Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches
Abstract
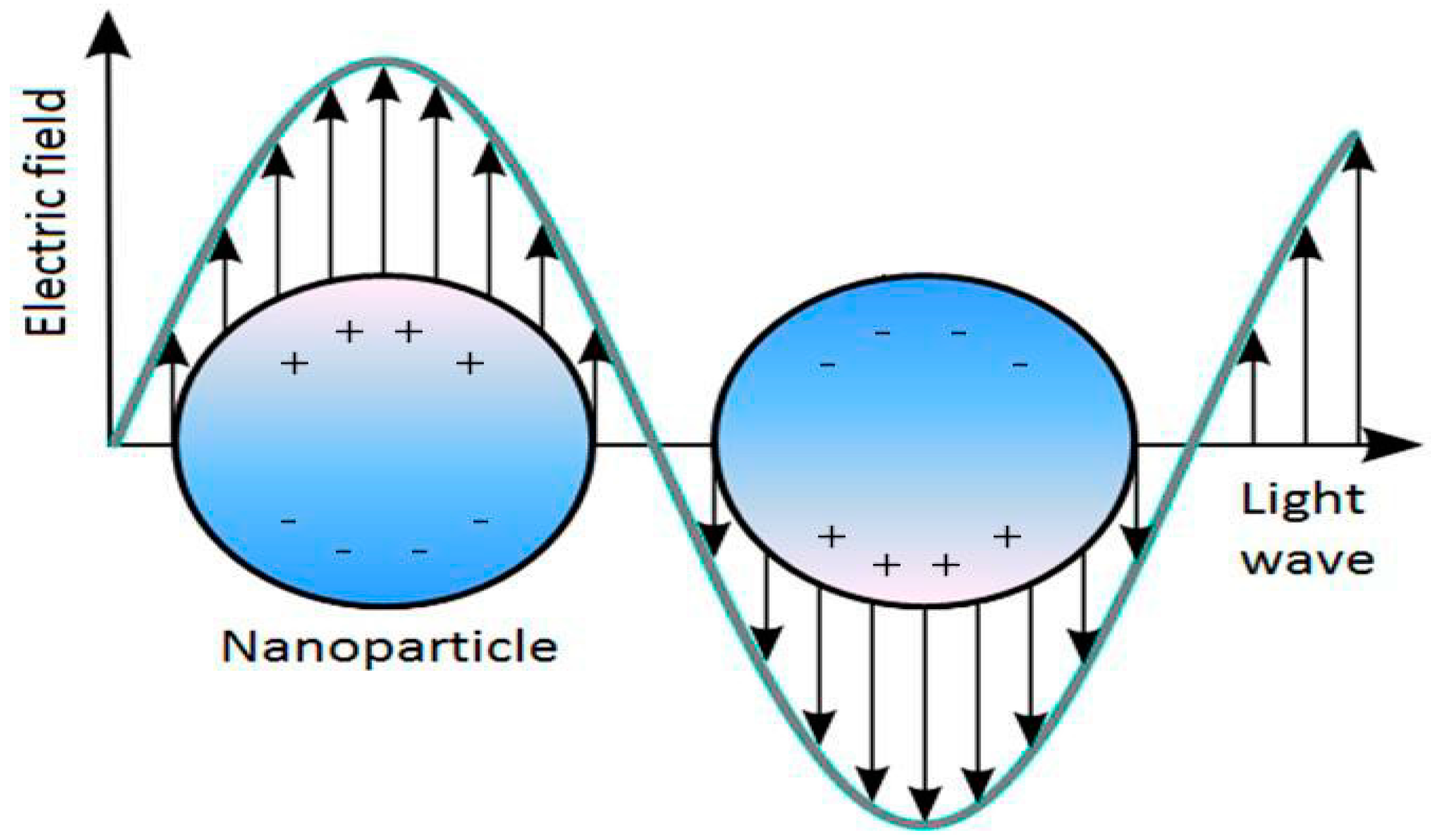
:1. Introduction
General Principles of Localized Surface Plasmon Resonance
2. The Challenge of Improving Limit of Detection
2.1. Enzymatic Amplification
2.2. Plasmonic Nanoparticle Coupling-Mediated Amplification
2.3. Biomolecular Conformationally-Gated Amplification

3. The Challenge of Improving Selectivity in Complex Solution
3.1. Improving Selectivity through Functionalization Layers
3.2. Improving Selectivity through Biological Scaffolds

3.3. Increasing Selectivity through Size-Selective Films or Shape Complementarity

4. The Challenge of Detecting of Membrane-Associated Species
4.1. Supported Lipid Membranes
4.2. LSPR Based Membrane Biosensors Using Supported Lipid Bilayers

| Sensor Substrate | Bulk Sensitivity (nm/RIU) | Coating Thickness (nm) | Reference |
|---|---|---|---|
| Au nanoholes | 113 | 20 | [120] |
| Ag nanoholes | 75 | 20 | [120] |
| Flat Au nanodisks | [4.5 nm/(nm of Al2O3)] 150 (approximate) | 10 | [122] |
| Ag nanocubes | 123 | 3.9 | [121] |
| Protruding Au nanodisks | 110 | 10 | [116] |
5. The Challenge of Incorporating LSPR Biosensing into Point-of-Care Diagnostic Devices
5.1. Plasmonic Point-of-Care Diagnostics


5.2. Multiplexed LSPR Platforms
5.2.1. Multiplexed Plasmonic Arrays

5.2.2. Multiplexed Single Nanoparticle LSPR Sensing
5.3. Microfluidic LSPR Biosensing Devices

6. Prospective and Conclusions
Acknowledgments
Conflicts of Interest
References
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absoprtion and Scattering Properties of Gold Nanoparticles of Different Size, Shape and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Schlucker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Adleman, J.R.; Boyd, D.A.; Goodwin, D.G.; Psaltis, D. Heterogenous Catalysis Mediated by Plasmon Heating. Nano Lett. 2009, 9, 4417–4423. [Google Scholar] [CrossRef]
- Nielsen, M.P.; Ashfar, A.; Cadien, K.; Elezzabi, A.Y. Plasmonic materials for metal-insulator-semiconductor-insulator-metal nanoplasmonic waveguides on silicon-on-insulator platform. Opt. Mater. 2013, 36, 294–298. [Google Scholar] [CrossRef]
- Sun, M.T.; Xu, H.X. A Novel Application of Plasmonics: Plasmon-Driven Surface-Catalyzed Reactions. Small 2012, 8, 2777–2786. [Google Scholar] [CrossRef]
- Kang, B.; Afifi, M.M.; Austin, L.A.; EI-Sayed, M.A. Exploiting the Nanoparticle Plasmon Effect: Observing Drug Delivery Dynamics in Single Cells via Raman/Fluorescence Imaging Spectroscopy. ACS Nano 2013, 7, 7420–7427. [Google Scholar] [CrossRef]
- Sagle, L.B.; Ruvuna, L.K.; Ruemmele, J.A.; van Duyne, R.P. Advances in localized surface plasmon resonance spectroscopy biosensing. Nanomedicine 2011, 6, 1447–1462. [Google Scholar] [CrossRef]
- Vilela, D.; Gonzalez, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Ameen, A.; Gartia, M.R.; Hsiao, A.; Chang, T.W.; Xu, Z.D.; Liu, G.L. Ultra-Sensitive Colorimetric Plasmonic Sensing and Microfluidics for Biofluid Diagnostics Using Nanohole Array. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Hammond, J.L.; Bhalla, N.; Rafiee, S.D.; Estrela, P. Localized Surface Plasmon Resonance as a Biosensing Platform for Developing Countries. Biosensors 2014, 4, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Myroshnychenko, V.; Rodriguez-Fernandez, J.; Pastoriza-Santos, I.; Funston, A.M.; Novo, C.; Mulvaney, P.; Liz-Marzan, L.M.; de Abajo, F.J.G. Modelling the optical response of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1792–1805. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtulus, O.; Lee, S.H.; Lindquist, N.C.; Oh, S.H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Wang, H.N.; Scaffidi, J. Plasmonic nanoprobes for SERS biosensing and bioimaging. J. Biophotonics 2010, 3, 89–102. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloider Metallösungen. Ann. Phys. 1908, 25, 377–445. [Google Scholar] [CrossRef]
- Yang, W.H.; Schatz, G.C.; Vanduyne, R.P. Discrete Dipole Approximation for Calculating Extinction and Raman Intensities for Small Particles with Arbitrary Shapes. J. Chem. Phys. 1995, 103, 869–875. [Google Scholar] [CrossRef]
