Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis
Abstract
:1. Electronic Nose and Metal Oxide Semi-Conductor Sensors
2. Application of MOS to Food
2.1. Meat
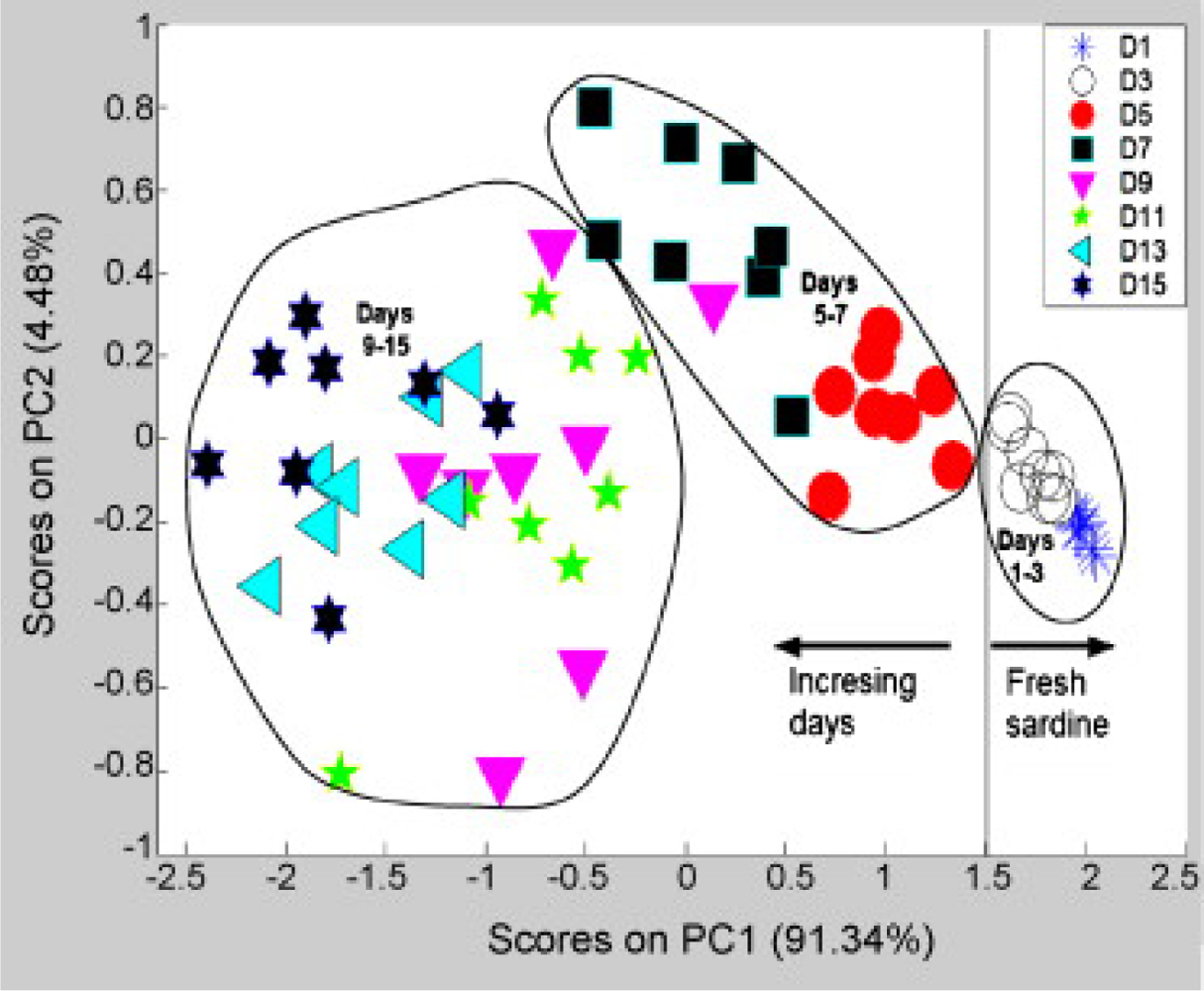
2.2. Fish
2.3. Milk and Dairy Products
2.3.1. Adulteration/ contamination of milk and off-flavors
2.3.2. Ageing of milk
2.3.3. Ripening of cheese and cheese types
2.3.4. Lactic acid bacteria
2.3.5. Off-flavors in cheese
2.3.6. Geographical origin of dairy product
2.4. Eggs
2.5. Grains
2.6. Fruits
2.7. Olive Oils
2.8. Alcoholic Drinks
2.8.1. Discrimination of wines by denomination of origin and vineyard
2.8.2. Aging of wines and beers
2.8.3. Classification of alcoholic drinks
2.8.4. Detection of aromatic compounds and off-favors in wines
2.9. Non-Alcoholic Beverages
2.10. Other Food
3. Conclusions
Acknowledgments
References
- Gardner, J.; Bartlett, P.N. Electronic Nose. Principles and Applications; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Schaller, E.; Bosset, J.O.; Escher, F. ‘Electronic noses’ and their application to food. Food Sci. Technol-Leb 1998, 31, 305–316. [Google Scholar]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuat. B 1999, 57, 1–16. [Google Scholar]
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar]
- Berna, A.Z.; Anderson, A.R.; Trowell, S.C. Bio-benchmarking of electronic nose sensors. Plos One 2009, 4, e6406. [Google Scholar]
- Sears, W.M.; Colbow, K.; Slamka, R.; Consadori, F. Selective thermally cycled gas sensing using fast fourier-transform techniques. Sens. Actuat. B 1990, 2, 283–289. [Google Scholar]
- Fort, A.; Gregorkiewitz, M.; Machetti, N.; Rocchi, S.; Serrano, B.; Tondi, L.; Ulivieri, N.; Vignoli, V.; Faglia, G.; Comini, E. Selectivity enhancement of SnO2 sensors by means of operating temperature modulation. Thin Solid Films 2002, 418, 2–8. [Google Scholar]
- Gutierrez-Osuna, R.; Gutierrez-Galvez, A.; Powar, N. Transient response analysis for temperature-modulated chemoresistors. Sens. Actuat. B 2003, 93, 57–66. [Google Scholar]
