Towards Low Energy Atrial Defibrillation
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
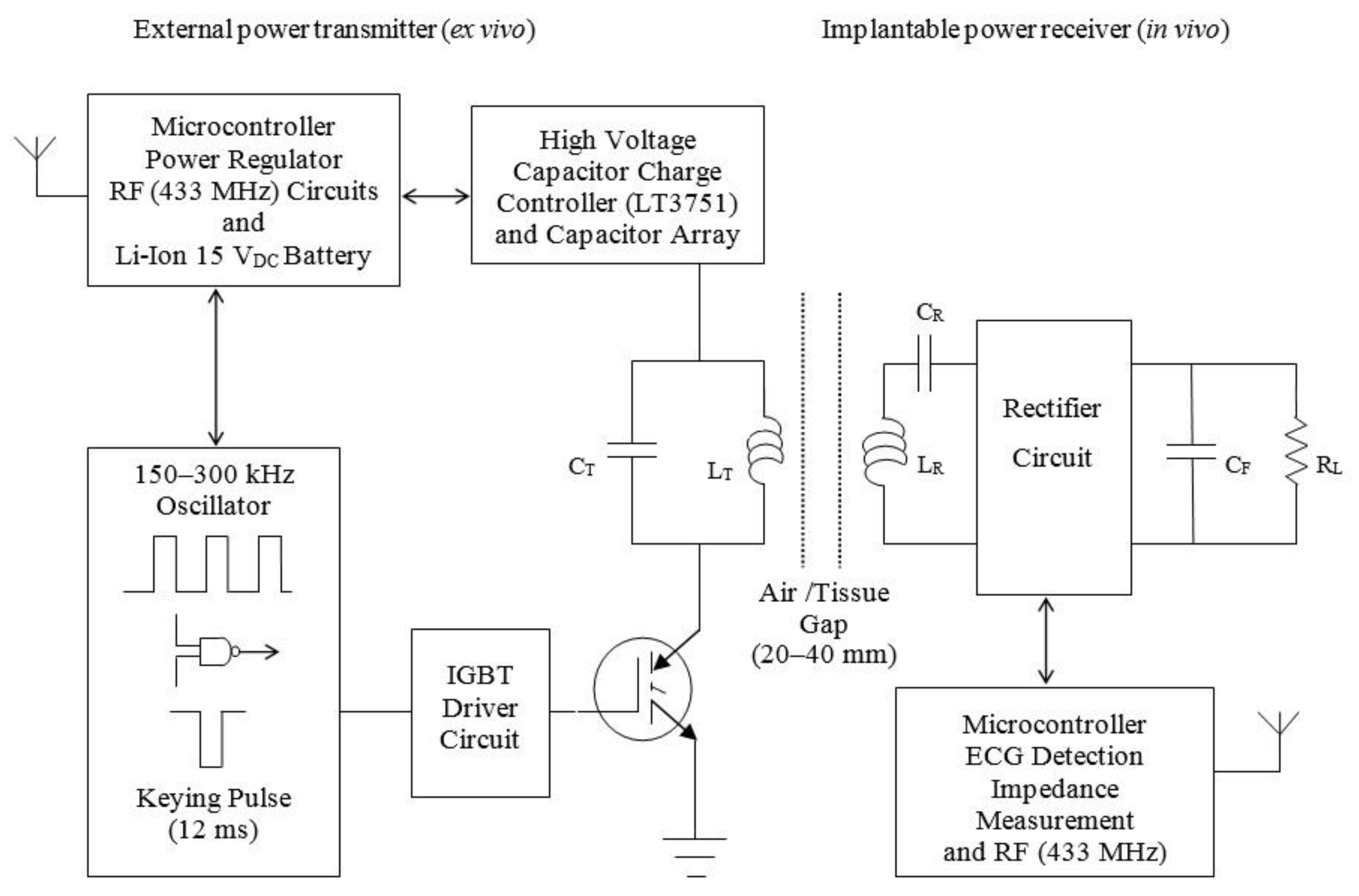

2.2. Simulation and Characterisation

2.3. Clinical Study

| Study Arm 1 Biphasic | Study Arm 2 Monophasic | |||
|---|---|---|---|---|
| Step | Voltage | Energy (J) | Voltage | Energy (J) |
| S1 | 50 | 0.38 | 50 | 0.60 |
| S2 | 100 | 1.50 | 100 | 2.40 |
| S3 | 150 | 3.38 | 150 | 5.40 |
| S4 | 200 | 6.00 | 200 | 9.60 |
| S5 | 240 | 8.62 | 240 | 13.80 |
| S6 | 280 | 11.75 | 280 | 18.81 |
| S7 | 300 | 18.50 | 300 | 21.60 |
Time and Frequency Domain Signal Processing
3. Results and Discussion
3.1. Simulation and Experimental Characterisation
| Wire Diameter (mm) | Self-Inductance (µH) [Wheeler's Method] | Self-Inductance (µH) [Grover's Method] | Mutual-Inductance (µH) [Grover's Method] | ||
|---|---|---|---|---|---|
| 0.75 0.85 0.40 | 39.65 38.32 34.89 | 39.08 38.20 34.43 | 3.52 2.93 1.29 | ||
| Resonant Frequency (kHz) | Tx/Rx Wire Diameter (mm) | Tx/Rx Resonant Capacitor (nF) | Estimated Link Efficiency with and without alternating current effects | ||
| 208 211 212 | 0.85 0.75 0.40 | 17.40/17.41 14.89/13.57 16.38/16.38 | 0.76/0.63 0.65/0.54 0.12/0.10 | ||
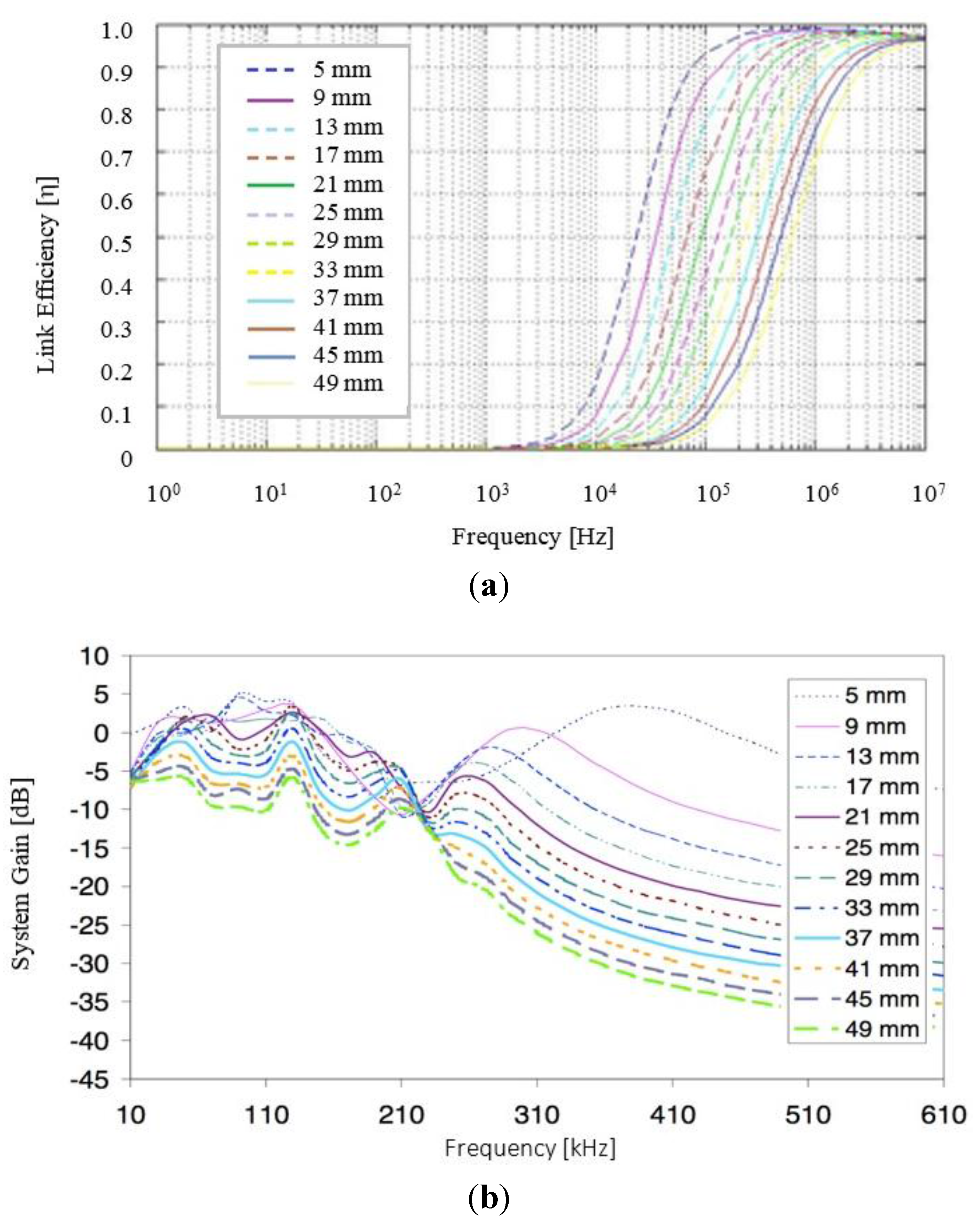

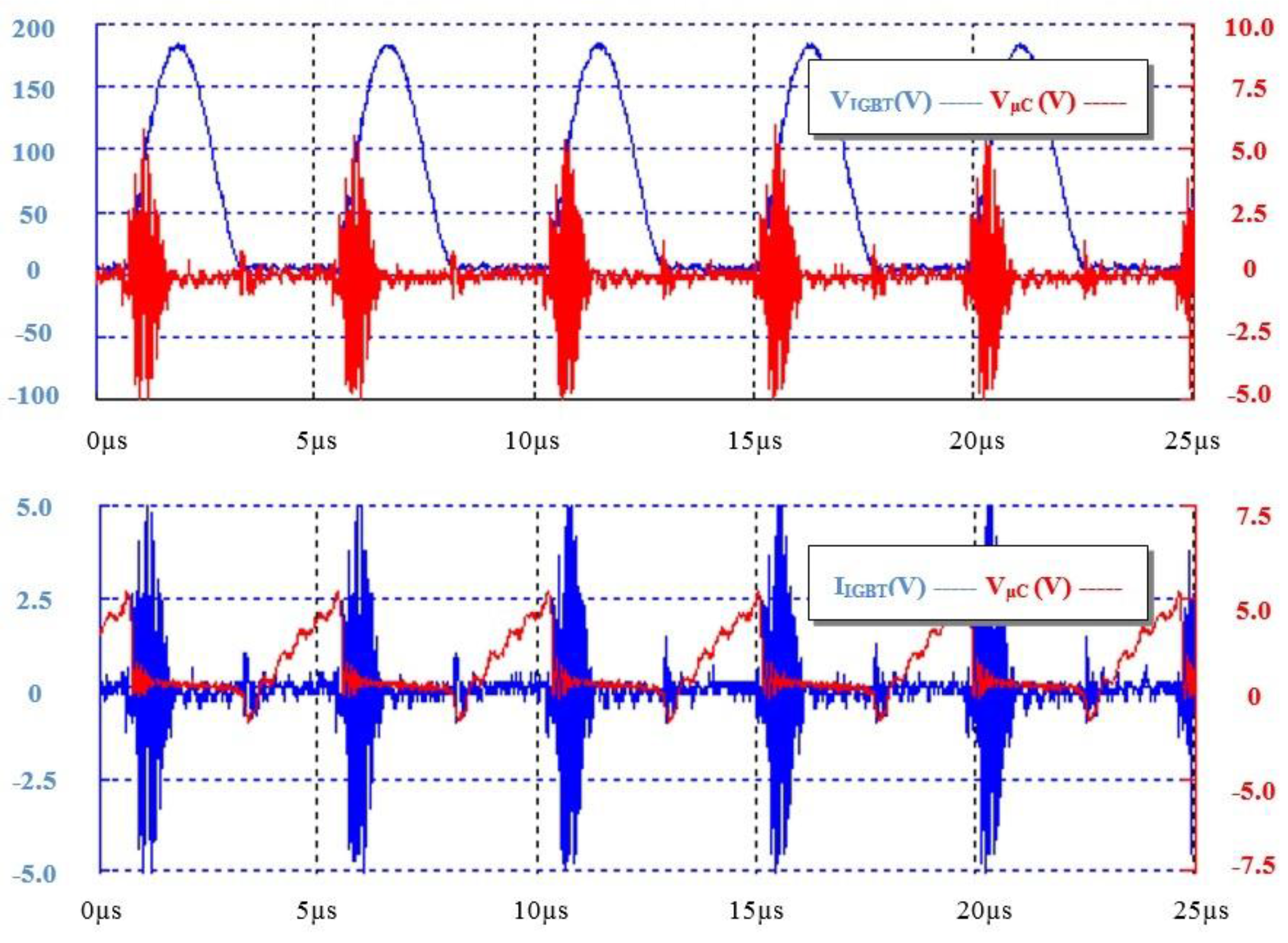
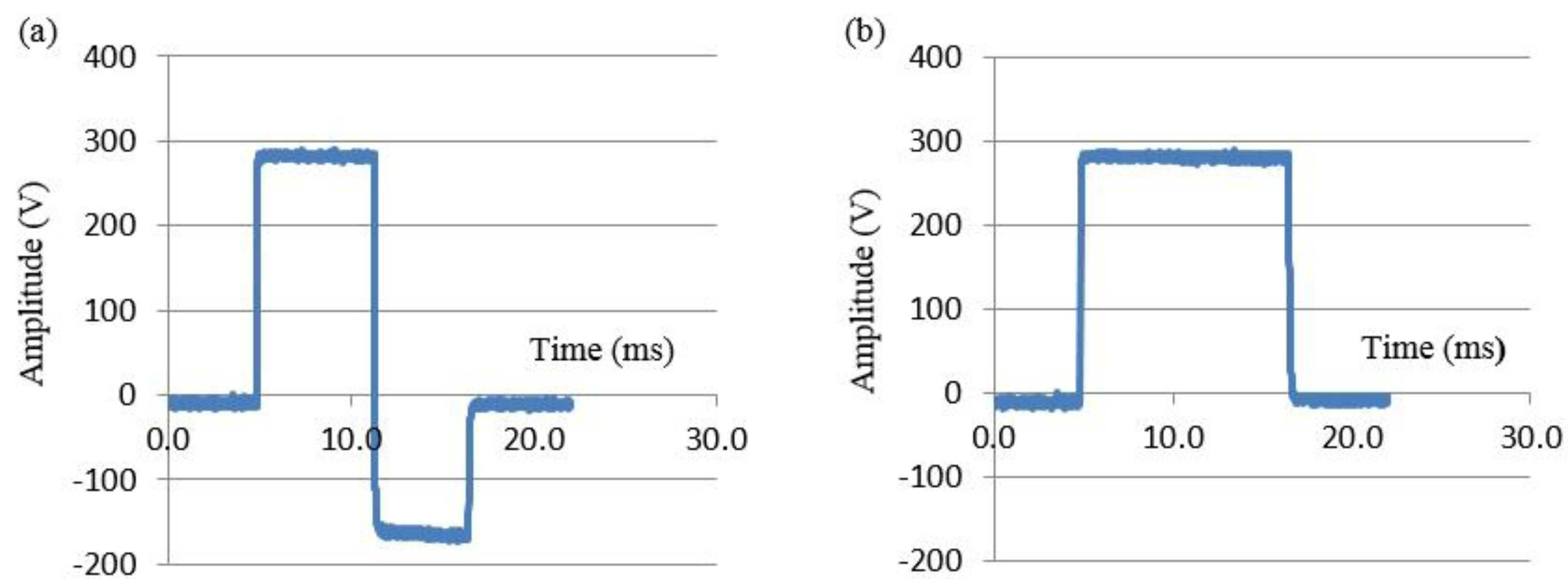
3.2. Clinical Study Outcomes and Results
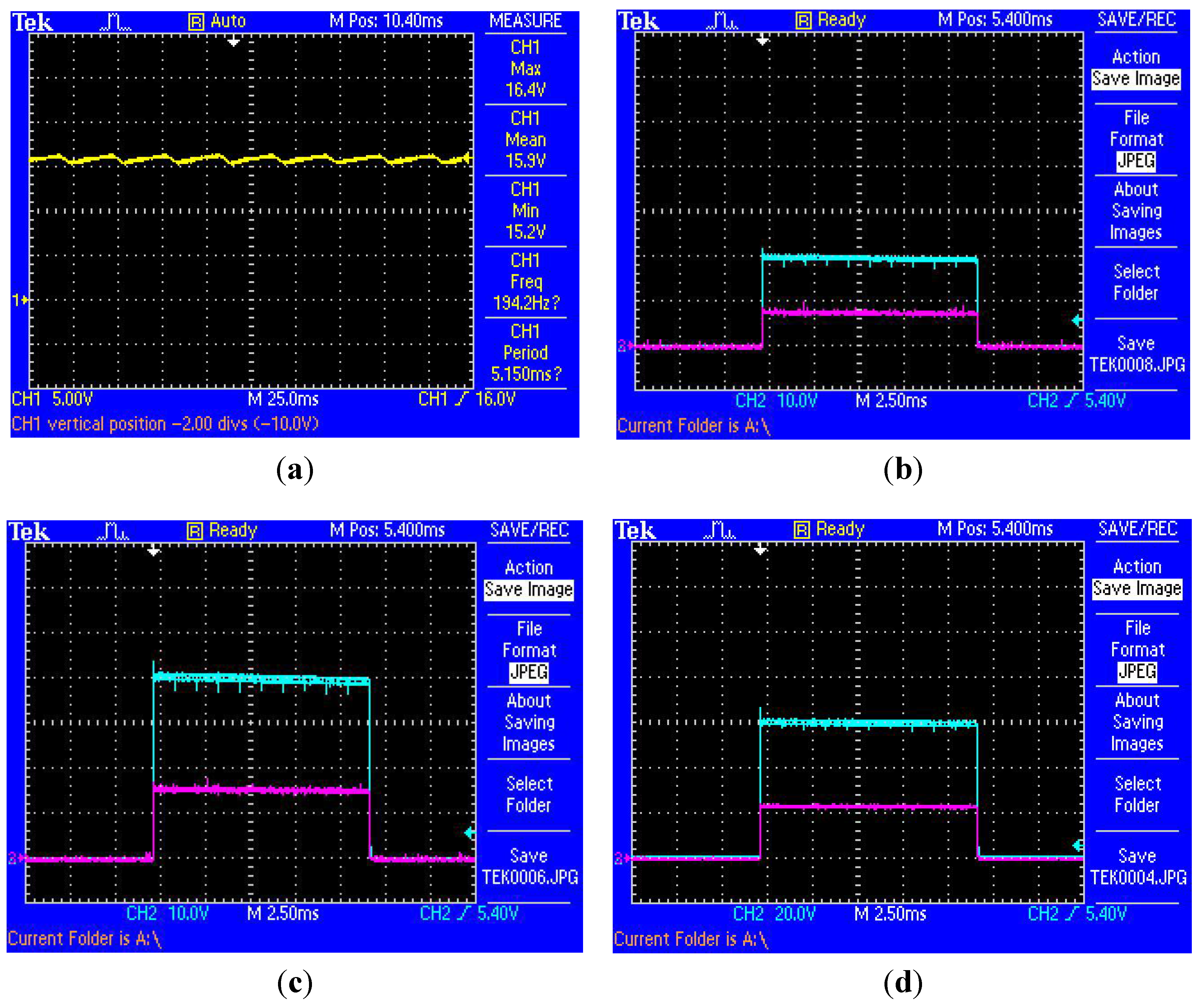

3.2.1. Quantitative Time Domain Findings
| Shock Waveform | Group-I B-VLTR Success (N=7) | Group-II B-VLTR Fail (N=8) | Group-III M-VLTR Success (N=7) | Group-IV M-VLTR Fail (N=8) |
|---|---|---|---|---|
