Elimination of Flammable Gas Effects in Cerium Oxide Semiconductor-Type Resistive Oxygen Sensors for Monitoring Low Oxygen Concentrations
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of CeZr10 Nanoparticles
2.2. Preparation of Thick Film Sensors
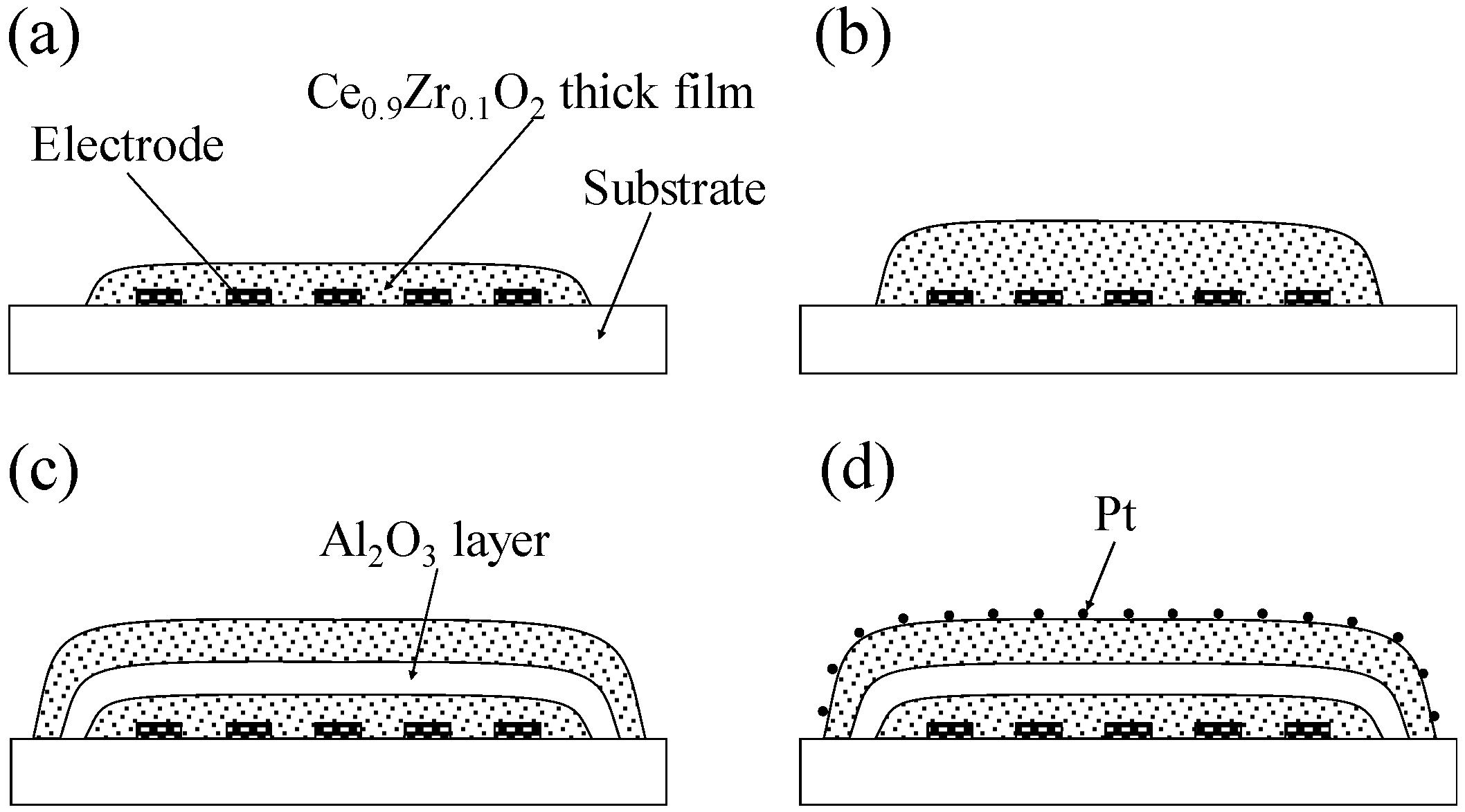
2.3. Analysis of the Thick Films
2.4. Preparation of Sensor Modules

2.5. Sensor Response Measurements
2.6. Conversion of the Resistance Measurements to Oxygen Concentration Values
3. Results and Discussion
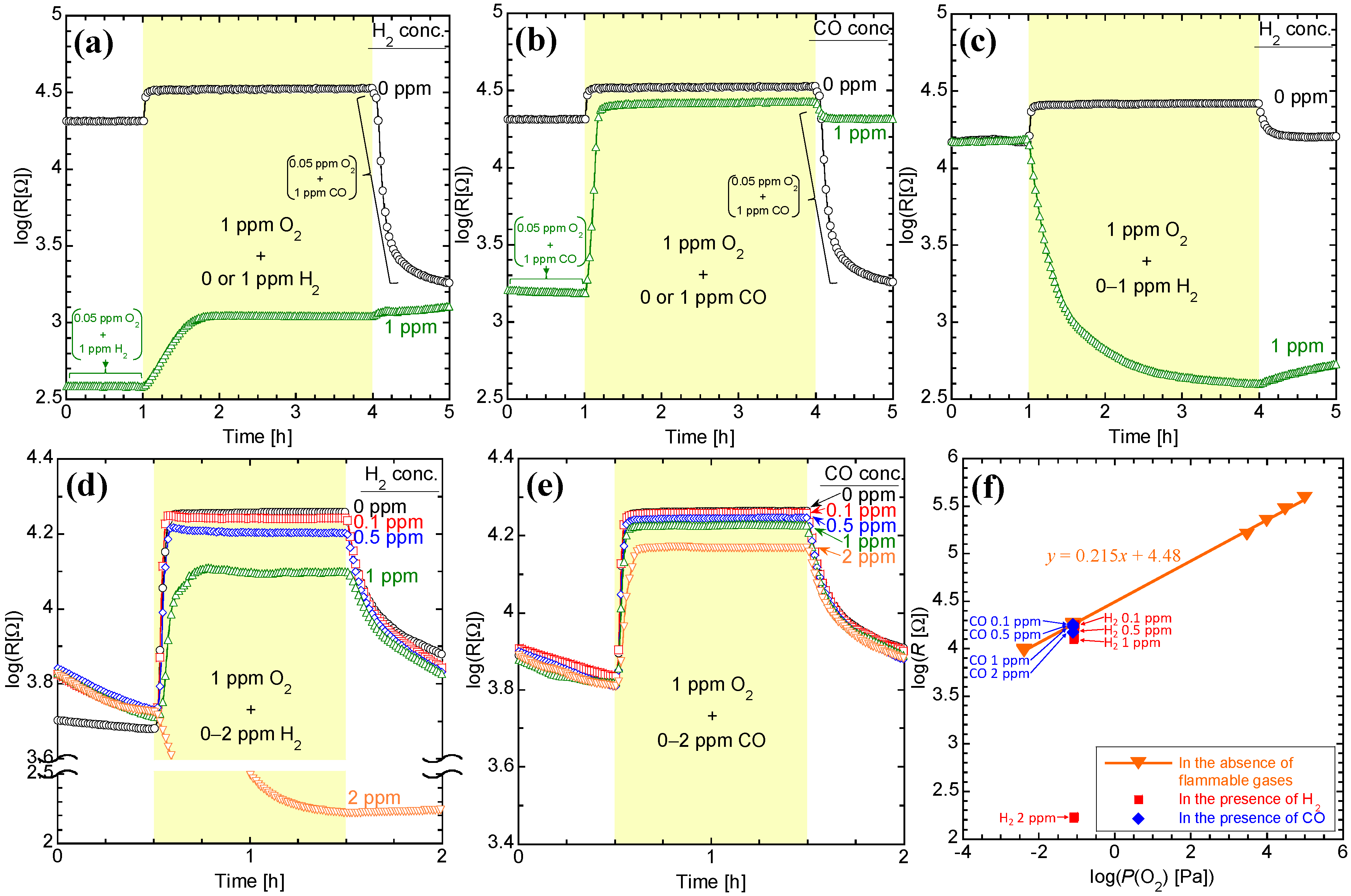
3.1. Effects of an Alumina Thick Film as an Insulator



3.2. Sensing Properties in Low Oxygen Concentrations
| Flammable Gas | CeZr10 | CeZr10/Al2O3/CeZr10 | CeZr10/Al2O3/Pt-CeZr10 | ||||
|---|---|---|---|---|---|---|---|
| Conc. (ppm) | log(R (Ω)) 1) | Conv. O2 Conc. (ppm) 2) | log(R (Ω)) 1) | Conv. O2 Conc. (ppm) 2) | log(R (Ω)) 1) | Conv. O2 Conc. (ppm) 2) | |
| - | 0 | 4.55 | 0.61 | 4.42 | 0.66 | 4.26 | 0.88 |
| H2 | 0.1 | - | - | - | - | 4.25 | 0.85 |
| 0.5 | - | - | - | - | 4.22 | 0.57 | |
| 1 | 3.04 | 5.7 × 10−10 | 2.60 | 5.8 × 10−11 | 4.11 | 0.19 | |
| 2 | - | - | - | - | 2.23 | 3.5 × 10−10 | |
| CO | 0.1 | - | - | - | - | 4.26 | 0.87 |
| 0.5 | - | - | - | - | 4.25 | 0.78 | |
| 1 | 4.43 | 0.11 | - | - | 4.23 | 0.64 | |
| 2 | - | - | - | - | 4.17 | 0.36 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ISO 2533:1975. In Standard Atmosphere; ISO: Geneva, Switzerland, 1975.
- Iio, A.; Ikeda, H.; Anggraini, S.A.; Miura, N. Potentiometric YSZ-based oxygen sensor using BaFeO3 sensing-electrode. Electrochem. Commun. 2014, 48, 134–137. [Google Scholar] [CrossRef]