- Haes, A.J.; van Duyne, R.P. A nanoscale optical blosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604. [Google Scholar] [CrossRef]
- Jung, L.S.; Campbell, C.T.; Chinowsky, T.M.; Mar, M.N.; Yee, S.S. Quantitative interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 1998, 14, 5636–5648. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Bingham, J.M.; Paige Hall, W.; van Duyne, R.P. Exploring the Unique Characteristics of LSPR Biosensing. In Nanoplasmonic Sensors; Dmitriev, A., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Haes, A.J.; Zou, S.L.; Schatz, G.C.; van Duyne, R.P. A nanoscale optical biosensor: The long range distance dependence of the localized surface plasmon resonance of noble metal nanoparticles. J. Phys. Chem. B 2004, 108, 109–116. [Google Scholar] [CrossRef]
- Miller, M.M.; Lazarides, A.A. Sensitivity of metal nanoparticle surface plasmon resonance to the dielectric environment. J. Phys. Chem. B 2005, 109, 21556–21565. [Google Scholar] [CrossRef]
- Miller, M.M.; Lazarides, A.A. Sensitivity of metal nanoparticle plasmon resonance band position to the dielectric environment as observed in scattering. J. Opt. A Pure Appl. Opt. 2006, 8, S239–S249. [Google Scholar] [CrossRef]
- Sepulveda, B.; Angelome, P.C.; Lechuga, L.M.; Liz-Marzan, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Haynes, C.L.; van Duyne, R.P. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Nordlander, P.; Oubre, C.; Prodan, E.; Li, K.; Stockman, M.I. Plasmon hybridizaton in nanoparticle dimers. Nano Lett. 2004, 4, 899–903. [Google Scholar] [CrossRef]
- Jain, P.K.; EI-Sayed, M.A. Plasmonic coupling in noble metal nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Dahlin, A.B. Size Matters: Problems and Advantages Associated with Highly Miniaturized Sensors. Sensors 2012, 12, 3018–3036. [Google Scholar] [CrossRef]
- Dahlin, A.B.; Wittenberg, N.J.; Hook, F.; Oh, S.H. Promises and challenges of nanoplasmonic devices for refractometric biosensing. Nanophotonics 2013, 2, 83–101. [Google Scholar] [CrossRef]
- El-Dessouky, R.; Georges, M.; Azzazy, H.M.E. Silver Nanostructures: Properties, Synthesis, and Biosensor Applications. In Functional Nanoparticles for Bioanalysis, Nanomedicine, and Bioelectronic Devices; American Chemical Society: Washington, DC, USA, 2012; Volume I. [Google Scholar]
- Kedem, O.; Vaskevich, A.; Rubinstein, I. Critical Issues in Localized Plasnnon Sensing. J. Phys. Chem. C 2014, 118, 8227–8244. [Google Scholar] [CrossRef]
- Tong, L.M.; Wei, H.; Zhang, S.P.; Xu, H.X. Recent Advances in Plasmonic Sensors. Sensors 2014, 14, 7959–7973. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; O’Neil, D.; EI-Sayed, M.A. Hollow and Solid Metallic Nanoparticles in Sensing and in Nanocatalysis. Chem. Mater. 2014, 26, 44–58. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.N.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, K.S.; Ahn, J.; Lee, J.J.; Kim, M.G.; Shin, Y.B. Highly Sensitive Biosensing Using Arrays of Plasmonic Au Nanodisks Realized by Nanoimprint Lithography. ACS Nano 2011, 5, 897–904. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, S.W.; Jung, J.A.; Ahn, J.; Kim, M.G.; Shin, Y.B. Signal Amplification by Enzymatic Reaction in an Immunosensor Based on Localized Surface Plasmon Resonance (LSPR). Sensors 2010, 10, 2045–2053. [Google Scholar] [CrossRef]
- Saa, L.; Coronado-Puchau, M.; Pavlov, V.; Liz-Marzan, L.M. Enzymatic etching of gold nanorods by horseradish peroxidase and application to blood glucose detection. Nanoscale 2014, 6, 7405–7409. [Google Scholar] [CrossRef]
- Xia, Y.S.; Ye, J.J.; Tan, K.H.; Wang, J.J.; Yang, G. Colorimetric Visualization of Glucose at the Submicromole Level in Serum by a Homogenous Silver Nanoprism-Glucose Oxidase System. Anal. Chem. 2013, 85, 6241–6247. [Google Scholar] [CrossRef]
- Yang, A.K.; Huntington, M.D.; Cardinal, M.F.; Masango, S.S.; van Duyne, R.P.; Odom, T.W. Hetero-oligomer Nanoparticle Arrays for Plasmon-Enhanced Hydrogen Sensing. ACS Nano 2014, 8, 7639–7647. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; de la Rica, R.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M.; Stevens, M.M. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat. Mater. 2012, 11, 604–607. [Google Scholar] [CrossRef]