| ZAV @S1 (Ω ± SD(σ)) | 79.20 ± 9.16 | 71.00 ± 17.55 | 80.50 ± 10.34 | 74.92 ± 22.68 |
| ZAV@ S2 (Ω ± SD(σ)) | 76.43 ± 10.02 | 69.28 ± 15.02 | 78.22 ± 10.16 | 68.02 ± 22.76 |
| ZAV @ S3(Ω ± SD(σ)) | 74.25 ± 8.59 | 67.70 ± 12.95 | 75.50 ± 9.43 | 66.80 ± 21.68 |
| |SS − ∆ZT|: S1 -> S3 (Ω ± SD(σ)),(p-value) | 4.95 ± 2.71 p < 0.009 | 3.30 ± 5.63 p > 0.154 | 5.00 ± 1.62 p < 0.003 | 8.12 ± 6.61 p > 0.051 |
| ZAV @ S4 (Ω ± SD(σ)) | 74.14 ± 7.84 | 67.68 ± 13.32 | 75.54 ± 9.80 | 65.88 ± 20.07 |
| |SS - ∆ZAV|: S3 -> S4 (Ω ± SD(σ)), (p-value) | 0.11 ± 2.04 p > 0.147 | 0.02 ± 1.61 p > 0.891 | −0.04 ± 2.80 p > 0.952 | 0.92 ± 3.40 p > 0.404 |
| ZAV @ S5 (Ω ± SD(σ)) | 71.92 ± 6.46 | 68.86 ± 12.80 | 74.51 ± 9.69 | 66.00 ± 21.00 |
| |SS - ∆ZAV|: S4 -> S5 (Ω ± SD(σ), (p-value) | 2.22 ± 1.61 p > 0.100 | −1.18 ± 3.50 p > 0.254 | 1.03 ± 3.20 p > 0.264 | −0.12 ± 3.35 p > 0.775 |
3.2.2. Quantitative Frequency Domain Findings


| Group/IAMSA Value (Ω-Hz) | Group-I and III (n = 15(Patients)) B-VLTR & M-VTLR Success | Group-II and IV (n = 15 (Patients)) B-VLTR & M-VTLR Fail | ||
|---|---|---|---|---|
| Shock | S1 | S3 | S1 | S3 |
| IAMSA(fRANGE-1)1 Hz–1 kHz (Ω-Hz) | 104.42 | 95.47 | 85.04 | 79.99 |
| IAMSA(fRANGE-2)1 kHz–10 kHz (Ω-Hz) | 8.25 | 2.98 | 4.91 | 1.62 |
| IAMSA(fRANGE-3)10 kHz–20 kHz (Ω-Hz) | 9.25 | 2.65 | 6.29 | 1.71 |
| IAMSA(1 Hz–20 kHz) (Ω-Hz) | 121.92 | 101.10 | 96.24 | 83.32 |
| ΔIAMSA: S1-S3 1 Hz–20 kHz (Ω-Hz ± SD(σ), p value) | 20.82 ± 10.77 p < 0.002 (n = 45 (waveforms)) | 12.92 ± 10.47 p< 0.030 (n = 45 (waveforms)) | ||
4. Discussion
4.1. Experimental Findings
4.2. Findings Appraisal in View of Previous Reports
