Ultra-High Sensitivity Zinc Oxide Nanocombs for On-Chip Room Temperature Carbon Monoxide Sensing
Abstract
:1. Introduction
2. Sensing Mechanism
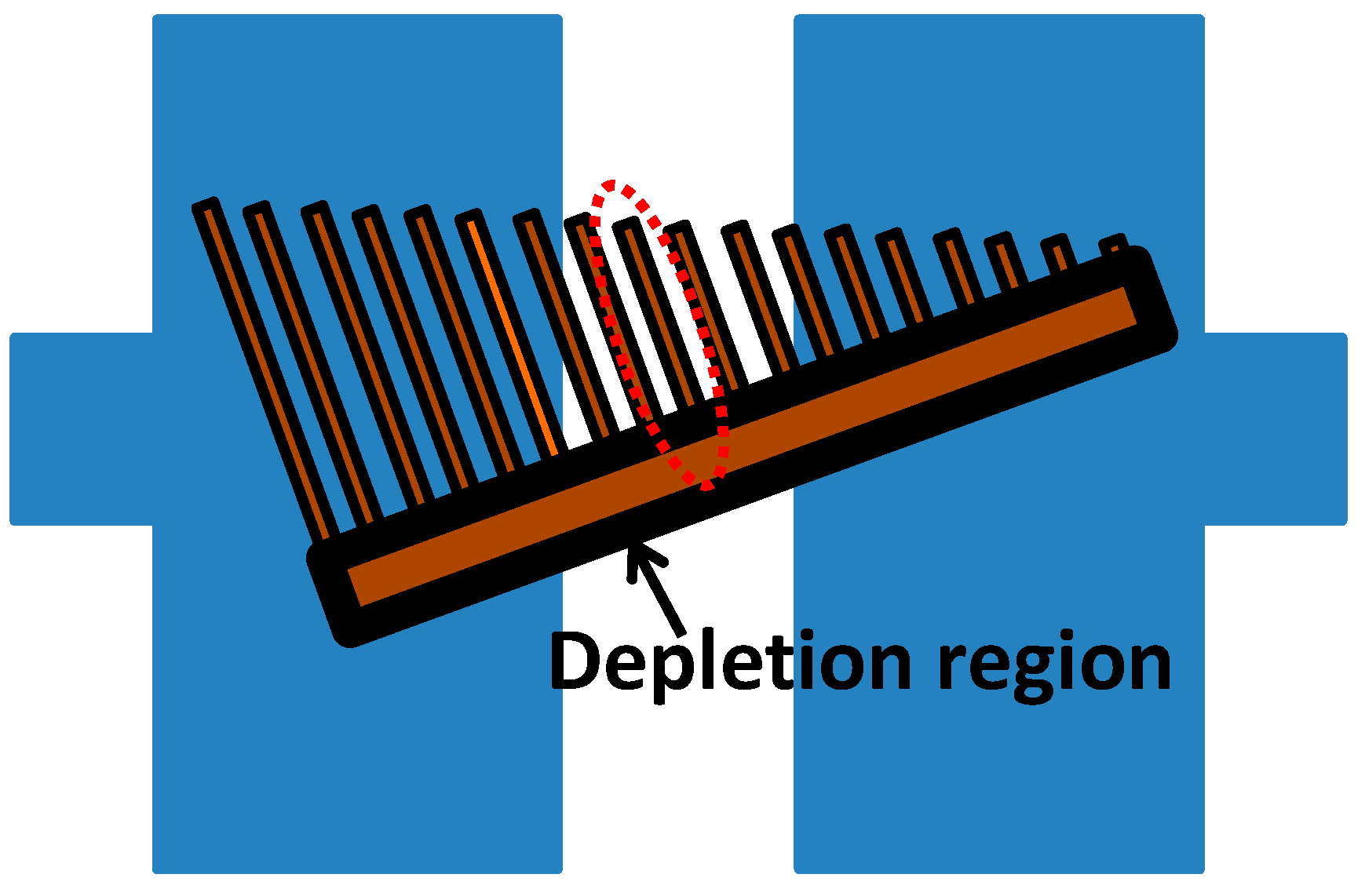

3. Device Fabrication
- An Au layer with 2 nm thickness is evaporated on top of a clean silicon substrate as the catalyst of the following CVD process.
- The evaporated Au catalyst is annealed at 700 °C for 30 min to form a layer of Au nanopaticles.
- Half a gram of Zn powder (99.9%, from Sigma-Aldrich Inc.) is placed at the bottom of the vial as the source material; while the previously prepared silicon substrate with Au catalyst is mounted at the bunghole of the vial (Figure 3).