- Sharpe, J.C.; Mitchell, J.S.; Lin, L.; Sedoglavich, H.; Blaikie, R.J. Gold nanohole array substrates as immunobiosensors. Anal. Chem. 2008, 80, 2244–2249. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Liang, H.Y.; Suri, S.; Ma, D.L.; Wu, N.Q. Plasmonic Nanorice Antenna on Triangle Nanoarray for Surface-Enhanced Raman Scattering Detection of Hepatitis B Virus DNA. Anal. Chem. 2013, 85, 2072–2078. [Google Scholar] [CrossRef]
- Hall, W.P.; Ngatia, S.N.; van Duyne, R.P. LSPR Biosensor Signal Enhancement Using Nanoparticle-Antibody Conjugates. J. Phys. Chem. C 2011, 115, 1410–1414. [Google Scholar] [CrossRef]
- Sudeep, P.K.; Joseph, S.T.S.; Thomas, K.G. Selective detection of cysteine and glutathione using gold nanorods. J. Am. Chem. Soc. 2005, 127, 6516–6517. [Google Scholar] [CrossRef]
- Wang, C.G.; Chen, Y.; Wang, T.T.; Ma, Z.F.; Su, Z.M. Biorecognition-driven self-assembly of gold nanorods: A rapid and sensitive approach toward antibody sensing. Chem. Mater. 2007, 19, 5809–5811. [Google Scholar] [CrossRef]
- Wang, L.B.; Zhu, Y.Y.; Xu, L.G.; Chen, W.; Kuang, H.; Liu, L.Q.; Agarwal, A.; Xu, C.L.; Kotov, N.A. Side-by-Side and End-to-End Gold Nanorod Assemblies for Environmental Toxin Sensing. Angew. Chem. Int. Ed. 2010, 49, 5472–5475. [Google Scholar] [CrossRef]
- Jana, D.; Matti, C.; He, J.; Sagle, L. Capping Agent-Free Gold Nanostars Show Greatly Increased Versatility and Sensitivity for Biosensing. Anal. Chem. 2015, 87, 3964–3972. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Storhoff, J.J.; Elghanian, R.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L. One-pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J. Am. Chem. Soc. 1998, 120, 1959–1964. [Google Scholar] [CrossRef]
- Bailey, R.C.; Nam, J.M.; Mirkin, C.A.; Hupp, J.T. Real-time multicolor DNA detection with chemoresponsive diffraction gratings and nanoparticle probes. J. Am. Chem. Soc. 2003, 125, 13541–13547. [Google Scholar] [CrossRef]
- Jin, R.C.; Wu, G.S.; Li, Z.; Mirkin, C.A.; Schatz, G.C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2003, 125, 1643–1654. [Google Scholar] [CrossRef]
- Xu, X.Y.; Han, M.S.; Mirkin, C.A. A gold-nanoparticle-based real-time colorimetric screening method for endonuclease activity and inhibition. Angew. Chem. Int. Ed. 2007, 46, 3468–3470. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Fan, Q.; Li, G.X. A new strategy for a DNA assay based on a target-triggered isothermal exponential degradation reaction. Chem. Commun. 2011, 47, 5262–5264. [Google Scholar] [CrossRef]
- Xie, X.J.; Xu, W.; Liu, X.G. Improving Colorimetric Assays through Protein Enzyme-Assisted Gold Nanoparticle Amplification. Acc. Chem. Res. 2012, 45, 1511–1520. [Google Scholar] [CrossRef]
- Guarise, C.; Pasquato, L.; de Filippis, V.; Scrimin, P. Gold nanoparticles-based protease assay. Proc. Natl. Acad. Sci. USA 2006, 103, 3978–3982. [Google Scholar] [CrossRef]
- Hall, W.P.; Anker, J.N.; Lin, Y.; Modica, J.; Mrksich, M.; van Duyne, R.P. A calcium-modulated plasmonic switch. J. Am. Chem. Soc. 2008, 130, 5836–5837. [Google Scholar] [CrossRef]
- Pandya, A.; Sutariya, P.G.; Menon, S.K. A non enzymatic glucose biosensor based on an ultrasensitive calix[4]arene functionalized boronic acid gold nanoprobe for sensing in human blood serum. Analyst 2013, 138, 2483–2490. [Google Scholar] [CrossRef]
- Xia, F.; Zuo, X.L.; Yang, R.Q.; Xiao, Y.; Kang, D.; Vallee-Belisle, A.; Gong, X.; Yuen, J.D.; Hsu, B.B.Y.; Heeger, A.J.; et al. Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc. Natl. Acad. Sci. USA 2010, 107, 10837–10841. [Google Scholar] [CrossRef]
- Zeng, S.W.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Rosman, C.; Prasad, J.; Neiser, A.; Henkel, A.; Edgar, J.; Sonnichsen, C. Multiplexed Plasmon Sensor for Rapid Label-Free Analyte Detection. Nano Lett. 2013, 13, 3243–3247. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Cao, X.N.; Wang, L.H.; Li, D.; Song, S.P.; Ye, B.C.; Fan, C.H. Adenosine detection by using gold nanoparticles and designed aptamer sequences. Analyst 2009, 134, 1355–1360. [Google Scholar] [CrossRef]