- Ngo, K.A.; Lauque, P.; Aguir, K. High performance of a gas identification system using sensor array and temperature modulation. Sens. Actuat. B 2007, 124, 209–216. [Google Scholar]
- Shahidi, F. Headspace volatile aldehydes as indicators of lipid oxidation in foods. Adv. Exp. Med. Biol 2001, 488, 113–123. [Google Scholar]
- Berna, A.Z.; Clifford, D.; Boss, P.; Trowell, T. Selection of optimal sensor/temperature conditions for winegrape analysis using generalized additive modeling of thermally cycled metal oxide sensors. Proceedings of the 8th IEEE Conference of Sensors, Christchurch, New Zealand, October 25–28, 2009; pp. 1117–1120.
- Binions, R.; Afonja, A.; Dungey, S.; Lewis, D.; Parkin, I.P.; Williams, D.E. Zeotite modification: towards discriminating metal oxide gas sensors. ECS Trans 2009, 19, 241–250. [Google Scholar]
- Binions, R.; Davies, H.; Afonja, A.; Dungey, S.; Lewis, D.; Williams, D.E.; Parkin, I.P. Zeolite-modified discriminating gas sensors. J. Electrochem. Soc 2009, 156, J46–J51. [Google Scholar]
- Vilaseca, M.; Coronas, J.; Cirera, A.; Cornet, A.; Morante, J.R.; Santamaria, J. Gas detection With SnO2 sensors modified by zeolite films. Sens. Actuat. B 2007, 124, 99–110. [Google Scholar]
- Mann, D.P.; Pratt, K.F.E.; Paraskeva, T.; Parkin, I.P.; Williams, D.E. Transition metal exchanged zeolite layers for selectivity enhancement of metal-oxide semiconductor gas sensors. IEEE Sens. J 2007, 7, 551–556. [Google Scholar]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.M. Time course of volatile compound formation during refrigerated storage of naturally contaminated beef in air. J. Appl. Bacteriol 1985, 59, 303–309. [Google Scholar]
- Mayr, D.; Hartungen, E.; Mark, T.; Margesin, R.; Schinner, F. Determination of the spoilage status of meat by aroma detection using proton-transfer-reaction mass-spectrometry. Proceedings of the 10th Weurman Flavour Research Symposium, Beaune, France, June 25−28, 2002; pp. 757–760.