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schamroth, L. An Introduction to Electrocardiography, 7th ed.; Blackwell Scientific Publications: London, UK, 1990. [Google Scholar]
- Heeringa, J.; van verKuip, D.A.; Hofman, A.; Kors, J.A.; van Herpen, G.; Stricker, B.H.; Stijnen, T.; Lip, G.Y.; Witterman, J.C. Prevalence, Incidence and Lifetime risk of Atrial Fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.; Rydén, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Le Huezey, J.Y.; Kay, G.N.; Lowe, J.E.; et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006, 8, 651–745. [Google Scholar] [PubMed]
- Wann, L.S.; Curtis, A.B.; January, C.T.; Ellenbogen, K.A.; Lowe, J.E.; Estes, N.A.; Page, R.L.; Ezekowitz, M.D.; Slotwiner, D.J.; Jackman, W.M.; et al. ACCF/AHA/HRS 2011 focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline). J. Am. Coll Cardiol. 2011, 57, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Johnston, S.S.; Chu, B.C.; Dalal, M.R.; Schulman, M.A. Estimation of Total Incremental Health Care Costs in Patients With Atrial Fibrillation in the United States. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Bonso, A.; Themistoclakis, S.; Giada, F.; Raviele, A. Low energy internal cardioversion in patients with long-lasting atrial fibrillation refractory to external electrical cardioversion: results and long-term follow-up. Europace 2001, 3, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Pavin, D.; Legrand, H.; Leclercq, C.; Crocq, P.; Daubert, J.C. Transvenous low energy internal cardioversion for atrial fibrillation refractory to external cardioversion: do non obese patients benefit ? Heart 2004, 90, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.J.; Wellens, M.D.; Lau, C.P.; Luderitz, B.; Akhtar, M.; Waldo, A.L.; Camm, A.J.; Timmermans, M.D.; Tse, H.F.; Jung, W.; et al. The Atrioverter: An Implantable Device for the Treatment of Atrial Fibrillation. Circulation 1998, 98, 1651–1656. [Google Scholar]
- Tse, H.F.; Lau, C.P.; Sra, J.S.; Crijns, H.; Edvardsson, N.; Kacet, S.; Wyse, G. Atrial fibrillation detection and R-wave synchronization by Metrix implantable atrial defibrillator: implications for long-term efficacy and safety. Circulation 1999, 99, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, C.; Lévy, S.; Ayers, G.M.; Jung, W.; Jordaens, L.; Rosenqvist, M.; Thibault, B.; Camm, J.; Rodriquez, L.M.; Wellens, H.J. Spontaneous episodes of atrial fibrillation after Implantation of the Metrix Atrioverter: Observations on treated and non-treated episodes. J. Am. CollCardiol. 2000, 35, 1428–1433. [Google Scholar] [CrossRef]
- Pedersen, S.S.; van Domburg, R.T.; Theuns, D.A.; Jordaens, L.; Erdman, R.A. Concerns about the implantable cardioverter defibrillator: A determinant of anxiety and depressive symptoms independent of experienced shocks. Am. Heart J. 2005, 149, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Undavia, M.; Goldstein, N.E.; Cohen, P.; Sinthawanarong, K.; Singson, M.; Bhutani, D.; Munson, T.; Gomes, J.A.; Fischer, A.; Mehta, D. Impact of implantable cardioverter-defibrillator recalls on patients’ anxiety, depression, and quality of life. PacingClin. Electrophysiol. 2008, 31, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, B.J.; Allen, D.J.; Harbinson, M.T.; McIntyre, A.; Anderson, J.; Adgey, A.A.J. Waveform Optimization for Internal Atrial Defibrillation: Effects of Waveform Rounding, Phase Duration, and Voltage Swing. PacingClin. Electrophysiol. 2001, 24, 1198–1207. [Google Scholar] [CrossRef]