- Liu, J.C.; Bai, W.H.; Niu, S.C.; Zhu, C.; Yang, S.M.; Chen, A.L. Highly sensitive colorimetric detection of 17 beta-estradiol using split DNA aptamers immobilized on unmodified gold nanoparticles. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Xu, H.; Mao, X.; Zeng, Q.X.; Wang, S.F.; Kawde, A.N.; Liu, G.D. Aptamer-Functionalized Gold Nanoparticles as Probes in a Dry-Reagent Strip Biosensor for Protein Analysis. Anal. Chem. 2009, 81, 669–675. [Google Scholar] [CrossRef]
- Liu, G.D.; Mao, X.; Phillips, J.A.; Xu, H.; Tan, W.H.; Zeng, L.W. Aptamer-Nanoparticle Strip Biosensor for Sensitive Detection of Cancer Cells. Anal. Chem. 2009, 81, 10013–10018. [Google Scholar] [CrossRef]
- Lu, W.T.; Arumugam, R.; Senapati, D.; Singh, A.K.; Arbneshi, T.; Khan, S.A.; Yu, H.T.; Ray, P.C. Multifunctional Oval-Shaped Gold-Nanoparticle-Based Selective Detection of Breast Cancer Cells Using Simple Colorimetric and Highly Sensitive Two-Photon Scattering Assay. ACS Nano 2010, 4, 1739–1749. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.H.; Kim, I.A.; Lee, S.J.; Jurng, J.; Gu, M.B. A novel colorimetric aptasensor using gold nanoparticle for a highly sensitive and specific detection of oxytetracycline. Biosens. Bioelectron. 2010, 26, 1644–1649. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.H.; Pan, D.; Song, S.P.; Boey, F.Y.C.; Zhang, H.; Fan, C.H. Visual cocaine detection with gold nanoparticles and rationally engineered aptamer structures. Small 2008, 4, 1196–1200. [Google Scholar] [CrossRef]
- Smith, J.E.; Griffin, D.K.; Leny, J.K.; Hagen, J.A.; Chavez, J.L.; Kelley-Loughnane, N. Colorimetric detection with aptamer-gold nanoparticle conjugates coupled to an android-based color analysis application for use in the field. Talanta 2014, 121, 247–255. [Google Scholar] [CrossRef]
- Song, K.M.; Cho, M.; Jo, H.; Min, K.; Jeon, S.H.; Kim, T.; Han, M.S.; Ku, J.K.; Ban, C. Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal. Biochem. 2011, 415, 175–181. [Google Scholar] [CrossRef]
- Deng, B.; Lin, Y.W.; Wang, C.; Li, F.; Wang, Z.X.; Zhang, H.Q.; Li, X.F.; Le, X.C. Aptamer binding assays for proteins: The thrombin example-A review. Anal. Chim. Acta 2014, 837, 1–15. [Google Scholar] [CrossRef]
- Gooding, J.J.; Ciampi, S. The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 2011, 40, 2704–2718. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Mrksich, M.; Whitesides, G.M. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Biophys. Biomol. Struct. 1996, 25, 55–78. [Google Scholar] [CrossRef]
- Ostuni, E.; Yan, L.; Whitesides, G.M. The interaction of proteins and cells with self-assembled monolayers of alkanethiolates on gold and silver. Colloid Surf. B 1999, 15, 3–30. [Google Scholar] [CrossRef]
- Menz, B.; Knerr, R.; Gopferich, A.; Steinem, C. Impedance and QCM analysis of the protein resistance of self-assembled PEGylated alkanethiol layers on gold. Biomaterials 2005, 26, 4237–4243. [Google Scholar] [CrossRef]
- Elbert, L.; Hubbell, J.A. Surface Treatments of Polymers for Biocompatibility. Ann. Rev. Mater. Sci. 1996, 26, 365–394. [Google Scholar] [CrossRef]
- Li, J.T.; Caldwell, K.D.; Rapaport, N. Surface properties of pluronic-coated polymeric colloids. Langmuir 1994, 10, 4475–4482. [Google Scholar] [CrossRef]
- Deng, L.; Mrksich, M.; Whitesides, G.M. Self-assembled monolayers of alkanethiolates presenting tri(propylene sulfoxide) groups resist the adsorption of protein. J. Am. Chem. Soc. 1996, 118, 5136–5137. [Google Scholar] [CrossRef]
- Fyrner, T.; Lee, H.H.; Mangone, A.; Ekblad, T.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Conlan, S.L.; Mutton, R.; Clare, A.S.; et al. Saccharide-Functionalized Alkanethiols for Fouling-Resistant Self-Assembled Monolayers: Synthesis, Monolayer Properties, and Antifouling Behavior. Langmuir 2011, 27, 15034–15047. [Google Scholar] [CrossRef]
- Luk, Y.Y.; Kato, M.; Mrksich, M. Self-assembled monolayers of alkanethiolates presenting mannitol groups are inert to protein adsorption and cell attachment. Langmuir 2000, 16, 9604–9608. [Google Scholar] [CrossRef]
- Siegers, C.; Biesalski, M.; Haag, R. Self-assembled monolayers of dendritic polyglycerol derivatives on gold that resist the adsorption of proteins. Chem. Eur. J. 2004, 10, 2831–2838. [Google Scholar] [CrossRef]