- The furnace temperature is then increased to 700 °C. As a result, the Zn powder is vaporized.
- A mixture of oxygen gas and argon gas (with a ratio of 1:49) is continuously blown into the furnace for 20 min under a pressure of 2.4 × 10−3 Torr.
- The furnace temperature is cooled-down to room temperature. The silicon substrate appears white due to the densely deposited ZnO nanocombs.
- The silicon substrate is immerged into isopropyl alcohol (IPA) solution to exfoliate the deposited ZnO nanocombs. In order to facilitate the exfoliation, it is always accompanied by light sonication.
- The IPA solution with diluted ZnO nanocombs is dripped on a prefabricated SiO2/p-Si substrate (1 μm SiO2) with patterned Ti/Au electrode array on top, which is composed of a layer of Ti (10 nm) and a layer of Au (90 nm). Lift-off technique is utilized to pattern this dual layer electrode array with a 2 μm gap formed between each electrode pair.
- Finally, the SiO2/p-Si substrate is annealed at 300 °C for 30 min to form ohmic contacts between the nanocomb’ two ends and the metal electrodes.

4. Experimental Results and Discussions




| Ref. | Materials | CO Concentration (ppm) | Operating Temperature (°C) | Peak Sensitivity | Response Time (s) | Recovery Time (s) | Additional Heating Component |
|---|---|---|---|---|---|---|---|
| [24] | Cu-doped ZnO thin film | 250 | 300 | 4 | 50 | 100 | Needed |
| [25] | Mesoporous In2O3 nanofiber | 100 | 300 | 5.3 | 600 | 600 | Needed |
| [26] | ZnO | 500 | 200 | 2.8 | 600 | 120~180 | Needed |
| [27] | Zinc ferrite particles | 1660 | 350 | 2.22 | Not reported | Not reported | Needed |
| [28] | SnO2 thin film sensor | 260 | 350 | 1.5 | 28 | 94 | Needed |
| [29] | Co-doped ZnO Nanorods | 300 | 350 | 5.5 | ~60 | ~60 | Needed |
| This work | ZnO nanocomb | 250 | 25(RT) | 7.22 | 200 | 50 | No need |
| 500 | 8.93 | 400 | 55 |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eranna, G.; Joshi, B.; Runthala, D.; Gupta, R. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Gardner, J.W.; Guha, P.K.; Udrea, F.; Covington, J.A. CMOS interfacing for integrated gas sensors: A review. IEEE Sens. J. 2010, 10, 1833–1848. [Google Scholar] [CrossRef]
- Barbria, N.E.; Llobetb, E.; Baric, N.E.; Correigb, X.; Bouchikhia, B. Application of a portable electronic nose system to assess the freshness of Moroccan sardines. Mater. Sci. Eng. 2008, 28, 666–670. [Google Scholar] [CrossRef]
- Penza, M.; Aversa, P.; Cassano, G.; Suriano, D.; Wlodarski, W.; Benetti, M.; Cannatà, D.; di Pietrantonio, F.; Verona, E. Thin-film bulk-acoustic-resonator gas sensor functionalized with a nanocomposite Langmuir–Blodgett layer of carbon nanotubes. IEEE Trans. Electron. Devices 2008, 55, 1237–1243. [Google Scholar] [CrossRef]
- Lavrik, N.V.; Sepaniak, M.J.; Datskos, P.G. Cantilever transducers as a platform for chemical and biological sensors. Rev. Sci. Instrum. 2004, 75, 2229–2253. [Google Scholar] [CrossRef]
- Lu, C.J.; Steinecker, W.H.; Tian, W.C.; Oborny, M.C.; Nichols, J.M.; Agah, M.; Potkay, J.A.; Chan, H.K.L.; Driscoll, J.; Sacks, R.D.; et al. First-generation hybrid MEMS gas chromatograph. Lab Chip 2005, 5, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Spannhake, J.; Schulz, O.; Helwig, A.; Krenkow, A.; Müller, G.; Doll, T. High-temperature MEMS heater platforms: Long-term performance of metal and semiconductor heater materials. Sensors 2006, 6, 405–419. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef]
- Guo, B.; Bermak, A.; Chan, P.C.H.; Yan, G. An integrated surface micromachined convex microhotplate structure for tin oxide gas sensor array. IEEE Sens. J. 2007, 7, 1720–1726. [Google Scholar] [CrossRef]
- Guo, B.; Bermak, A.; Chan, P.C.H.; Yan, G. A monolithically integrated 4 × 4 Tin Oxide gas sensor array with on-chip multiplexing and differential readout circuits. Solid-State Electron. 2007, 51, 69–76. [Google Scholar] [CrossRef]
