Neurological Tremor: Sensors, Signal Processing and Emerging Applications
Abstract
:1. Introduction
General recommendations
2. Sensors and Instrumentation for the Characterization of Tremor
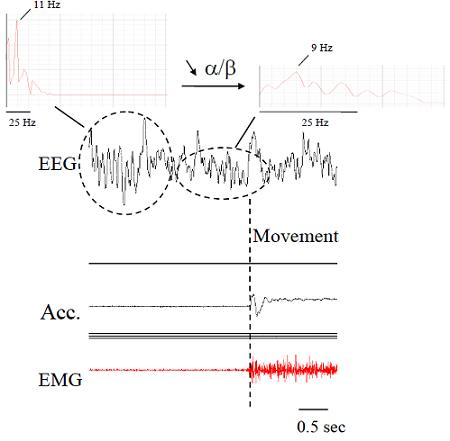
2.1. Electromyography (EMG)
2.2. Accelerometers
2.3. Gyroscopes
2.4. Flexible Angular Sensors and Goniometers
2.5. Videos
2.6. Optoelectronic Devices
2.7. Force Sensors
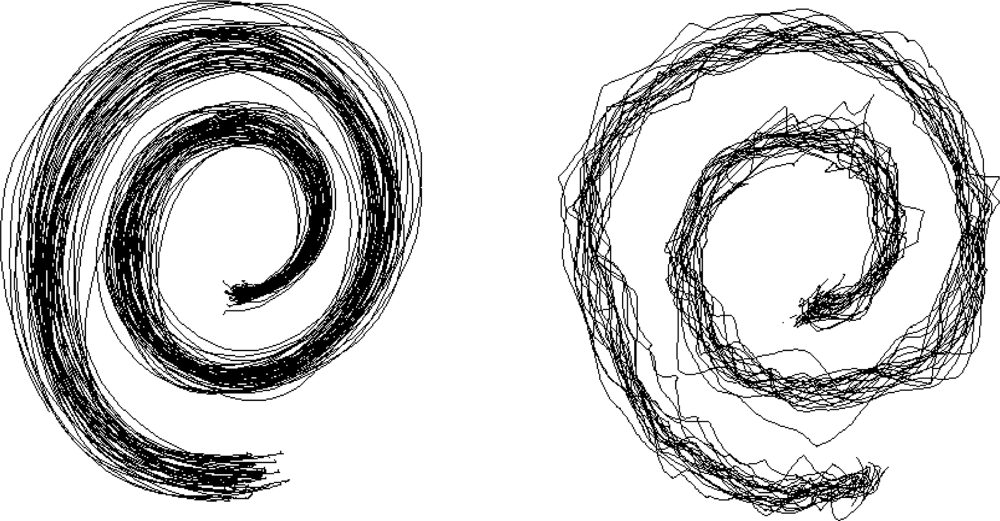
2.8. Evaluation of Handwriting and Drawing
2.9. Wearable Orthosis
2.10. Other Sensors
2.11. Choice of the Sensor and Future Developments in the Assessment of Tremor
3. Methods of Signal Processing for Tremor
3.1. Editing
3.2. Noise Minimization and Wavelets
3.3. Spectral Estimations
3.4. Parameters Commonly Extracted for Spectral Estimations
- -spectrum shape with identification of single or multiple peaks
- -peak frequency
- -median frequency
- -power spectral density (PSD), in particular in the band 1–20 Hz (PSD1-20Hz) and 1–33 Hz (PSD1-33Hz), with identification of the peak intensity (PI)
- -power in specific frequency bands (α band: 8–13 Hz, β1 band: 13–20 Hz, β2 band 20–26 Hz; β3 band: 26–33 Hz) and power ratios for each frequency band as compared to total PSD
- -crest factor: ratio of the PI divided by PSD1-20Hz or PSD1-33Hz
- -center frequency (F50): frequency of the power spectrum dividing the area under the spectrum in two equal parts
- -harmonic index (HI): index comparing the tremor frequency pattern with the pattern of single harmonic oscillation (HI = 1 in case of single harmonic)
- -frequency dispersion: corresponds to center frequency ± SD. Frequency dispersion is low for regular tremors.