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MM)—medical applications, food control and environmental research. Int. J. Mass Spectrom 1998, 173, 191–241. [Google Scholar]
- Winquist, F.; Hornsten, E.G.; Sundgren, H.; Lundstrom, I. Performance of an electronic nose for quality estimation of ground meat. Meas. Sci. Technol 1993, 4, 1493–1500. [Google Scholar]
- Balasubramanian, S.; Panigrahi, S.; Logue, C.M.; Doetkott, C.; Marchello, M.; Sherwood, J.S. Independent component analysis-processed electronic nose data for predicting Salmonella Typhimurium populations in contaminated beef. Food Control 2008, 19, 236–246. [Google Scholar]
- Kermit, M.; Tomic, O. Independent component analysis applied on gas sensor array measurement data. IEEE Sens. J 2003, 3, 218–228. [Google Scholar]
- Vernat-Rossi, V.; Garcia, C.; Talon, R.; Denoyer, C.; Berdague, J.L. Rapid discrimination of meat products and bacterial strains using semiconductor gas sensors. Sens. Actuat. B 1996, 37, 43–48. [Google Scholar]
- Patterson, R.L.S. 5 alpha-androst-16-Ene-3-1: compound responsible for taint in boar fat. J. Sci. Food. Agric 1968, 19, 31–37. [Google Scholar]
- Rius, M.A.; Hortos, M.; Garcia-Regueiro, J.A. Influence of volatile compounds on the development of off-flavours in pig back fat samples classified with boar taint by a test panel. Meat Sci 2005, 71, 595–602. [Google Scholar]
- Bourrounet, B.; Talou, T.; Gaset, A. Application of a multi-gas-sensor device in the meat industry for boar-taint detection. Sens. Actuat. B 1995, 27, 250–254. [Google Scholar]
- Bene, A.; Hayman, A.; Reynard, E.; Luisier, J.L.; Villettaz, J.C. A new method for the rapid determination of volatile substances: the SPME-direct method—Part II. Determination of the freshness of fish. Sens. Actuat. B 2001, 72, 204–207. [Google Scholar]
- Olafsdottir, G.; Chanie, E.; Westad, F.; Jonsdottir, R.; Thalmann, C.R.; Bazzo, S.; Labreche, S.; Marcq, P.; Lundby, F.; Haugen, J.E. Prediction of microbial and sensory quality of cold smoked atlantic salmon (Salmo Salar) by electronic nose. J. Food Sci 2005, 70, S563–S574. [Google Scholar]
- Haugen, J.E.; Chanie, E.; Westad, F.; Jonsdottir, R.; Bazzo, S.; Labreche, S.; Marcq, P.; Lundby, F.; Olafsdottir, G. Rapid control of smoked Atlantic salmon (Salmo salar) quality by electronic nose: Correlation with classical evaluation methods. Sens. Actuat. B 2006, 116, 72–77. [Google Scholar]
- El Barbri, N.; Amari, A.; Vinaixa, M.; Bouchikhi, B.; Correig, X.; Llobet, E. Building of a metal oxide gas sensor-based electronic nose to assess the freshness of sardines under cold storage. Sens. Actuat. B 2007, 128, 235–244. [Google Scholar]
- El Barbri, N.; Llobet, E.; El Bari, N.; Correig, X.; Bouchikhi, B. Application of a portable electronic nose system to assess the freshness of moroccan sardines. Mat. Sci. Eng. C-Bio S 2008, 28, 666–670. [Google Scholar]
- Fox, P.F. Advanced Dairy Chemistry; Chapman & Hall: London, UK/New York, NY, USA, 1992. [Google Scholar]
- Yu, H.C.; Wang, J.; Xu, Y. Identification of Adulterated Milk Using Electronic Nose. Sens. Mater 2007, 19, 275–285. [Google Scholar]
- Benedetti, S.; Bonomi, F.; Iametti, S.; Mannino, S.; Cosio, M.S. Detection of aflatoxin M1 in ewe milk by using an electronic nose. Proceedings of the 2nd Central European Meeting 5th Croatian Congress of Food Technologists, Biotechnologists and Nutritionists, Opatija, Croatia, October 17–20, 2004; pp. 101–105.
- Ampuero, S.; Bosset, J.O. The Electronic nose applied to dairy products: a review. Sens. Actuat. B 2003, 94, 1–12. [Google Scholar]
- Mulville, T. UHT the nose knows. Food Manufact 2000, 27–28. [Google Scholar]
- Mariaca, R.; Bosset, J.O. Instrumental analysis of volatile (flavour) compounds in milk and dairy products. Lait 1997, 77, 13–40. [Google Scholar]
- Capone, S.; Epifani, M.; Quaranta, F.; Siciliano, P.; Taurino, A.; Vasanelli, L. Monitoring of rancidity of milk by means of an electronic nose and a dynamic PCA analysis. Sens. Actuat. B 2001, 78, 174–179. [Google Scholar]
- Capone, S.; Siciliano, P.; Quaranta, F.; Rella, R.; Epifani, M.; Vasanelli, L. Analysis of vapours and foods by means of an electronic nose based on a sol-gel metal oxide sensors array. Sens. Actuat. B 2000, 69, 230–235. [Google Scholar]
- Labreche, S.; Bazzo, S.; Cade, S.; Chanie, E. Shelf life determination by electronic nose: application to milk. Sens. Actuat. B 2005, 106, 199–206. [Google Scholar]
- Schaller, E.; Bosset, J.O.; Escher, F. Practical experience with ‘Electronic Nose’ systems for monitoring the quality of dairy products. Chimia 1999, 53, 98–102. [Google Scholar]
- Jou, K.D.; Harper, W.J. Pattern recognition of Swiss cheese aroma compounds by SPME/GC and an electronic nose. Milchwissenschaft 1998, 53, 259–263. [Google Scholar]
- Gutierrez-Mendez, N.; Vallejo-Cordoba, B.; Gonzalez-Cordova, A.F.; Nevarez-Moorillon, G.V.; Rivera-Chavira, B. Evaluation of aroma generation of Lactococcus Lactis with an electronic nose and sensory analysis. J. Dairy Sci 2008, 91, 49–57. [Google Scholar]
- Schaller, E.; Bosset, J.O.; Escher, F. Feasibility study: detection of rind taste off-flavour in Swiss emmental cheese using an ‘electronic nose’ and a GC-MS. Mitt. Lebensm. Hyg 2000, 91, 610–615. [Google Scholar]
- Application Note 55 Comparison of Suppliers and QC Monitoring-Application with Caseinate. Available online: http://www.alpha-mos.com (accessed on 15 April 2010).