- Ahn, M.W.; Park, K.S.; Heo, J.H.; Kim, D.W.; Choi, K.; Park, J.G. On-chip fabrication of ZnO-nanowire gas sensor with high gas sensitivity. Sens. Actuators B Chem. 2009, 138, 168–173. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Ponzoni, A.; Comini, E.; Sberveglieri, G.; Zhou, J.; Deng, S.Z.; Xu, N.S.; Ding, Y.; Wang, Z.L. Ultrasensitive and highly selective gas sensors using three-dimensional tungsten oxide nanowire networks. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Hwang, S.H.; Kim, S.; Park, H. Preparation of ZnO nanorods by microemulsion synthesis and their application as a CO gas sensor. Sens. Actuators B Chem. 2011, 160, 94–98. [Google Scholar] [CrossRef]
- Tian, W.; Zhai, T.; Zhang, C.; Li, S.L.; Wang, X.; Liu, F.; Liu, D.; Cai, X.; Tsukagoshi, K.; Golberg, D.; et al. Low-cost fully transparent ultraviolet photodetectors based on electrospun ZnO-SnO2 heterojunction nanofi bers. Adv. Mater. 2013, 25, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Cao, X.C.; Sun, J.; Yuan, B.; Li, Q.H.; Zhu, Z.; Zhang, Y. A vacuum pressure sensor based on ZnO nanobelt film. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Changa, S.P.; Changa, S.J.; Lua, C.Y.; Lib, M.J.; Hsub, C.L.; Chiouc, Y.Z.; Hsuehd, T.J.; Chene, I.C. A ZnO nanowire-based humidity sensor. Superlattices Microstruct. 2010, 47, 772–778. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Ikohura, K.; Watson, J. The Stannic Oxide Gas Sensor: Principles and Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Fan, Z.; Lu, J.G. Chemical sensing with ZnO nanowire field-effect transistor. IEEE Trans. Nanotechnol. 2006, 5, 393–396. [Google Scholar] [CrossRef]
- Morrison, S. The Chemical Physics of Surfaces, 1st ed.; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- Chang, P.C.; Fan, Z.; Wang, D.; Tseng, W.Y.; Chiou, W.A.; Hong, J.; Lu, J.G. ZnO nanowires synthesized by vapor trapping CVD method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Gonga, H.; Hua, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2006, 115, 247–251. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.H.; Chang, D.; Kim, S. Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens. Actuators B Chem. 2010, 149, 28–33. [Google Scholar] [CrossRef]
- Barrecaa, D.; Bekermannb, D.; Cominic, E.; Devib, A.; Fischerb, R.A.; Gasparottod, A.; Maccatod, C.; Sberveglieric, G.; Tondellod, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Mukherjee, K.; Majumder, S.B. Synthesis process induced improvement on the gas sensing characteristics of nano-crystalline magnesium zinc ferrite particles. Sens. Actuators B Chem. 2012, 162, 229–236. [Google Scholar] [CrossRef]
- Bruneta, E.; Maiera, T.; Mutinatia, G.C.; Steinhauera, S.; Köcka, A.; Gspanb, C.; Groggerb, W. Comparison of the gas sensing performance of SnO2 thin film and SnO2 nanowire sensors. Sens. Actuators B Chem. 2012, 165, 110–118. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, K.M.; Wang, C.Y.; Kuob, C.I.; Chen, L.J. Low-temperature electrodeposited Co-doped ZnO nanorods with enhanced ethanol and CO sensing properties. Sens. Actuators B Chem. 2012, 161, 734–739. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Zhao, X. Ultra-High Sensitivity Zinc Oxide Nanocombs for On-Chip Room Temperature Carbon Monoxide Sensing. Sensors 2015, 15, 8919-8930. https://doi.org/10.3390/s150408919
Pan X, Zhao X. Ultra-High Sensitivity Zinc Oxide Nanocombs for On-Chip Room Temperature Carbon Monoxide Sensing. Sensors. 2015; 15(4):8919-8930. https://doi.org/10.3390/s150408919
Chicago/Turabian StylePan, Xiaofang, and Xiaojin Zhao. 2015. "Ultra-High Sensitivity Zinc Oxide Nanocombs for On-Chip Room Temperature Carbon Monoxide Sensing" Sensors 15, no. 4: 8919-8930. https://doi.org/10.3390/s150408919
APA StylePan, X., & Zhao, X. (2015). Ultra-High Sensitivity Zinc Oxide Nanocombs for On-Chip Room Temperature Carbon Monoxide Sensing. Sensors, 15(4), 8919-8930. https://doi.org/10.3390/s150408919