5. Conclusions
Acknowledgments
References and Notes
- Louis, E.D.; Marder, K.; Cote, L. Differences in the prevalence of essential tremor among elderly African Americans, whites, and Hispanics in northern Manhattan, NY. Arch. Neurol 1995, 52, 1201–1205. [Google Scholar]
- Rocon, E.; Manto, M.; Pons, J.; Camut, S.; Belda, J.M. Mechanical suppression of essential tremor. Cerebellum 2007, 6, 73–78. [Google Scholar]
- Deuschl, G.; Bain, P.; Brin, M. Consensus statement of the Movement Disorder Society on. Tremor. Ad Hoc Scientific Committee. Mov. Disord 1998, 13, 2–23. [Google Scholar]
- Elble, R.J. Characteristics of physiologic tremor in young and elderly adults. Clin. Neurophysiol 2003, 114, 624–35. [Google Scholar]
- Elble, R.J. Central mechanisms of tremor. J. Clin. Neurophysiol 1996, 13, 133–144. [Google Scholar]
- Dietz, V.; Hillesheimer, W.; Freund, H.J. Correlation between tremor, voluntary contraction, and firing pattern of motor units in Parkinson’s disease. J. Neurol Neurosurg. Psychiatry 1974, 37, 927–937. [Google Scholar]
- Findley, L.J.; Koller, W.C. Handbook of Tremor Disorders; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Grimaldi, G.; Manto, M. Tremor: From Pathogenesis to Treatment; Morgan & Claypool: San Rafael, CA, USA, 2008. [Google Scholar]
- Kronenbuerger, M.; Konczak, J.; Ziegler, W.; Buderath, P.; Frank, B.; Coenen, V.A.; Kiening, K.; Reinacher, P.; Noth, J.; Timmann, D. Balance and motor speech impairment in essential tremor. Cerebellum 2009, 8, 389–398. [Google Scholar]
- Tsuji, S.; Onodera, O.; Goto, J.; Nishizawa, M. Study Group on Ataxic Diseases. Sporadic ataxias in Japan--a population-based epidemiological study. Cerebellum 2008, 7, 189–197. [Google Scholar]
- Timmann, D.; Brandauer, B.; Hermsdörfer, J.; Ilg, W.; Konczak, J.; Gerwig, M.; Gizewski, E.R.; Schoch, B. Lesion-symptom mapping of the human cerebellum. Cerebellum 2008, 7, 602–606. [Google Scholar]
- Döhlinger, S.; Hauser, T.K.; Borkert, J.; Luft, A.R.; Schulz, J.B. Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum 2008, 7, 204–214. [Google Scholar]
- Mathiowetz, V.; Weber, K.; Kashman, N.; Volland, G. Adult norms for the nine hole peg test of finger dexterity. Occup. Ther. J. Res 1985, 5, 24–37. [Google Scholar]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the box and block test of manual dexterity. Am. J. Occup. Ther 1985, 39, 386–391. [Google Scholar]
- Wong, W.Y.; Wong, M.S.; Lo, K.H. Clinical applications of sensors for human posture and movement analysis: a review. Prosthet. Orthot. Int 2007, 31, 62–75. [Google Scholar]
- Luinge, H.J.; Veltink, P.H. Measuring orientation of human body segments using miniature gyroscopes and accelerometers. Med. Biol. Eng. Comput 2005, 43, 273–282. [Google Scholar]
- Raethjen, J.; Lauk, M.; Köster, B.; Fietzek, U.; Friege, L.; Timmer, J.; Lücking, C.H.; Deuschl, G. Tremor analysis in two normal cohorts. Clin. Neurophysiol 2004, 9, 2151–2156. [Google Scholar]
- Wastensson, G.; Lamoureux, D.; Sällsten, G.; Beuter, A.; Barregård, L. Quantitative assessment of neuromotor function in workers with current low exposure to mercury vapor. Neurotoxicology 2008, 29, 596–604. [Google Scholar]
- Safronov, V.A.; Shevelev, I.N.; Shabalov, V.A.; Panin, V.V. Localization of a Tremor Generator. Human Physiol 2001, 4, 454–457. [Google Scholar]
- Daube, J.R.; Rubin, D.I. Needle electromyography. Muscle Nerve 2009, 39, 244–270. [Google Scholar]