- Application Note 34 Aroma Differentiation Based on Process and Origin-Application to the Dairy Industry. Available online: http://www.alpha-mos.com (accessed on 15 April 2010).
- Wang, Y.W.; Wang, J.; Zhou, B.; Lu, Q.J. Monitoring storage time and quality attribute of egg based on electronic nose. Anal. Chim. Acta 2009, 650, 183–188. [Google Scholar]
- Dutta, R.; Hines, E.L.; Gardner, J.W.; Udrea, D.D.; Boilot, P. Non-destructive egg freshness determination: an electronic nose based approach. Meas. Sci. Technol 2003, 14, 190–198. [Google Scholar]
- Brown, M.L.; Holbrook, D.M.; Hoerning, E.F.; Legendre, M.G.; Stangelo, A.J. Volatile indicators of deterioration in liquid egg products. Poult. Sci 1986, 65, 1925–1933. [Google Scholar]
- Suman, M.; Riani, G.; Dalcanale, E. MOS-based artificial olfactory system for the assessment of egg products freshness. Sens. Actuat. B 2007, 125, 40–47. [Google Scholar]
- Campagnoli, A.; Dell’orto, V.; Savoini, G.; Cheli, F. Screening cereals quality by electronic nose: the example of mycotoxins naturally contaminated maize and durum wheat. Proceedings of the 13th International Symposium on Olfaction and Electronic Nose, Brescia, Italy, April 15–17, 2009; pp. 507–510.
- Olsson, J.; Borjesson, T.; Lundstedt, T.; Schnurer, J. Detection and quantification of ochratoxin A and deoxynivalenol in barley grains by GC-MS and electronic nose. Int. J. Food Microbiol 2002, 72, 203–214. [Google Scholar]
- Borjesson, T.; Eklov, T.; Jonsson, A.; Sundgren, H.; Schnurer, J. Electronic nose for odor classification of grains. Cereal Chem 1996, 73, 457–461. [Google Scholar]
- Jonsson, A.; Winquist, F.; Schnurer, J.; Sundgren, H.; Lundstrom, I. Electronic nose for microbial quality classification of grains. Int. J. Food Microbiol 1997, 35, 187–193. [Google Scholar]
- Gomez, A.H.; Hu, G.X.; Wang, J.; Pereira, A.G. Evaluation of tomato maturity by electronic nose. Comput. Electron. Agri 2006, 54, 44–52. [Google Scholar]
- Concina, I.; Falasconi, M.; Gobbi, E.; Bianchi, F.; Musci, M.; Mattarozzi, M.; Pardo, M.; Mangia, A.; Careri, M.; Sberveglieri, G. Early detection of microbial contamination in processed tomatoes by electronic nose. Food Control 2009, 20, 873–880. [Google Scholar]
- Simon, J.E.; Hetzroni, A.; Bordelon, B.; Miles, G.E.; Charles, D.J. Electronic sensing of aromatic volatiles for quality sorting of blueberries. J. Food Sci 1996, 61, 967–970. [Google Scholar]
- Supriyadi; Shimazu, K.; Susuki, M.; Yoshida, K.; Muto, T.; Fujita, A.; Tomita, N.; Watanabe, N. Maturity discrimination of snake fruit (Salacca edulis Reinw.) cv. Pondoh based on volatiles analysis using an electronic nose device equipped with a sensor array and fingerprint mass spectrometry. Flavour Frag. J 2004, 19, 44–50. [Google Scholar]
- Gomez, A.H.; Wang, J.; Hu, G.X.; Pereira, A.G. Discrimination of storage shelf-life for mandarin by electronic nose technique. Lwt-Food Sci. Technol 2007, 40, 681–689. [Google Scholar]
- Gomez, A.H.; Wang, J.; Pereira, A.G. Mandarin ripeness monitoring and quality attribute evaluation using an electronic nose technique. Trans. ASABE 2007, 50, 2137–2142. [Google Scholar]
- Steinmetz, V.; Crochon, M.; Talou, T.; Bourrounet, B. Sensor fusion for fruit quality assessment: application to melons. Proceedings of International Conference on Harvest and Postharvest Technologies for Fresh Fruits and Vegetables, Guanajuato, Gto, Mexico, February 20–24, 1995; pp. 488–496.