- Wyszogrodzka, M.; Haag, R. Synthesis and Characterization of Glycerol Dendrons, Self-Assembled Monolayers on Gold: A Detailed Study of Their Protein Resistance. Biomacromolecules 2009, 10, 1043–1054. [Google Scholar] [CrossRef]
- Chen, S.F.; Cao, Z.Q.; Jiang, S.Y. Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials 2009, 30, 5892–5896. [Google Scholar] [CrossRef]
- Statz, A.R.; Meagher, R.J.; Barron, A.E.; Messersmith, P.B. New peptidomimetic polymers for antifouling surfaces. J. Am. Chem. Soc. 2005, 127, 7972–7973. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Phosphorylcholine-containing polymers for biomedical applications. Anal. Bioanal. Chem. 2005, 381, 534–546. [Google Scholar] [CrossRef]
- Chen, S.F.; Zheng, J.; Li, L.Y.; Jiang, S.Y. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: Insights into nonfouling properties of zwitterionic materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Chen, X.X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Reinhard, B.M.; Siu, M.; Agarwal, H.; Alivisatos, A.P.; Liphardt, J. Calibration of dynamic molecular rule based on plasmon coupling between gold nanoparticles. Nano Lett. 2005, 5, 2246–2252. [Google Scholar] [CrossRef]
- Sonnichsen, C.; Reinhard, B.M.; Liphardt, J.; Alivisatos, A.P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 2005, 23, 741–745. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Chen, Y.; Ginger, D.S. Plasmonic Nanoparticle Dimers for Optical Sensing of DNA in Complex Media. J. Am. Chem. Soc. 2010, 132, 9600–9601. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Durkee, H.; Traxler, B.; Ginger, D.S. Optical Detection of Protein in Complex Media with Plasmonic Nanoparticle Dimers. Small 2011, 7, 1993–1997. [Google Scholar] [CrossRef]
- Reinhard, B.M.; Sheikholeslami, S.; Mastroianni, A.; Alivisatos, A.P.; Liphardt, J. Use of plasmon coupling to reveal the dynamics of DNA bending and cleavage by single EcoRV restriction enzymes. Proc. Natl. Acad. Sci. USA 2007, 104, 2667–2672. [Google Scholar] [CrossRef]
- Jun, Y.W.; Sheikholeslami, S.; Hostetter, D.R.; Tajon, C.; Craik, C.S.; Alivisatos, A.P. Continuous imaging of plasmon rulers in live cells reveals early-stage caspase-3 activation at the single-molecule level. Proc. Natl. Acad. Sci. USA 2009, 106, 17735–17740. [Google Scholar] [CrossRef]
- Liu, N.; Hentschel, M.; Weiss, T.; Alivisatos, A.P.; Giessen, H. Three-Dimensional Plasmon Rulers. Science 2011, 332, 1407–1410. [Google Scholar] [CrossRef]
- Hong, C.; Lee, J.; Zheng, H.; Hong, S.S.; Lee, C. Porous silicon nanoparticles for cancer photothermotherapy. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef]
- Hong, S.; Lee, S.; Yi, J. Sensitive and molecular size-selective detection of proteins using a chip-based and heteroliganded gold nanoisland by localized surface plasmon resonance spectroscopy. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef]
- Kreno, L.E.; Hupp, J.T.; van Duyne, R.P. Metal-Organic Framework Thin Film for Enhanced Localized Surface Plasmon Resonance Gas Sensing. Anal. Chem. 2010, 82, 8042–8046. [Google Scholar] [CrossRef]
- Lu, G.; Farha, O.K.; Kreno, L.E.; Schoenecker, P.M.; Walton, K.S.; van Duyne, R.P.; Hupp, J.T. Fabrication of Metal-Organic Framework-Containing Silica-Colloidal Crystals for Vapor Sensing. Adv. Mater. 2011, 23, 4449–4452. [Google Scholar] [CrossRef]
- Lopez-Puente, V.; Abalde-Cela, S.; Angelome, P.C.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Plasmonic Mesoporous Composites as Molecular Sieves for SERS Detection. J. Phys. Chem. Lett. 2013, 4, 2715–2720. [Google Scholar] [CrossRef]
- Anderson, B.D.; Tracy, J.B. Nanoparticle conversion chemistry: Kirkendall effect, galvanic exchange, and anion exchange. Nanoscale 2014, 6, 12195–12216. [Google Scholar] [CrossRef]
- Fredriksson, H.; Alaverdyan, Y.; Dmitriev, A.; Langhammer, C.; Sutherland, D.S.; Zaech, M.; Kasemo, B. Hole-mask colloidal lithography. Adv. Mater. 2007, 19. [Google Scholar] [CrossRef]
- Jana, D.; Lehnoff, E.; Bruzas, I.; Robinson, J.; Lum, W.; Sagle, L. Tunable Au-Ag Nanobowl Arrays for Size-Selective Plasmonic Biosensing. Unpublished results.
