Google Scholar] [CrossRef]
- Fan, P.; Fan, S.; Wang, H.; Mao, J.; Shi, Y.; Ibrahim, M.M.; Ma, W.; Yu, X.; Hou, Z.; Wang, B. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res. Ther. 2013, 4, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims-Mourtada, J.; Opdenaker, L.M.; Davis, J.; Arnold, K.M.; Flynn, D. Taxane-induced hedgehog is linked to expansion of breast cancer stem-like populations after chemotherapy. Mol. Carcinog. 2015, 54, 1480–1493. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chao, K.; Liao, H.-F.; Chen, Y.-J. Targeting sonic hedgehog by compounds and derivatives from natural products. Evid.-Based Complement. Altern. Med. 2013, 2013, 748587. [Google Scholar] [CrossRef] [Green Version]
- Hui, Z.; Tretiakova, M.; Zhang, Z.; Li, Y.; Wang, X.; Zhu, J.X.; Gao, Y.; Mai, W.; Furge, K.; Qian, C.-N. Radiosensitization by inhibiting STAT1 in renal cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 288–295. [Google Scholar] [CrossRef]
- Yu, W.; Sun, H.; Zha, W.; Cui, W.; Xu, L.; Min, Q.; Wu, J. Apigenin attenuates adriamycin-induced cardiomyocyte apoptosis via the PI3K/AKT/mTOR pathway. Evid.-Based Complement. Altern. Med. 2017, 2017, 2590676. [Google Scholar] [CrossRef] [Green Version]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P. Targeting AKT/mTOR in oral cancer: Mechanisms and advances in clinical trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Huang, S.-M.; Harari, P.M. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: Inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin. Cancer Res. 2000, 6, 2166–2174. [Google Scholar]
- Rashmi, K.V.; Shah, N.B.; Kumar, P.V. Optimal exact-regenerating codes for distributed storage at the MSR and MBR points via a product-matrix construction. IEEE Trans. Inf. Theory 2011, 57, 5227–5239. [Google Scholar] [CrossRef] [Green Version]
- Boreddy, S.R.; Sahu, R.P.; Srivastava, S.K. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-α/VEGF/Rho-GTPases: Pivotal role of STAT-3. PLoS ONE 2011, 6, e25799. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, E.-W.; Mo, S.-J.; Rhee, D.-K.; Pyo, S. Inhibition of ICAM-1 expression by garlic component, allicin, in gamma-irradiated human vascular endothelial cells via downregulation of the JNK pathway. Int. Immunopharmacol. 2006, 6, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Rajendra Prasad, N.; Kanimozhi, G.; Agilan, B. Apigenin prevents gamma radiation-induced gastrointestinal damages by modulating inflammatory and apoptotic signalling mediators. Nat. Prod. Res. 2021, 1–5. [Google Scholar] [CrossRef]
- Lee, G.T.; Cha, H.J.; Lee, K.S.; Lee, K.K.; Hong, J.T.; Ahn, K.J.; An, I.-S.; An, S.; Bae, S. Arctiin induces an UVB protective effect in human dermal fibroblast cells through microRNA expression changes. Int. J. Mol. Med. 2014, 33, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Mahran, Y.F.; Badr, A.M.; Aldosari, A.; Bin-Zaid, R.; Alotaibi, H.N. Carvacrol and thymol modulate the cross-talk between TNF-α and IGF-1 signaling in radiotherapy-induced ovarian failure. Oxid. Med. Cell. Longev. 2019, 2019, 3173745. [Google Scholar] [CrossRef]
- Gerić, M.; Gajski, G.; Mihaljević, B.; Miljanić, S.; Domijan, A.-M.; Garaj-Vrhovac, V. Radioprotective properties of food colorant sodium copper chlorophyllin on human peripheral blood cells in vitro. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2019, 845, 403027. [Google Scholar] [CrossRef]