- Steinmetz, V.; Sevila, F.; Bellon-Maurel, V. A Methodology for sensor fusion design: Application to fruit quality assessment. J. Agr. Eng. Res 1999, 74, 21–31. [Google Scholar]
- Berna, A.Z.; Trowell, T.; Clifford, D.; Stone, G.; Lovell, D. Fast aroma analysis of Cabernet Sauvignon and Riesling grapes using an electronic nose. Am. J. Enol. Vitic 2007, 58, 416A–417A. [Google Scholar]
- Sayago, I.; Horrillo, M.D.; Ares, L.; Fernandez, M.J.; Gutierrez, J. Tin oxide multisensor for detection of grape juice and fermented wine varieties. Sens. Mater 2003, 15, 165–176. [Google Scholar]
- Sayago, I.; Horrillo, M.C.; Getino, J.; Gutierrez, J.; Ares, L.; Robla, J.I.; Fernandez, M.J.; Rodrigo, J. Discrimination of grape juice and fermented wine using a tin oxide multisensor. Sens. Actuat. B 1999, 57, 249–254. [Google Scholar]
- Smejkalova, D.; Piccolo, A. High-power gradient diffusion NMR spectroscopy for the rapid assessment of extra-virgin olive oil adulteration. Food Chem 2010, 118, 153–158. [Google Scholar]
- Gonzalez Martin, Y.; Cerrato Oliveros, M.C.; Perez Pavon, J.L.; Garcia Pinto, C.; Moreno Cordero, B. Electronic nose based on metal oxide semiconductor sensors and pattern recognition techniques: characterisation of vegetable oils. Anal. Chim. Acta 2001, 449, 69–80. [Google Scholar]
- IOOC (International Olive Oil Council). COI/T.20/Document 15/Rev. 1 Organoleptic Assessment of Olive Oil; Resolution RES-3/75-IV/96.; 20 November 1996. [Google Scholar]
- Garcia-Gonzalez, D.L.; Aparicio, R. Detection of vinegary defect in virgin olive oils by metal oxide sensors. J. Agric. Food Chem 2002, 50, 1809–1814. [Google Scholar]
- Garcia-Gonzalez, D.L.; Aparicio, R. Detection of defective virgin olive oils by metal-oxide sensors. Eur. Food. Res. Technol 2002, 215, 118–123. [Google Scholar]
- Marti, M.; Boque, R.; Busto, O.; Guasch, J. Electronic noses in the quality control of alcoholic beverages. Trends Anal. Chem 2005, 24, 57–66. [Google Scholar]
- Herbele, I.; Liebminger, A.; Weimar, U.; Gopel, W. Optimised sensor arrays with chromatographic preseparation: characterisation of alcoholic beverages. Sens. Actuat. B 2000, 68, 53–57. [Google Scholar]
- Garcia, M.; Aleixandre, M.; Gutierrez, J.; Horrillo, M. Electronic nose for wine discrimination. Sens. Actuat. B 2006, 113, 911–916. [Google Scholar]
- Aishima, T. Discrimination of liquor aromas by pattern-recognition analysis of responses from a gas sensor array. Anal. Chim. Acta 1991, 243, 293–300. [Google Scholar]
- Schafer, T.; Serrano-Santos, M.; Rocchi, S.; Fuoco, R. Pervaporation membrane separation process for enhancing the selectivity of an artificial olfactory system (“electronic nose”). Anal. Bioanal. Chem 2006, 384, 860–866. [Google Scholar]
- Pinheiro, C.; Rodrigues, C.; Schafer, T.; Crespo, J. Monitoring the aroma production during wine-must fermentation with an electronic nose. Biotechnol. Bioeng 2002, 77, 632–640. [Google Scholar]
- Guadarrama, A.; Fernandez, J.; Iniguez, M.; Souto, J.; Saja, J. Array of conducting polymer sensors for the characterisation of wines. Anal. Chim. Acta 2000, 411, 193–200. [Google Scholar]
- McKellar, R.; Young, J.; Johnston, A.; Knight, K.; Lu, X.; Buttenham, S. Use of the electronic nose and gas chromatography-mass spectrometry to determine the optimum time for aging beer. Master Brewers Assoc. Amer 2002, 39, 99–105. [Google Scholar]
- McKellar, R.; Rupasinghe, H.; Lu, X.; Knight, K. The electronic nose as a tool for the classification of fruit and grape wines from different Ontario wines. J. Sci. Food. Agric 2005, 85, 2391–2396. [Google Scholar]