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Jacoby, E.; Bouhelal, R.; Gerspacher, M.; Seuwen, K. The 7TM G-protein-coupled receptor target family. ChemMedChem 2006, 1, 760–782. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, X. Tools for GPCR drug discovery. Acta Pharmacol. Sin. 2012, 33, 372–384. [Google Scholar] [CrossRef]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Maynard, J.A.; Lindquist, L.C.; Sutherland, J.N.; Lesuffleur, A.; Warrington, A.E.; Rodriguez, M.; Oh, S. Surface plasmon resonance for high-thoughput ligand screening of membrane-bound proteins. Biotechnol. J. 2009, 4, 1542–1558. [Google Scholar] [CrossRef]
- Konradi, R.; Textor, M.; Reimhult, E. Using Complementary Acoustic and Optical Tehcniques for Quantitative Monitoring of Biomolecular Adsorption at Interfaces. Biosensors 2012, 2, 341–376. [Google Scholar] [CrossRef]
- Dahlin, A.B.; Jonsson, M.P.; Hook, F. Specific self-assembly of single lipid vesicles in nanoplasmonic apertures in gold. Adv. Mater. 2008, 20. [Google Scholar] [CrossRef]
- Kumar, K.; Dahlin, A.B.; Sannomiya, T.; Kaufmann, S.; Isa, L.; Reimhult, E. Embedded Plasmonic Nanomenhirs as Location-Specific Biosensors. Nano Lett. 2013, 13, 6122–6129. [Google Scholar] [CrossRef]
- Cremer, P.S.; Boxer, S.G. Formation and spreading of lipid bilayers on planar glass supports. J. Phys. Chem. B 1999, 103, 2554–2559. [Google Scholar] [CrossRef]
- Richter, R.P.; Brisson, A.R. Following the formation of supported lipid bilayers on mica: A study combining AFM, QCM-D, and ellipsometry. Biophys. J. 2005, 88, 3422–3433. [Google Scholar] [CrossRef]
- Khan, M.S.; Dosoky, N.S.; Williams, J.D. Engineering Lipid Bilayer Membranes for Protein Studies. Int. J. Mol. Sci. 2013, 14, 21561–21597. [Google Scholar] [CrossRef]
- Jackman, J.A.; Tabaei, S.R.; Zhao, Z.L.; Yorulmaz, S.; Cho, N.J. Self-Assembly Formation of Lipid Bilayer Coatings on Bare Aluminum Oxide: Overcoming the Force of Interfacial Water. ACS Appl. Mater. Interfaces 2015, 7, 959–968. [Google Scholar] [CrossRef]
- Zan, G.H.; Jackman, J.A.; Kim, S.O.; Cho, N.J. Controlling Lipid Membrane Architecture for Tunable Nanoplasmonic Biosensing. Small 2014, 10, 4828–4832. [Google Scholar] [CrossRef]
- Tanaka, M.; Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 2005, 437, 656–663. [Google Scholar] [CrossRef]
- Parikh, A.N.; Groves, J.T. Materials science of supported lipid membranes. MRS Bull. 2006, 31, 507–512. [Google Scholar] [CrossRef]
- Dahlin, A.; Zach, M.; Rindzevicius, T.; Kall, M.; Sutherland, D.S.; Hook, F. Localized surface plasmon resonance sensing of lipid-membrane-mediated biorecognition events. J. Am. Chem. Soc. 2005, 127, 5043–5048. [Google Scholar] [CrossRef]
- Jonsson, M.P.; Jonsson, P.; Dahlin, A.B.; Hook, F. Supported lipid bilayer formation and lipid-membrane-mediated biorecognition reactions studied with a new nanoplasmonic sensor template. Nano Lett. 2007, 7, 3462–3468. [Google Scholar] [CrossRef]
- Wu, H.J.; Henzie, J.; Lin, W.C.; Rhodes, C.; Li, Z.; Sartorel, E.; Thorner, J.; Yang, P.D.; Groves, J.T. Membrane-protein binding measured with solution-phase plasmonic nanocube sensors. Nat. Methods 2012, 9, 1189–1191. [Google Scholar] [CrossRef]
- Jose, J.; Jordan, L.R.; Johnson, T.W.; Lee, S.H.; Wittenberg, N.J.; Oh, S.H. Topographically Flat Substrates with Embedded Nanoplasmonic Devices for Biosensing. Adv. Funct. Mater. 2013, 23, 2812–2820. [Google Scholar] [CrossRef]
- Langhammer, C.; Larsson, E.M.; Kasemo, B.; Zoric, I. Indirect Nanoplasmonic Sensing: Ultrasensitive Experimental Platform for Nanomaterials Science and Optical Nanocalorimetry. Nano Lett. 2010, 10, 3529–3538. [Google Scholar] [CrossRef]
- Baciu, C.L.; Becker, J.; Janshoff, A.; Sonnichsen, C. Protein-membrane interaction probed by single plasmonic nanoparticles. Nano Lett. 2008, 8, 1724–1728. [Google Scholar] [CrossRef]
- Garrenton, L.S.; Young, S.L.; Thorner, J. Function of the MAPK scaffold protein, Ste5, requires a cryptic PH domain. Gene Dev. 2006, 20, 1946–1958. [Google Scholar] [CrossRef]