- Mori, M.; Itagaki, Y.; Sadaoka, Y.; Nakagawa, S.; Kida, M.; Kojima, T. Detection of offensive odorant in air with a planar-type potentiometric gas sensor based on YSZ with Au and Pt electrodes. Sens. Actuators B Chem. 2014, 191, 351–355. [Google Scholar] [CrossRef]
- Dunst, K.; Jasinski, G.; Jasinski, P. Potentiometric oxygen sensor with solid state reference electrode. Metrol. Meas. Syst. 2014, 21, 205–216. [Google Scholar] [CrossRef]
- Sekharz, P.K.; Subramaniyam, K. Detection of Harmful Benzene, Toluene, Ethylbenzene, Xylenes (BTEX) Vapors Using Electrochemical Gas Sensors. ECS Electrochem. Lett. 2014, 3, B1–B4. [Google Scholar] [CrossRef]
- Fadeyev, G.; Kalakin, A.; Demin, A.; Volkov, A.; Brouzgou, A.; Tsiakaras, P. Electrodes for solid electrolyte sensors for the measurement of CO and H2 content in air. Int. J. Hydrog. Energy 2013, 38, 13484–13490. [Google Scholar] [CrossRef]
- Plashnitsa, V.V.; Elumalaia, P.; Fujio, Y.; Miura, N. Zirconia-based electrochemical gas sensors using nano-structured sensing materials aiming at detection of automotive exhausts. Electrochim. Acta 2009, 54, 6099–6106. [Google Scholar] [CrossRef]
- Miura, N.; Jin, H.; Wama, R.; Nakakubo, S.; Elumalai, P.; Plashnitsa, V.V. Novel solid-state manganese oxide-based reference electrode for YSZ-based oxygen sensors. Sens. Actuators B Chem. 2011, 152, 261–266. [Google Scholar] [CrossRef]
- Han, J.; Zhou, F.; Bao, J.; Wang, X.; Song, X. A high performance limiting current oxygen sensor with Ce0.8Sm0.2O1.9 electrolyte and La0.8Sr0.2Co0.8Fe0.2O3 diffusion barrier. Electrochim. Acta 2013, 108, 763–768. [Google Scholar] [CrossRef]
- Inaba, T.; Saji, K. Low temperature operation of thin-film limiting-current type oxygen sensor using graded-composition layer electrodes. Sens. Actuators B Chem. 2008, 129, 874–880. [Google Scholar] [CrossRef]