- Berna, A.Z.; Trowell, S.; Cynkar, W.; Cozzolino, D. Comparison of metal oxide-based electronic nose and mass spectrometry-based electronic nose for the prediction of red wine spoilage. J. Agric. Food Chem 2008, 56, 3238–3244. [Google Scholar]
- Berna, A.Z.; Trowell, S.; Clifford, D.; Cynkar, W.; Cozzolino, D. Geographical origin of Sauvignon Blanc wines predicted by mass spectrometry and metal oxide based electronic nose. Anal. Chim. Acta 2009, 648, 146–152. [Google Scholar]
- Pinho, O.; Ferreira, I.; Santos, L. Method optimization by solid-phase microextraction in combination with gas chromatography with mass spectrometry for analysis of beer volatile fraction. J. Chromatogr. A 2006, 1121, 145–153. [Google Scholar]
- Pearce, T.C.; Gardner, J.W.; Friel, S.; Bartlett, P.N.; Blair, N. Electronic nose for monitoring the flavor of beers. Analyst 1993, 118, 371–377. [Google Scholar]
- Sakuma, S.; Amano, H.; Ohkochi, M. Identification of off-flavor compounds in beer. J. Am. Soc. Brew. Chem 2000, 58, 26–29. [Google Scholar]
- Penza, M.; Cassano, G. Chemometric characterization of Italian wines by thin-film multisensors array and artificial neural networks. Food Chem 2004, 86, 283–296. [Google Scholar]
- Santos, J.; Arroyo, T.; Aleixandre, M.; Lozano, J.; Sayago, I.; Garcia, M.; Fernandez, M.; Ares, L.; Gutierrez, J.; Cabellos, J.; Gil, M.; Horrillo, M. A comparative study of sensor array and GC-MS: application to Madrid wines characterisation. Sens. Actuat. B 2004, 102, 299–307. [Google Scholar]
- Buratti, S.; Benedetti, S.; Scampicchio, M.; Pangerod, E. Characterization and classification of Italian Barbera wines by using an electronic nose and an amperometric electronic tongue. Anal. Chim. Acta 2004, 525, 133–139. [Google Scholar]
- Di Natale, C.; Davide, F.A.M.; D’Amico, A.; Nelli, P. An electronic nose for the recogition of the vineyard of a red wine. Sens. Actuat. B 1996, 33, 83–88. [Google Scholar]
- Di Natale, C.; D’Amico, A. The electronic nose: a new instrument for wine analysis. Ital. Food Bever. Tech 1998, 14, 17–19. [Google Scholar]
- Villanueva, S.; Guadarrama, A.; Rodriguez-Mendez, M.L.; De Saja, J.A. Use of an array of metal oxide sensors coupled with solid phase microextraction for characterisation of wines study of the role of the carrier gas. Sens. Actuat. B 2008, 132, 125–133. [Google Scholar]
- Ragazzo-Sanchez, J.A.; Chalier, P.; Chevalier, D.; Ghommidh, C. Electronic nose discrimination of aroma compounds in alcoholised solutions. Sens. Actuat. B 2006, 114, 665–673. [Google Scholar]
- Ragazzo-Sanchez, J.A.; Chalier, P.; Chevalier-Lucia, D.; Calderon-Santoyo, M.; Ghommidh, C. Off-flavours detection in alcoholic beverages by electronic nose coupled to GC. Sens. Actuat. B 2009, 140, 29–34. [Google Scholar]
- Ibeas, J.; Lozano, I.; Perdigones, F.; Jimenez, J. Detection of Dekkera-Brettanomyces strains in sherry by a nested PCR method. Appl. Environ. Microbiol 1996, 62, 998–1003. [Google Scholar]
- Lozano, J.; Santos, J.P.; Horrillo, M.C. Classification of white wine aromas with an electronic nose. Talanta 2005, 67, 610–616. [Google Scholar]
- Arroyo, T.; Lozano, J.; Cabellos, J.M.; Gil-Diaz, M.; Santos, J.P.; Horrillo, C. Evaluation of wine aromatic compounds by a sensory human panel and an electronic nose. J. Agric. Food Chem 2009, 57, 11543–11549. [Google Scholar]
- Santos, J.P.; Lozano, J.; Aleixandre, M.; Arroyo, T.; Cabellos, J.M.; Gil, M.; Horrillo, M.C. Threshold detection of aromatic compounds in wine with an electronic nose and a human sensory panel. Talanta 2010, 80, 1899–1906. [Google Scholar]
- Bhuyan, M.; Borah, S. Use of electronic nose in tea industry. Proceedings of International Conference on Energy, Automation and Information Technology, Kharagpur, India, December 2001; pp. 848–853.