- Wittenberg, N.J.; Im, H.; Xu, X.H.; Wootla, B.; Watzlawik, J.; Warrington, A.E.; Rodriguez, M.; Oh, S.H. High-Affinity Binding of Remyelinating Natural Autoantibodies to Myelin-Mimicking Lipid Bilayers Revealed by Nanohole Surface Plasmon Resonance. Anal. Chem. 2012, 84, 6031–6039. [Google Scholar] [CrossRef]
- Im, H.; Wittenberg, N.J.; Lesuffleur, A.; Lindquist, N.C.; Oh, S.H. Membrane protein biosensing with plasmonic nanopore arrays and pore-spanning lipid membranes. Chem. Sci. 2010, 1, 688–696. [Google Scholar] [CrossRef]
- Im, H.; Sutherland, J.N.; Maynard, J.A.; Oh, S.H. Nanohole-Based Surface Plasmon Resonance Instruments with Improved Spectral Resolution Quantify a Broad Range of Antibody-Ligand Binding Kinetics. Anal. Chem. 2012, 84, 1941–1947. [Google Scholar] [CrossRef]
- Tokel, O.; Inci, F.; Demirci, U. Advances in Plasmonic Technologies for Point of Care Applications. Chem. Rev. 2014, 114, 5728–5752. [Google Scholar] [CrossRef]
- Haes, A.J.; Chang, L.; Klein, W.L.; van Duyne, R.P. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc. 2005, 127, 2264–2271. [Google Scholar] [CrossRef]
- Cecchin, D.; de la Rica, R.; Bain, R.E.S.; Finnis, M.W.; Stevens, M.M.; Battaglia, G. Plasmonic ELISA for the detection of gp120 at ultralow concentrations with the naked eye. Nanoscale 2014, 6, 9559–9562. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, C.Z. One-step conjugation chemistry of DNA with highly scattered silver nanoparticles for sandwich detection of DNA. Analyst 2012, 137, 3434–3436. [Google Scholar] [CrossRef]
- Wabuyele, M.B.; vo-Dinh, T. Detection of human immunodeficiency virus type 1 DNA sequence using plasmonics nanoprobes. Anal. Chem. 2005, 77, 7810–7815. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.C.; Oh, B.K.; Choi, J.W. Highly sensitive localized surface plasmon resonance immunosensor for label-free detection of HIV-1. Nanomedcine 2013, 9, 1018–1026. [Google Scholar] [CrossRef]
- Inci, F.; Tokel, O.; Wang, S.Q.; Gurkan, U.A.; Tasoglu, S.; Kuritzkes, D.R.; Demirci, U. Nanoplasmonic Quantitative Detection of Intact Viruses from Unprocessed Whole Blood. ACS Nano 2013, 7, 4733–4745. [Google Scholar] [CrossRef]
- Lee, J.; Ahmed, S.R.; Oh, S.; Kim, J.; Suzuki, T.; Parmar, K.; Park, S.S.; Lee, J.; Park, E.Y. A plasmon-assisted fluoro-immunoassay using gold nanoparticle-decorated carbon nanotubes for monitoring the influenza virus. Biosens. Bioelectron. 2015, 64, 311–317. [Google Scholar] [CrossRef]
- Chang, Y.F.; Wang, S.F.; Huang, J.C.; Su, L.C.; Yao, L.; Li, Y.C.; Wu, S.C.; Chen, Y.M.A.; Hsieh, J.P.; Chou, C.; et al. Detection of swine-origin influenza A (H1N1) viruses using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosens. Bioelectron. 2010, 26, 1068–1073. [Google Scholar] [CrossRef]
- Draz, M.S.; Fang, B.A.; Li, L.J.; Chen, Z.; Wang, Y.J.; Xu, Y.H.; Yang, J.; Killeen, K.; Chen, F.F. Hybrid Nanocluster Plasmonic Resonator for Immunological Detection of Hepatitis B Virus. ACS Nano 2012, 6, 7634–7643. [Google Scholar] [CrossRef]
- Zheng, S.; Kim, D.K.; Park, T.J.; Lee, S.J.; Lee, S.Y. Label-free optical diagnosis of hepatitis B virus with genetically engineered fusion proteins. Talanta 2010, 82, 803–809. [Google Scholar] [CrossRef]
- Tian, L.M.; Morrissey, J.J.; Kattumenu, R.; Gandra, N.; Kharasch, E.D.; Singamaneni, S. Bioplasmonic Paper as a Platform for Detection of Kidney Cancer Biomarkers. Anal. Chem. 2012, 84, 9928–9934. [Google Scholar] [CrossRef]
- Chen, S.; Svedendahl, M.; Kall, M.; Gunnarsson, L.; Dmitriev, A. Ultrahigh sensitivity made simple: Nanoplasmonic label-free biosensing with an extremely low limit-of-detection for bacterial and cancer diagnostics. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.H.; el-Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar] [CrossRef]
- Leonor Guariguata, T.N.; Beagley, J.; Linnekamp, U.; Jacqmain, O. (Eds.) I.D. Federation, about Diabetes; International Diabetes Federation. Available online: http://www.idf.org (accessed on 5 September 2014).