- Niebauer, M.J.; Chung, M.K.; Brewer, J.E.; Tchou, P.J. Reduced Cardioversion Thresholds for Atrial Fibrillation and Flutter Using The Rectilinear Biphasic Waveform. J. Interv. Card. Electrophysiol. 2005, 13, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.J.; Manoharan, G.; Escalona, O.J.; Santos, J.; Evans, N.; Anderson, J.; Stevenson, M.; Allen, J.D.; Adgey, A.A. Novel rectangular biphasic and monophasic waveforms delivered by a radio frequency powered defibrillator compared with conventional capacitor based waveforms in transvenous cardioversion of atrial fibrillation. Europace 2006, 10, 873–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glover, B.M.; McCann, C.; Manoharan, G.; Walsh, S.J.; Moore, M.J.; Allen, J.D.; Escalona, O.; Anderson, J.M.; Truton, T.G.; Stevenson, M.; et al. A pilot study of a low-tilt biphasic waveform for transvenous cardioversion of atrial fibrillation. Pacing Clin. Electrophysiol. 2008, 31, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Kerber, R.E.; Martins, J.B.; Kienzle, M.G.; Constantin, L.; Olshansky, B.; Hopson, R.; Charbonnier, F. Energy, current, and success in defibrillation and cardioversion: Clinical studies using an automated impedance-based method of energy adjustment. Circulation 1988, 77, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Escalona, O.J.; Kodoth, V.; Castro, N.C.; Xavier, S.; Walsh, P.R.; Glover, B.M.; Lau, E.; Manoharan, G. Linear variation analysis of intracardiac atrial impedance during internal cardioversion using rectilinear waveforms and energy step up protocol. Comput. Cardiol. 2012, 39, 721–724. [Google Scholar]