- López-Gándara, C.; Ramos, F.M.; Cirera, A.; Cornet, A. A model of the behavior of the limiting current oxygen sensors. Sens. Actuators B Chem. 2009, 140, 432–438. [Google Scholar] [CrossRef]
- Komachiya, M.; Suzuki, S.; Fujita, T.; Tsuruki, M.; Ohuchi, S.; Nakazawa, T. Limiting-current type air-fuel ratio sensor using porous zirconia layer without inner gas chambers: Proposal for a quick-startup sensor. Sens. Actuators B Chem. 2001, 73, 40–48. [Google Scholar] [CrossRef]
- Takeuchi, T.; Watanabe, S.; Hatano, Y.; Kuwano, M.; Eguchi, Y.; Yoshida, K.; Ishihara, T.; Takita, Y. Current-Voltage Characteristics of Various Metal Electrodes in Limiting-Current-Type Zirconia Cells. J. Electrochem. Soc. 2001, 148, H132–H138. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.; Härdtl, K.H.; Menesklou, W.; Riegel, J. Principles of solid state oxygen sensors for lean combustion gas control. Electrochim. Acta 2001, 47, 807–814. [Google Scholar] [CrossRef]
- Ogino, H.; Asakura, K. Development of a highly sensitive galvanic cell oxygen sensor. Talanta 1995, 42, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Nei, L.; Compton, R.G. An improved Clark-type galvanic sensor for dissolved oxygen. Sens. Actuators B Chem. 1996, 30, 83–87. [Google Scholar] [CrossRef]
- Ito, K. Yellow Phosphorus Luminous Type Trace Oxygen Analyzer “TOA-IIS”. Nippon Sanso Gihou 1993, 12, 61. (In Japanese) [Google Scholar]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Development of Resistive Oxygen Sensors Based on Cerium Oxide Thick Film. J. Electroceram. 2004, 13, 703–706. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Matsubara, I.; Murayama, N. Evaluation of response characteristics of resistive oxygen sensors based on porous cerium oxide thick film using pressure modulation method. Sens. Actuators B Chem. 2006, 113, 207–213. [Google Scholar] [CrossRef]