- Manto, M.; Sauvage, C.; Roark, R.M. Unifying hypothesis for the motoneuronal code in neurological disorders. Biosci. Hypoth 2008, 1, 93–99. [Google Scholar]
- McAuley, J.; Rothwell, J. Identification of psychogenic, dystonic, and other organic tremors by a coherence entrainment test. Mov. Disord 2004, 19, 253–267. [Google Scholar]
- Breit, S.; Spieker, S.; Schulz, J.B.; Gasser, T. Long-term EMG recordings differentiate between parkinsonian and essential tremor. J. Neurol 2008, 255, 103–11. [Google Scholar]
- Engin, M. A recording and analysis system for human tremor. Measurement 2007, 40, 288–293. [Google Scholar]
- Roetenberg, D.; Luinge, H.J.; Baten, C.T.M.; Veltink, P.H. Compensation of magnetic disturbances improves inertial and magnetic sensing of human body segment orientation. IEEE Trans. Neural. Syst. Rehab. Eng 2005, 13, 395–405. [Google Scholar]
- Morrison, S.; Newell, K.M. Postural and resting tremor in the upper limb. Clin. Neurophysiol 2000, 111, 651–663. [Google Scholar]
- Patel, S.; Lorincz, K.; Hughes, R.; Huggins, N.; Growdon, J.; Standaert, D.; Akay, M.; Dy, J.; Welsh, M.; Bonato, P. Monitoring motor fluctuations in patients with Parkinson’s disease using wearable sensors. IEEE Trans. Inf. Technol. Biomed 2009, 13, 864–873. [Google Scholar]
- Journee, H.L.; Postma, A.A.; Staal, M.J. Intraoperative neurophysiological assessment of disabling symptoms in DBS surgery. Neurophysiol. Clin 2007, 37, 467–475. [Google Scholar]
- Manto, M.; Marmolino, D. Animal models of human cerebellar ataxias: a cornerstone for the therapies of the twenty-first century. Cerebellum 2009, 8, 137–154. [Google Scholar]
- Metni, N.; Pflimlin, J.M.; Hamel, T.; Souères, P. Attitude and gyro bias estimation for a VTOL UAV. Contr. Eng. Pract 2006, 14, 1511–1520. [Google Scholar]
- Salarian, A.; Russmann, H.; Wider, C.; Burkhard, PR.; Vingerhoets, FJG.; Aminian, K. Quantification of tremor and bradykinesia in Parkinson’s disease using a novel ambulatory monitoring system. IEEE Trans. Biomed. Eng 2007, 2, 313–322. [Google Scholar]
- Zhou, H.; Hu, H.; Tao, Y. Inertial measurements of upper limb motion. Med. Bio. Eng. Comput 2006, 44, 479–487. [Google Scholar]
- Zhang, T.; Wei, G.; Yan, Z.; Ding, M.; Li, C.; Ding, H.; Xu, S. Quantitative assessment of Parkinson’s disease deficits. Chin. Med. J. (English) 1999, 112, 812–815. [Google Scholar]
- Swider, M. The application of video image processing to quantitative analysis of extremity tremor in humans. J. Neurosci. Methods 1998, 84, 167–172. [Google Scholar]
- Louis, E.D.; Levy, G.; Côte, L.J.; Mejia, H.; Fahn, S.; Marder, K. Diagnosing Parkinson’s disease using videotaped neurological examinations: validity and factors that contribute to incorrect diagnoses. Mov. Disord 2002, 17, 513–517. [Google Scholar]
- Louis, E.D.; Ford, B.; Bismuth, B. Reliability between two observers using a protocol for diagnosing essential tremor. Mov. Disord 1998, 13, 287–293. [Google Scholar]
- Stacy, M.A.; Elble, R.J.; Ondo, W.G.; Wu, S.C.; Hulihan, J.; TRS study group. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov. Disord 2007, 22, 833–838. [Google Scholar]
- Louis, E.D.; Barnes, L.; Wendt, K.J.; Ford, B.; Sangiorgio, M.; Tabbal, S.; Lewis, L.; Kaufmann, P.; Moskowitz, C.; Comella, C.L.; Goetz, C.C.; Lang, A.E. A teaching videotape for the assessment of essential tremor. Mov. Disord 2001, 16, 89–93. [Google Scholar]