- Ngoepe, M.; Choonara, Y.E.; Tyagi, C.; Tomar, L.K.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K.; Pillay, V. Integration of Biosensors and Drug Delivery Technologies for Early Detection and Chronic Management of Illness. Sensors 2013, 13, 7680–7713. [Google Scholar] [CrossRef]
- Radhakumary, C.; Sreenivasan, K. Naked Eye Detection of Glucose in Urine Using Glucose Oxidase Immobilized Gold Nanoparticles. Anal. Chem. 2011, 83, 2829–2833. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Montelongo, Y.; Vasconcellos, F.D.; Martinez-Hurtado, J.L.; Neupane, S.; Butt, H.; Qasim, M.M.; Blyth, J.; Burling, K.; Carmody, J.B.; et al. Reusable, Robust, and Accurate Laser-Generated Photonic Nanosensor. Nano Lett. 2014, 14, 3587–3593. [Google Scholar] [CrossRef]
- Joshi, G.K.; Johnson, M.A.; Sardar, R. Novel pH-responsive nanoplasmonic sensor: Controlling polymer structural change to modulate localized surface plasmon resonance response. RSC Adv. 2014, 4, 15807–15815. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.Y.; Tan, P.L.; Zhou, J.; Huang, Y.; Nie, Z.; Yao, S.Z. A plasmonic blood glucose monitor based on enzymatic etching of gold nanorods. Chem. Commun. 2013, 49, 1856–1858. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Lin, Y.Q.; Zhu, N.N.; Ma, Y.R.; Mao, L.Q. Colorimetric Detection of Glucose in Rat Brain Using Gold Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 4800–4804. [Google Scholar] [CrossRef]
- Unser, S.; Campbell, I.; Jana, D.; Sagle, L. Direct glucose sensing in the physiological range through plasmonic nanoparticle formation. Analyst 2015, 140, 590–599. [Google Scholar] [CrossRef]
- Yu, C.; Irudayaraj, J. Quantitative evaluation of sensitivity and selectivity of multiplex nanoSPR biosensor assays. Biophys. J. 2007, 93, 3684–3692. [Google Scholar] [CrossRef]
- Endo, T.; Kerman, K.; Nagatani, N.; Hiepa, H.M.; Kim, D.-K.; Yonezawa, Y.; Nakano, K.; Tamiya, E. Multiple label-free detection of antigen-antibody reaction using localized surface plasmon resonance-based core-shell structured nanoparticle layer nanochip. Anal. Chem. 2006, 78, 6465–6475. [Google Scholar] [CrossRef]
- Jia, K.; Bijeon, J.L.; Adam, P.M.; Ionescu, R.E. Sensitive localized surface plasmon resonance multiplexing protocols. Anal. Chem. 2012, 84, 8020–8027. [Google Scholar] [CrossRef]
- Ruemmele, J.A.; Hall, W.P.; Ruvuna, L.K.; van Duyne, R.P. A localized surface plasmon resonance imaging instrument for multiplexed biosensing. Anal. Chem. 2013, 85, 4560–4566. [Google Scholar] [CrossRef]
- Cetin, A.E.; Coskun, A.F.; Galarreta, B.C.; Huang, M.; Herman, D.; Ozcan, A.; Altug, H. Handheld high-throughput plasmonic biosensor using computational on-chip imaging. Light Sci. Appl. 2014, 3. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; Alvarez-Puebla, R.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stephan, O.; Kociak, M.; Liz-Marzan, L.M.; de Abajo, F.J.G. Zeptomol Detection Through Controlled Ultrasensitive Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618. [Google Scholar] [CrossRef]
- Klar, T.; Perner, M.; Grosse, S.; von Plessen, G.; Spirkl, W.; Feldmann, J. Surface-plasmon resonances in single metallic nanoparticles. Phys. Rev. Lett. 1998, 80. [Google Scholar] [CrossRef]
- Taton, T.A.; Lu, G.; Mirkin, C.A. Two-color labeling of oligonucleotide arrays via size-selective scattering of nanoparticle probes. J. Am. Chem. Soc. 2001, 123, 5164–5165. [Google Scholar] [CrossRef]
- Hu, R.; Yong, K.-T.; Roy, I.; Ding, H.; He, S.; Prasad, P.N. Metallic nanostructures as localized plasmon resonance enhanced scattering probes for multiplex dark-field targeted imaging of cancer cells. J. Phys. Chem. C 2009, 113, 2676–2684. [Google Scholar] [CrossRef]
- Ahijado-Guzmán, R.; Prasad, J.; Rosman, C.; Henkel, A.; Tome, L.; Schneider, D.; Rivas, G.; Sönnichsen, C. Plasmonic Nanosensors for Simultaneous Quantification of Multiple Protein-Protein Binding Affinities. Nano Lett. 2014, 14, 5528–5532. [Google Scholar] [CrossRef]
- Acimovic, S.S.; Ortega, M.A.; Sanz, V.; Berthelot, J.; Garcia-Cordero, J.L.; Renger, J.; Maerkl, S.J.; Kreuzer, M.P.; Quidant, R. LSPR chip for parallel, rapid, and sensitive detection of cancer markers in serum. Nano Lett. 2014, 14, 2636–2641. [Google Scholar] [CrossRef]
- Lee, S.H.; Lindquist, N.C.; Wittenberg, N.J.; Jordan, L.R.; Oh, S.H. Real-time full-spectral imaging and affinity measurements from 50 microfluidic channels using nanohole surface plasmon resonance. Lab Chip 2012, 12, 3882–3890. [Google Scholar] [CrossRef]
- Chen, P.; Chung, M.T.; McHugh, W.; Nidetz, R.; Liu, Y.; Fu, J.; Cornell, T.T.; Shanley, T.P.; Kurabayashi, K. Multiplex Serum Cytokine Immunoassay Using Nanoplasmonic Biosensor Microarrays. ACS Nano 2015, 9, 4173–4181. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684-15716. https://doi.org/10.3390/s150715684
Unser S, Bruzas I, He J, Sagle L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors. 2015; 15(7):15684-15716. https://doi.org/10.3390/s150715684
Chicago/Turabian StyleUnser, Sarah, Ian Bruzas, Jie He, and Laura Sagle. 2015. "Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches" Sensors 15, no. 7: 15684-15716. https://doi.org/10.3390/s150715684
APA StyleUnser, S., Bruzas, I., He, J., & Sagle, L. (2015). Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors, 15(7), 15684-15716. https://doi.org/10.3390/s150715684





