Enhanced Sensitive Love Wave Surface Acoustic Wave Sensor Designed for Immunoassay Formats
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Apparatus


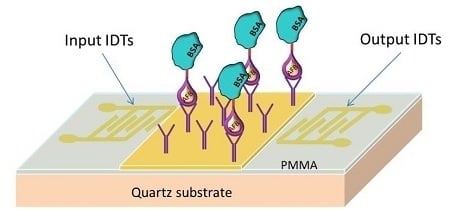
2.3. Configuration of the LW-SAW Sensor
2.3.1. Waveguide Mode and Deposition of the Sensitive Layers
2.3.2. Oscillating Circuit of the LW-SAW Device
2.3.3. Design of the LW-SAW Sensor Testing Cell

2.3.4. Preparation of Self-Assembled Monolayer (SAM) of 11-Mercaptoundecanoic Acid
2.4. Antibody Immobilization onto SAM Functionalized Surfaces
- -
- The activation of the terminal carboxylic groups of the linker via EDC/NHS esters was performed by three time injecting 100 μL of a 1:1 mixture of 0.4 M EDC and 0.1 M NHS into the cuvette for 15 min, thus avoiding long time incubations of EDC/NHS mixtures which could have led to the hydrolysis of the unstable NHS esters;
- -
- Anti-AFB1 antibody solutions were prepared in a 10 mM acetate buffer (pH = 4.5); the value of the working pH was chosen after performing several pre-concentration experiments at pH 3.5, 4.5, 5.5 and 6, in order to avoid repulsive interactions between the negatively charged carboxyl groups of the sensor’s functionalized surface and the negatively charged antibody.
- -
- Anti-AFB1 antibody was immobilized by injecting 50 μL of 1:1000 diluted stock solution (1 µg/mL) for 40 min in the SPR and LW-SAW assays;
- -
- The remaining active sites of the sensors were blocked by injection of 50 μL of 1 M ethanolamine (pH = 8.5) for 15 min, followed by rinsing with PBS 0.1 M, pH 7.5.
2.5. AFB1-BSA/Anti-AFB1 Antibody Binding Assays
- (1)
- 50 μL of AFB1-BSA in PBS solutions (pH 7.5), with concentrations ranging from 3 up to 100 nM for the SAW sensor and 6.5 nM up to 265 nM for the SPR sensor (in PBS, pH 7.5) were injected onto sensors surface. The solutions were kept in contact with the immobilized antibody for 40 min during the association phase in SPR assays and for 20 min in the LW-SAW assays, in order to reach the thermodynamic equilibrium. Then, the solutions were drained out and 50 μL PBS was injected for the dissociation phase. Since the SPR protocol uses a double channel system, in order to extract any potential contributions to the SPR signal resulting from the interaction between the protein part of the bioconjugate and the SAM modified surface, the antibody was immobilized in both sample and reference channels. During the interaction, a volume of 50 μL AFB1-BSA solution was injected in the sample cell, while 50 μL of BSA solution of the same concentration was passed through the reference cell. In these conditions, the increase of SPR signal after subtracting the reference signal from the sample signal can be assigned only to the binding of the AFB1 part of the bioconjugate to the antibody epitope.In the case of the LW-SAW sensor, separate assays with BSA were performed as blank experiments and the LW-SAW signal corresponding to BSA non-specific binding was extracted from the LW-SAW signal of AFB1-BSA. Each interaction experiment was followed by a dissociation step with 0.1 M PBS pH 7.5 and a regeneration step with 0.1 M HCl incubated for 10 min over the sensor surface.
- (2)
- The same ranges of AFB1-BSA concentrations as depicted above was kept for both SPR and SAW assays but, in order to avoid the time-consuming regeneration steps required after each interaction assay, we used a successive additions format. Thus, the dissociation and the regeneration sequences were carried out after the stabilization of the SPR/LW-SAW signal corresponding to the highest concentration of AFB1-BSA. All SPR/LW-SAW measurements were made at a constant temperature (25 °C).
3. Results and Discussion
3.1. Paired Interaction Assays with SPR and LW-SAW Sensors
3.1.1. Interaction Monitoring with the SPR Sensor
3.1.2. Optimization of the Regeneration Procedure during the SRP Assays
3.1.3. Interaction Monitoring with the LW-SAW Sensor


3.1.4. Optimization of the Gold Layer Thickness of the LW-SAW Sensor

3.1.5. Optimization of the Regeneration Procedure during the LW-SAW Assays
3.2. Calibration of the SPR and LW-SAW Sensors