- Dutta, R.; Hines, E.L.; Gardner, J.W.; Kashwan, K.R.; Bhuyan, A. Tea quality prediction using a tin oxide-based electronic nose: an artificial intelligence approach. Sens. Actuat. B 2003, 94, 228–237. [Google Scholar]
- Yu, H.; Wang, J.; Xiao, H.; Liu, M. Quality grade identification of green tea using the eigenvalues of PCA based on the E-nose signals. Sens. Actuat. B 2009, 140, 378–382. [Google Scholar]
- Gardner, J.W.; Shurmer, H.V.; Tan, T.T. Application of an electronic nose to the discrimination of coffees. Sens. Actuat. B 1992, 6, 71–75. [Google Scholar]
- Aishima, T. Aroma discrimination by pattern-recognition analysis of responses from semiconductor gas sensor array. J. Agric. Food Chem 1991, 39, 752–756. [Google Scholar]
- Pardo, M.; Niederjaufner, G.; Benussi, G.; Comini, E.; Faglia, G.; Sberveglieri, G.; Holmberg, M.; Lundstrom, I. Data preprocessing enhances the classification of different brands of espresso coffee with an electronic nose. Sens. Actuat. B 2000, 69, 397–403. [Google Scholar]
- Pardo, M.; Sberveglieri, G. Coffee analysis with an electronic nose. IEEE T. Instrum. Meas 2002, 51, 1334–1339. [Google Scholar]
- Falasconi, M.; Pardo, M.; Sberveglieri, G.; Ricco, I.; Bresciani, A. The novel EOS835 electronic nose and data analysis for evaluating coffee ripening. Sens. Actuat. B 2005, 110, 73–80. [Google Scholar]
- Riva, M.; Benedetti, S.; Mannino, S. Shelf life of fresh cut vegetables as measured by an electronic nose: preliminary study. Ital. Food Tech 2002, 27, 5–11. [Google Scholar]
- Pastorelli, S.; Torri, L.; Rodriguez, A.; Valzacchi, S.; Limbo, S.; Simoneau, C. Solid-phase micro-extraction (SPME-GC) and sensors as rapid methods for monitoring lipid oxidation in nuts. Food Addit. Contam 2007, 24, 1219–1225. [Google Scholar]
- Lee, S.Y.; Krochta, J.M. Accelerated shelf life testing of whey-protein-coated peanuts analyzed by static headspace gas chromatography. J. Agric. Food Chem 2002, 50, 2022–2028. [Google Scholar]
- Rossi, V.; Talon, R.; Berdague, J.L. Rapid discrimination of Micrococcaceae species using semiconductor gas sensors. J. Microbiol. Meth 1995, 24, 183–190. [Google Scholar]
- Nychas, G.J.E.; Arkoudelos, J.S. Staphylococci—their role in fermented sausages. J. Appl. Bacteriol 1990, 69, S167–S188. [Google Scholar]




| Commodity | Test | No. of MOS sensors | Material | Commercial (C) or experimental (E) E-nose | Ref. |
|---|---|---|---|---|---|
| Meat | Freshness and type of meat | 4* | SnO2 | C | [19] |
| Microbiological contamination | 7 | SnO2 thick film | E | [20] | |
| Origin of meats | 6 | SnO2 | C | [22] | |
| Taints | 5 | SnO2 | E | [25] | |
| Fish | Freshness | 6 | SnO2, CTO, WO3 | C | [27] |
| 6 | SnO2, CTO, WO3 | C | [28] | ||
| 6 | SnO2 | E | [29] | ||
| Milk and dairy products | Adulteration/ Contamination | 10 | SnO2 | C | [32] |
| 12* | SnO2 | C** | [33] | ||
| Off-flavor | n.d. | n.d. | E | [35] | |
| 12* | SnO2 | C** | [43] | ||
| Milk and dairy products | Aging/ripening | 5 | SnO2 thin film doped with Pd, Pt, Os, Ni | E | [37,38] |
| 18 | SnO2, CTO, WO3 | C | [39] | ||
| 5 | n.d. | C** | [40] | ||
| Cheese type | 6 | SnO2, CTO, WO3 | C | [41] | |
| Bacterial strains | 12 | SnO2, CTO, WO3 | C | [42] | |