- Walsh, P.R.; Escalona, O.J.; Kodoth, V.; McEneaney, D.; Lau, E.; Manoharan, G. Dynamic changes in intracardiac impedance as a predictive marker during internal cardioversion of atrial fibrillation. Comput. Cardiol. 2013, 40, 611–614. [Google Scholar]
- Kodoth, V.; Castro, N.C.; Glover, B.M.; Anderson, J.; Escalona, O.J.; Lau, E.; Manoharan, G. Waveform optimisation for internal cardioversion of atrial fibrillation. J. Electrocardiol. 2011, 44, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Tarantini, F.; Caldi, F.; Nakhanian, Y.; Padeletti, M.; Boncinelli, L.; Valoti, P.; Di Serio, C.; Pellerito, S.; Padeletti, L.; et al. Multiple Shocks Affect Thoracic Electrical Impedance During External Cardioversion of Atrial Fibrillation. Pacing Clin. Electrophysiol. 2009, 32, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Fenton, F.H.; Luther, S.; Cherry, E.M.; Otani, N.; Krinsky, V.; Pumir, A.; Bodenschatz, E.; Gilmour, R.F. Termination of Atrial Fibrillation Using Pulsed Low-Energy Far-Field Stimulation. Circulation 2009, 120, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.; Fenton, F.H.; Kornreich, B.G.; Squires, A.; Bittihn, P.; Hornung, D.; Zabel, M.; Flanders, J.; Gladuli, A.; Campoy, L.; Cherry, E.M.; et al. Low-energy control of electrical turbulence in the heart. Nature 2011, 475, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Escalona, O.J.; Velasquez, J.J.; Waterman, N.; Chirwa, L.; Anderson, J. Transcutaneous dual tuned RF coil system voltage gain and efficiency evaluation for a passive implantable atrial defibrillator. Comput. Cardiol. 2010, 37, 569–572. [Google Scholar]