- Izu, N.; Oh-hori, N.; Itou, M.; Shin, W.; Matsubara, I.; Murayama, N. Resistive oxygen gas sensors based on Ce1−xZrxO2 nano powder prepared using new precipitation method. Sens. Actuators B Chem. 2005, 108, 238–243. [Google Scholar] [CrossRef]
- Itoh, T.; Uchida, T.; Matsubara, I.; Izu, N.; Shin, W.; Miyazaki, H.; Tanjo, H.; Kanda, K. Preparation of γ-alumina large grain particles with large specific surface area via polyol synthesis. Ceram. Int. 2007, 41, 3631–3638. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itoh, T.; Izu, N.; Akamatsu, T.; Shin, W.; Miki, Y.; Hirose, Y. Elimination of Flammable Gas Effects in Cerium Oxide Semiconductor-Type Resistive Oxygen Sensors for Monitoring Low Oxygen Concentrations. Sensors 2015, 15, 9427-9437. https://doi.org/10.3390/s150409427
Itoh T, Izu N, Akamatsu T, Shin W, Miki Y, Hirose Y. Elimination of Flammable Gas Effects in Cerium Oxide Semiconductor-Type Resistive Oxygen Sensors for Monitoring Low Oxygen Concentrations. Sensors. 2015; 15(4):9427-9437. https://doi.org/10.3390/s150409427
Chicago/Turabian StyleItoh, Toshio, Noriya Izu, Takafumi Akamatsu, Woosuck Shin, Yusuke Miki, and Yasuo Hirose. 2015. "Elimination of Flammable Gas Effects in Cerium Oxide Semiconductor-Type Resistive Oxygen Sensors for Monitoring Low Oxygen Concentrations" Sensors 15, no. 4: 9427-9437. https://doi.org/10.3390/s150409427
APA StyleItoh, T., Izu, N., Akamatsu, T., Shin, W., Miki, Y., & Hirose, Y. (2015). Elimination of Flammable Gas Effects in Cerium Oxide Semiconductor-Type Resistive Oxygen Sensors for Monitoring Low Oxygen Concentrations. Sensors, 15(4), 9427-9437. https://doi.org/10.3390/s150409427