| Origin of caseinates | 18 | SnO2, CTO, WO3 | C | [44] | |
| Origin of milk | 18 | SnO2, CTO, WO3 | C | [45] | |
| Eggs | Freshness | 8 | n.d. | E | [46] |
| 4 | SnO2 | E | [47] | ||
| 12 | SnO2 thick film | C | [49] | ||
| Grains | Contamination by toxin | 6* | SnO2 | C* | [51] |
| 10 | SnO2 | C | [50] | ||
| Off-flavors | 4* | SnO2 | E | [52] | |
| 4* | SnO2 | E | [53] | ||
| Fruit | Ripening changes | 10 | SnO2 | C | [54] |
| 2 | SnO2 | E | [56] | ||
| 10 | SnO2 | C | [59] | ||
| 5 | SnO2 | E | [60,61] | ||
| 12 | SnO2, CTO, WO3 | C | [62] | ||
| Varieties | 16 | SnO2 thin film doped with Cr, Pt | E | [63,64] | |
| Microbial contamination | 6 | n.d. | C | [55] | |
| Shelf life | 18 | SnO2, CTO, WO3 | C | [57] | |
| 10 | SnO2 | C | [58] | ||
| Olive oil | Authenticity | 6 | SnO2, CTO, WO3 | C | [66] |
| Taints | 18 | SnO2, CTO, WO3 | C | [68,69] | |
| Alcoholic beverages | Denomination of origin and vineyard discrimination | 4 | WO3 | E | [84] |
| 16 | SnO2 thin film doped with Cr, In | E | [85] | ||
| 10 | SnO2 | C | [86] | ||
| 4 | SnO2 thin film doped with Pt, Pd | E | [87,88] | ||
| Aging of wines and beers | 16 | SnO2 thin film doped with Cr, In | E | [72] | |
| 20 | SnO2 | E | [89] | ||
| 12 | SnO2, CTO, WO3 | C | [77] | ||
| Alcoholic beverages | Classification of drinks | 12 | SnO2, CTO, WO3 | C | [78] |
| 6 | SnO2, CTO, WO3 | C | [90] | ||
| 6 | SnO2 | E | [73] | ||
| 6* | SnO2 | C | [71] | ||
| 18 | SnO2, CTO, WO3 | C | [91] | ||
| Off-flavors and aromatic compounds | 12 | SnO2, CTO, WO3 | C | [79] | |
| 18 | SnO2, CTO, WO3 | C | [91] | ||
| 16 | SnO2 | E | [93] | ||
| 16 | SnO2 thin film | E | [95] | ||
| 16 | SnO2 | E | [94] | ||
| Non alcoholic beverages | Grading | 4 | SnO2 | E | [97] |
| 10 | SnO2 | C | [98] | ||
| Quality/process control | 6 | SnO2 | E | [100] | |
| 12 | SnO2 | E | [99] | ||
| 4 | SnO2 thin film doped with Au, Pt | E | [101] | ||
| 5 | SnO2 thin film doped with Au, Pt, Pd | E | [102] | ||
| 6 | WO3, SnO2 | C | [103] | ||
| Other food | Shelf life of nuts | 10 | SnO2 | C | [105] |
| Shelf life of vegetables | 5* | n.d. | E | [104] | |
| Bacterial species classification | 6 | SnO2, CTO, WO3 | C | [107] |
| Compound | E-nose sensor type | Linear regression: a×conc. + b | Detection limit (μg L−1) | ||
|---|---|---|---|---|---|
| a | b | r2 | |||
| 4-Ethylphenol | SY/G | 6.80E-3 | −1.02 | 0.99 | 101.2 |
| SY/Gh | 6.05E-3 | −1.13 | 0.99 | 138.4 | |
| SY/gCT | 6.08E-3 | −1.34 | 0.99 | 43.8 | |
| 4-Ethylguaiacol | SY/Gh | 6.75E-4 | −0.59 | 0.81 | 93.5 |
| SY/gCT | 6.60E-4 | −0.37 | 0.78 | 91.1 | |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Berna, A. Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis. Sensors 2010, 10, 3882-3910. https://doi.org/10.3390/s100403882
Berna A. Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis. Sensors. 2010; 10(4):3882-3910. https://doi.org/10.3390/s100403882
Chicago/Turabian StyleBerna, Amalia. 2010. "Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis" Sensors 10, no. 4: 3882-3910. https://doi.org/10.3390/s100403882
APA StyleBerna, A. (2010). Metal Oxide Sensors for Electronic Noses and Their Application to Food Analysis. Sensors, 10(4), 3882-3910. https://doi.org/10.3390/s100403882