- Klebe, S.; Stolze, H.; Grensing, K.; Volkmann, J.; Wenzelburger, R.; Deuschl, G. Influence of alcohol on gait in patients with essential tremor. Neurology 2005, 65, 96–101. [Google Scholar]
- Pellegrini, B.; Faes, L.; Nollo, G.; Schena, F. Quantifying the contribution of arm postural tremor to the outcome of goal-directed pointing task by displacement measures. J. Neurosci. Meth 2004, 139, 185–193. [Google Scholar]
- Braune, W.; Fischer, O. The human gait; Springer: Berlin, Germany, 1987. [Google Scholar]
- Chiari, L.; Della Croce, U.; Leardini, A.; Cappozzo, A. Human movement analysis using stereophotogrammetry. Part 2: instrumental errors. Gait Posture 2005, 21, 197–211. [Google Scholar]
- Taylor, K.D.; Mottier, F.M.; Simmons, D.W.; Cohen, W.; Pavlak, R., Jr.; Cornell, D.P.; Hankins, G.B. An automated motion measurement system for clinical gait analysis. J. Biomech 1982, 15, 505–516. [Google Scholar]
- Ferrigno, G.; Borghese, N.A.; Pedotti, A. Pattern-recognition in 3-D automatic human motion analysis. ISPRS J. Photogramm 1990, 45, 227–246. [Google Scholar]
- Gazzani, F. Comparative assessment of two algorithms for calibrating stereophotogrammetric systems. J. Biomech 1993, 26, 1449–1454. [Google Scholar]
- Leardini, A.; Chiari, L.; Della Croce, U.; Cappozzo, A. Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait Posture 2005, 21, 212–25. [Google Scholar]
- Avizzano, C. A.; Bergamasco, M. Technological aids for the treatment of the tremor. International Conference on Rehabilitation Robotics (ICORR), Stanford University, Stanford, CA, USA; 1999. [Google Scholar]
- Grimaldi, G.; Lammertse, P.; Van Den Braber, N.; Meuleman, J.; Manto, M. Effects of inertia and wrist oscillations on contralateral neurological postural tremor using the wristalyzer, a new myohaptic device. IEEE Trans. Biomed. Circuits Syst 2008, 4, 269–279. [Google Scholar]
- Grimaldi, G.; Lammertse, P.; Manto, M. Effects of wrist oscillations on contralateral neurological postural tremor using a new myohaptic device (“wristalyzer”). Proceedings of the 4th IEEE/EMBS International Summer School and Symposium on Medical Devices and Biosensors, Cambridge, UK, August 2007; pp. 44–48.
- Rozman, J.; Bartolić, A.; Ribaric, S. A new method for selective measurement of joint movement in hand tremor in Parkinson’s disease patients. J. Med. Eng. Technol 2007, 31, 305–311. [Google Scholar]
- Miralles, F.; Tarongí, S.; Espino, A. Quantification of the drawing of an Archimedes spiral through the analysis of its digitized picture. J. Neurosci. Meth 2006, 152, 18–31. [Google Scholar]
- Bonato, P. Wearable sensors/systems and their impact on biomedical engineering. IEEE Eng. Med. Biol. Magazine 2003, 3, 18–20. [Google Scholar]
- Patel, S.; Mancinelli, C.; Hughes, R.; Dalton, A.; Shih, L.; Bonato, P. Optimizing deep brain stimulation settings using wearable sensing technology. Neural. Eng 2009, 6–9. [Google Scholar]
- Swallow, L.; Siores, E. Tremor Suppression Using Smart Textile Fibre Systems. J.F.B.I 2009, 4, 261–266. [Google Scholar]
- Timmann, D.; Lee, P.; Watts, S.; Hore, J. Kinematics of arm joint rotations in cerebellar and unskilled subjects associated with the inability to throw fast. Cerebellum 2008, 7, 366–378. [Google Scholar]
- Xiong, X.; Wu, Y.L.; Jone, W.B. Material Fatigue and Reliability of MEMS Accelerometers. Proceedings of the 2008 IEEE International Symposium on Defect and Fault Tolerance of VLSI Systems, Cambridge, MA, USA, October 1–3, 2008; pp. 314–322.