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gronewold, T.M.A. Surface acoustic wave sensors in the bioanalytical field: Recent trends and challenges. Anal. Chim. Acta 2007, 603, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Newton, M.I.; McHale, G.; Melzak, K.A.; Gizeli, E. Pulse mode shear horizontal-surface acoustic wave (SH-SAW) system for liquid based sensing applications. Biosens. Bioelectron. 2004, 19, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Blondeau-Patissier, V.; Boireau, W.; Cavallier, B.; Lengaigne, G.; Daniau, W.; Martin, G.; Ballandras, S. Integrated Love Wave Device Dedicated to Biomolecular Interactions Measurements in Aqueous Media. Sensors 2007, 7, 1992–2003. [Google Scholar] [CrossRef]
- Deobagkar, D.D.; Limaye, V.; Sinha, S.; Yadava, R.D.S. Acoustic wave immunosensing of Escherichia coli in water. Sens. Actuators B Chem. 2005, 104, 85–89. [Google Scholar] [CrossRef]
- Länge, K.; Rapp, B.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Hodnik, V.; Anderluh, G. Toxin Detection by Surface Plasmon Resonance. Sensors 2009, 9, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Olaru, A.; Bala, C.; Jaffrezic-Renault, N.; Aboul-Enein, H.Y. Surface Plasmon Resonance (SPR) Biosensors in Pharmaceutical Analysis. Crit. Rev. Anal. Chem. 2014, 45, 97–105. [Google Scholar] [CrossRef]
- Shephard, G. Aflatoxin analysis at the beginning of the twenty-first century. Anal. Bioanal. Chem. 2009, 395, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, G.; Skipper, P.L.; Büchi, G.; Tannenbaum, S.R. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis 1987, 8, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Puiu, M.; Istrate, O.; Rotariu, L.; Bala, C. Kinetic approach of aflatoxin B1–acetylcholinesterase interaction: A tool for developing surface plasmon resonance biosensors. Anal. Biochem. 2012, 421, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Acharya, D.; Roy, D.; Shrestha, D.; Dhar, T.K. Simultaneous enzyme immunoassay for the screening of aflatoxin B1 and ochratoxin A in chili samples. Anal. Chim. Acta 2007, 584, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, D.; Xu, X.; Ying, Y.; Li, Y.; Ye, Z.; Wang, J. Immunosensors for detection of pesticide residues. Biosens. Bioelectron. 2008, 23, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Gaso, M.-I.; March-Iborra, C.; Montoya-Baides, Á.; Arnau-Vives, A. Surface Generated Acoustic Wave Biosensors for the Detection of Pathogens: A Review. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Fu, C.; Kim, K.; Lee, K. Wireless and Simultaneous Detections of Multiple Bio-Molecules in a Single Sensor Using Love Wave Biosensor. Sensors 2014, 14, 21660–21675. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, D.S., Jr.; Martin, S.J.; Ricco, A.J.; Frye, G.C.; Wohltjen, H.; White, R.M.; Zellers, E.T. Chapter 6—Practical Aspects of Acoustic-Wave Sensors. In Acoustic Wave Sensors; Martin, S.J., Ballantine, D.S., Jr., Ricco, A.J., Zellers, E.T., Frye., G.C., Wohltjen, H., Eds.; Academic Press: Burlington, MA, USA, 1997; pp. 331–395. [Google Scholar]
- O’Shannessy, D.J.; Brigham-Burke, M.; Peck, K. Immobilization chemistries suitable for use in the Biacore surface plasmon resonance detector. Anal. Biochem. 1992, 205, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.A.; Wang, S.; Allan, R.D.; Kennedy, I.R. A Rapid Aflatoxin B1 ELISA: Development and Validation with Reduced Matrix Effects for Peanuts, Corn, Pistachio, and Soybeans. J. Agric. Food Chem. 2004, 52, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puiu, M.; Gurban, A.-M.; Rotariu, L.; Brajnicov, S.; Viespe, C.; Bala, C. Enhanced Sensitive Love Wave Surface Acoustic Wave Sensor Designed for Immunoassay Formats. Sensors 2015, 15, 10511-10525. https://doi.org/10.3390/s150510511
Puiu M, Gurban A-M, Rotariu L, Brajnicov S, Viespe C, Bala C. Enhanced Sensitive Love Wave Surface Acoustic Wave Sensor Designed for Immunoassay Formats. Sensors. 2015; 15(5):10511-10525. https://doi.org/10.3390/s150510511
Chicago/Turabian StylePuiu, Mihaela, Ana-Maria Gurban, Lucian Rotariu, Simona Brajnicov, Cristian Viespe, and Camelia Bala. 2015. "Enhanced Sensitive Love Wave Surface Acoustic Wave Sensor Designed for Immunoassay Formats" Sensors 15, no. 5: 10511-10525. https://doi.org/10.3390/s150510511
APA StylePuiu, M., Gurban, A.-M., Rotariu, L., Brajnicov, S., Viespe, C., & Bala, C. (2015). Enhanced Sensitive Love Wave Surface Acoustic Wave Sensor Designed for Immunoassay Formats. Sensors, 15(5), 10511-10525. https://doi.org/10.3390/s150510511