- Walsh, P.R.; Rodrigues, P.A.; Velasquez, J.J.; Watermann, N.; Escalona, O.J. Impedance compensated passive implantable atrial defibrilator. Electron. Lett. 2014, 50, 1192–1193. [Google Scholar] [CrossRef]
- Wheeler, H.A. Inductance formulas for circular and square coils. IEEE Proc. 1982, 70, 1449–1450. [Google Scholar] [CrossRef]
- Grover, F.W. Inductance Calculations: Working Formulas and Tables, 1st ed.; Dover Publications: New York, NY, USA, 1946; pp. 82–113. [Google Scholar]
- Terman, F. Radio Engineering Handbook, 1st ed.; McGraw-Hill Book Company: New York, NY USA, 1943; pp. 128–134. [Google Scholar]
- Knecht, O.; Bosshard, R.; Kolar, J.W.; Stark, C.T. Optimisation of Transcutaneous Energy Transfer coils for high power medical applications. In Proceedings of the 2014 IEEE 15th Workshop on Control and Modeling for Power Electronics (COMPEL), Santander, Spain, 22–25 June 2014; pp. 1–10.
- Zhang, W.; Wong, S.C.; Tse, C.K.; Chen, Q. Analysis and Comparison of Secondary Series and Parallel-Compensated Inductive Power Transfer Systems Operating for Optimal Efficiency and Load-Independent Voltage-Transfer Ratio. IEEE Trans. Power Electron. 2014, 29, 2979–2990. [Google Scholar] [CrossRef]
- Chen, Q.; Wong, S.C.; Tse, C.K.; Ruan, X. Analysis, Design, and Control of a Transcutaneous Power Regulator for Artificial Hearts. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 1932–1945. [Google Scholar] [CrossRef] [PubMed]