- Fei, J.; Batur, C. A novel adaptive sliding mode control with application to MEMS gyroscope. ISA Trans 2009, 48, 73–78. [Google Scholar]
- Batur, C.; Sreeramreddy, T.; Khasawneh, Q. Sliding mode control of a simulated MEMS gyroscope. ISA Trans 2006, 45, 99–108. [Google Scholar]
- Leland, R. Adaptive mode tuning for vibrational gyroscopes. IEEE Trans. Control Syst. Technol 2003, 11, 242–247. [Google Scholar]
- Park, S.; Horowitz, R. New adaptive mode of operation for MEMS gyroscopes. ASME Trans. Dyn. Syst. Meas. Control 2004, 126, 800–810. [Google Scholar]
- Brunetti, F.J.; Rocon, E.; Pons, J.L.; Manto, M. The tremor coherence analyzer (TCA): a portable tool to assess instantaneous inter-muscle coupling in tremor. Conf. Proc. IEEE Eng. Med. Biol. Soc 2004, 1, 61–64. [Google Scholar]
- Lin, C.T.; Ko, L.W.; Chang, M.H.; Duann, J.R.; Chen, J.Y.; Su, T.P.; Jung, T.P. Review of Wireless and Wearable Electroencephalogram Systems and Brain-Computer Interfaces—A Mini-Review. Gerontology 2009. (Epub ahead of print).. [Google Scholar]
- Gu, Y.; do Nascimento, O.F.; Lucas, M.F.; Farina, D. Identification of task parameters from movement-related cortical potentials. Med. Biol. Eng. Comput 2009. (Epub ahead of print).. [Google Scholar]
- Shibasaki, H.; Hallett, M. What is the Bereitschaftspotential? Clin. Neurophysiol 2006, 117, 2341–2356. [Google Scholar]
- The tremor project (ICT-2007-224051). Available online: http://www.iai.csic.es/tremor/ (accessed on 11 February 2010).
- Hyde, R.A.; Ketteringham, L.P.; Neild, S.A.; Jones, R.S. Estimation of upper-limb orientation based on accelerometer and gyroscope measurements. IEEE Trans. Biomed. Eng 2008, 55, 746–754. [Google Scholar]
- Manto, M.; Grimaldi, G.; Lorivel, T.; Farina, D.; Popovic, L.; Conforto, S.; D’Alessio, T.; Belda-Lois, J.M.; Pons, J.L.; Rocon, E. Bioinformatic Approaches Used in Modelling Human Tremor. Curr. Bioinf 2009, 2, 154–172. [Google Scholar]
- Journée, H.L.; Postmab, A.A.; Sunc, M.; Staala, M.J. Detection of tremor bursts by a running second order moment function and analysis using interburst histograms. Med. Eng. Physics 2008, 1, 75–83. [Google Scholar]
- Kim, S.; McNames, J. Tracking Tremor Frequency in Spike Trains Using the Extended Kalman Filter Engineering in Medicine and Biology Society. Proceedings of 27th Annual Conference of the IEEE EMBS, Shanghai, China, September 1–4; 2005; pp. 7576–7579. [Google Scholar]
- Strambi, S.K.; Rossi, B.; De Michele, G.; Sello, S. Effect of medication in Parkinson’s disease: a wavelet analysis of EMG signals. Med. Eng. Physics 2004, 26, 279–290. [Google Scholar]
- McAuley, J.H.; Rothwell, J.C.; Marsden, C.D. Frequency peaks of tremor, muscle vibration and electromyographic activity at 10 Hz, 20 Hz and 40 Hz during human finger muscle contraction may reflect rhythmicities of central neural firing. Exp. Brain Res 1997, 114, 525–541. [Google Scholar]
- Duval, C. Rest and postural tremors in patients with Parkinson’s disease. Brain Res. Bull 2006, 70, 44–48. [Google Scholar]
- Edwards, R.; Beuter, A. Indexes for identification of abnormal tremor using computer tremor evaluation systems. IEEE Trans. Biomed. Eng 1999, 7, 895–898. [Google Scholar]
- Rocon, E.; Pons, J.L.; Andrade, A.O.; Nasuto, S.J. Application of EMD as a novel technique for the study of tremor time series. Conf. Proc. IEEE Eng. Med. Biol. Soc 2006, (Suppl), 6533–6536. [Google Scholar]
- Matilla-Dueñas, A. The highly heterogeneous spinocerebellar ataxias: from genes to targets for therapeutic intervention. Cerebellum 2008, 7, 97–100. [Google Scholar]