- Roy, S.; Yu, Y.; Padhye, S.B.; Sarkar, F.H.; Majumdar, A.P. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS ONE 2013, 8, e68543. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-K.; Kim, E.-H.; Jung, J.-H.; Lee, H.-H.; Hyun, J.-W.; Surh, Y.-J. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Joshi, J.; Kumar, A.; Pandey, B.N.; Hazra, B.; Mishra, K.P. Radiosensitization by diospyrin diethylether in MCF-7 breast carcinoma cell line. Mol. Cell. Biochem. 2007, 304, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Shanthakumar, J.; Karthikeyan, A.; Bandugula, V.R.; Prasad, N.R. Ferulic acid, a dietary phenolic acid, modulates radiation effects in Swiss albino mice. Eur. J. Pharmacol. 2012, 691, 268–274. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Parasuraman, V.; Arunagiri, V.; Gunaseelan, S.; Chou, H.-Y.; Anbazhagan, R.; Lai, J.-Y.; Prasad, R. Radioprotective effect of self-assembled low molecular weight Fucoidan–Chitosan nanoparticles. Int. J. Pharm. 2020, 579, 119161. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Mahmoudzadeh, A.; Ahmadi, A.; Mohamadifar, S.; Akhlaghpoor, S. Radioprotective effects of hesperidin against genotoxicity induced by γ-irradiation in human lymphocytes. Mutagenesis 2009, 24, 233–235. [Google Scholar] [CrossRef]
- Puah, B.-P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-P.; Zhao, J.; Li, S.-S.; Yang, L.-J.; Zeng, L.-L.; Chen, Y.; Fang, J. Mangiferin activates Nrf2-antioxidant response element without reducing the sensitivity to etoposide of human myeloid leukemia cells in vitro. Acta Pharmacol. Sin. 2014, 35, 257–266. [Google Scholar] [CrossRef]
- Alonso-González, C.; González, A.; Menéndez-Menéndez, J.; Martínez-Campa, C.; Cos, S. Melatonin as a Radio-Sensitizer in Cancer. Biomedicines 2020, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.S.; Chin-Sinex, H.; Gomez-Millan, J.; Datzman, N.; Hardacre, M.; Comerford, K.; Nakshatri, H.; Nye, M.; Benjamin, L.; Mehta, S. Parthenolide sensitizes cells to X-ray-induced cell killing through inhibition of NF-κB and split-dose repair. Radiat. Res. 2007, 168, 689–697. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Park, S.-J.; Kwon, M.-J.; Jeong, T.-S.; Bok, S.-H.; Choi, W.-Y.; Jeong, W.-I.; Ryu, S.-Y.; Do, S.-H.; Lee, C.-S. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef]
- Thabet, N.M.; Moustafa, E.M. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: Role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch. Physiol. Biochem. 2018, 124, 185–193. [Google Scholar] [CrossRef]
- Wang, J.; Wakeman, T.P.; Lathia, J.D.; Hjelmeland, A.B.; Wang, X.F.; White, R.R.; Rich, J.N.; Sullenger, B.A. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010, 28, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Gu, M.-M.; Lang, Y.; Shi, J.; Chen, B.P.; Guan, H.; Yu, L.; Zhou, P.-K.; Shang, Z.-F. The vanillin derivative VND3207 protects intestine against radiation injury by modulating p53/NOXA pathway and restoring the balance of gut microbiota. Free Radic. Biol. Med. 2019, 145, 223–236. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jit, B.P.; Pradhan, B.; Dash, R.; Bhuyan, P.P.; Behera, C.; Behera, R.K.; Sharma, A.; Alcaraz, M.; Jena, M. Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways. Antioxidants 2022, 11, 49. https://doi.org/10.3390/antiox11010049
Jit BP, Pradhan B, Dash R, Bhuyan PP, Behera C, Behera RK, Sharma A, Alcaraz M, Jena M. Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways. Antioxidants. 2022; 11(1):49. https://doi.org/10.3390/antiox11010049
Chicago/Turabian StyleJit, Bimal Prasad, Biswajita Pradhan, Rutumbara Dash, Prajna Paramita Bhuyan, Chhandashree Behera, Rajendra Kumar Behera, Ashok Sharma, Miguel Alcaraz, and Mrutyunjay Jena. 2022. "Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways" Antioxidants 11, no. 1: 49. https://doi.org/10.3390/antiox11010049
APA StyleJit, B. P., Pradhan, B., Dash, R., Bhuyan, P. P., Behera, C., Behera, R. K., Sharma, A., Alcaraz, M., & Jena, M. (2022). Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways. Antioxidants, 11(1), 49. https://doi.org/10.3390/antiox11010049