- Nam, I.; Dougal, R.; Santi, E. Optimal design method for series LCLC resonant converter based on analytical solutions for voltage gain resonant peaks. In Proceedings of the 2013 Twenty-Eighth Annual IEEE Applied Power Electronics Conference and Exposition (APEC), Long Beach, CA, USA, 17–21 March 2013; pp. 1429–1437.
- Dissanayake, T.D.; Budgett, D.M; Hu, P.; Bennet, L.; Pyner, S.; Booth, L.; Amirapu, S.; Wu, Y.; Malpas, S.C. A Novel Low Temperature Transcutaneous Energy Transfer System Suitable for High Power Implantable Medical Devices: Performance and Validation in Sheep. Artif. Organs 2010, 34, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.S.; Myers, T.J. Transcutaneous Energy Transmission for Mechanical Circulatory Support Systems: History, Current Status,and Future Prospects. J. Card. Surg. 2010, 25, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.Y.; Budgett, D.M.; Hu, A.P. Minimizing Power Loss in Air-Cored Coils for TET Heart Pump Systems. IEEE J. Emerg. Sel. Top. Circuits Syst. 2011, 1, 412–419. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, P.; Kodoth, V.; McEneaney, D.; Rodrigues, P.; Velasquez, J.; Waterman, N.; Escalona, O. Towards Low Energy Atrial Defibrillation. Sensors 2015, 15, 22378-22400. https://doi.org/10.3390/s150922378
Walsh P, Kodoth V, McEneaney D, Rodrigues P, Velasquez J, Waterman N, Escalona O. Towards Low Energy Atrial Defibrillation. Sensors. 2015; 15(9):22378-22400. https://doi.org/10.3390/s150922378
Chicago/Turabian StyleWalsh, Philip, Vivek Kodoth, David McEneaney, Paola Rodrigues, Jose Velasquez, Niall Waterman, and Omar Escalona. 2015. "Towards Low Energy Atrial Defibrillation" Sensors 15, no. 9: 22378-22400. https://doi.org/10.3390/s150922378