- Underwood, B.R.; Rubinsztein, D.C. Spinocerebellar ataxias caused by polyglutamine expansions: a review of therapeutic strategies. Cerebellum 2008, 7, 215–221. [Google Scholar]







| -to move a glass full of water on a table | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| -to drink | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| -to eat (use of forks and knives) | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| -to shave | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| -to write words on a sheet of paper or to sign | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| -to read a book | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| -to drive a car | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| -to dress one-self | |
| No problem | 0 |
| Slight difficulties | 1 |
| Important difficulties | 2 |
| Impossible | 3 |
| Total Score:................................................../24 | |
| C-TES (Clinical-TES) |
| Anamnesis |
| Assessment of disability (Activities of Daily Living scales / ADL-T24) |
| Physical and Neurological examination |
Tremor evaluation
|
| Brain imaging (CT-scan - MRI - SPECT- PET) |
| Blood studies |
| N-TES (Neurophysiological-TES) |
| EMG and EEG recordings (time-frequency analysis, coherence, ERS/ERD) |
| Evaluation of pinch force |
| Analysis of writing (digitizing tablet) |
| F-TES (Functional-TES; see [13–14]) |
| Mechanical counters |
| Box and block Test |
| 9-Hole-Peg Test |
| Coin test |
| Assessment of kinematics** | EMG Surface EMG (SEMG) Needle electrodes Fine-wire electrodes Long-term recordings | Force transducers and force-feedback devices (haptic devices) | |||
|---|---|---|---|---|---|
| Accelerometer | Gyroscope | Video | |||
| Gravity effect influence | yes | no | no | no | no |
| Accuracy of frequency information | good | good | may be low | good | good |
| Signal-to-noise ratio | low to high | high | variable | high | high |
| Electrical contacts with subjects | no | no | no | yes | yes |
| Size | small | small | relatively large | small | Large |
| Painful | no | no | no | yes (needle EMG) | no |
| Cost | cheap | cheap | cheap to expensive | variable | expensive |
| Easy to use | yes | yes | yes | variable | relatively difficult |
| Data processing required | yes | yes | yes | yes | yes |
| Measurement of tremor amplitude | calculation from time/acceleration | measurement of inertial angular rate | from calibrated video frames | no | from force/mass or position encoder |
| Parameter | Piezoelectric | Piezoresistive | Capacitive |
|---|---|---|---|
| Gravitational component | No | Yes | Yes |
| Bandwidth | Wide | Low to moderate | Wide |
| Impedance | High | Low | Very high |
| Signal level | High | Low | Moderate |
| Ruggedness | Good | Moderate | Good |
| Cost | High | Low | High |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grimaldi, G.; Manto, M. Neurological Tremor: Sensors, Signal Processing and Emerging Applications. Sensors 2010, 10, 1399-1422. https://doi.org/10.3390/s100201399
Grimaldi G, Manto M. Neurological Tremor: Sensors, Signal Processing and Emerging Applications. Sensors. 2010; 10(2):1399-1422. https://doi.org/10.3390/s100201399
Chicago/Turabian StyleGrimaldi, Giuliana, and Mario Manto. 2010. "Neurological Tremor: Sensors, Signal Processing and Emerging Applications" Sensors 10, no. 2: 1399-1422. https://doi.org/10.3390/s100201399
APA StyleGrimaldi, G., & Manto, M. (2010). Neurological Tremor: Sensors, Signal Processing and Emerging Applications. Sensors, 10(2), 1399-1422. https://doi.org/10.3390/s100201399