One-Pot Hydrothermal Synthesis of Magnetite Prussian Blue Nano-Composites and Their Application to Fabricate Glucose Biosensor
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Instruments
2.3. One-Pot Synthesis of Magnetite PB Nanoparticles
2.4. Preparation of Glucose Oxidase Biosensor on Magnetite PB Modified Glassy Carbon Electrode
3. Results and Discussion
3.1. Possible Formation Mechanism of Magnetite PB Nanocomposite
3.2. Characterization
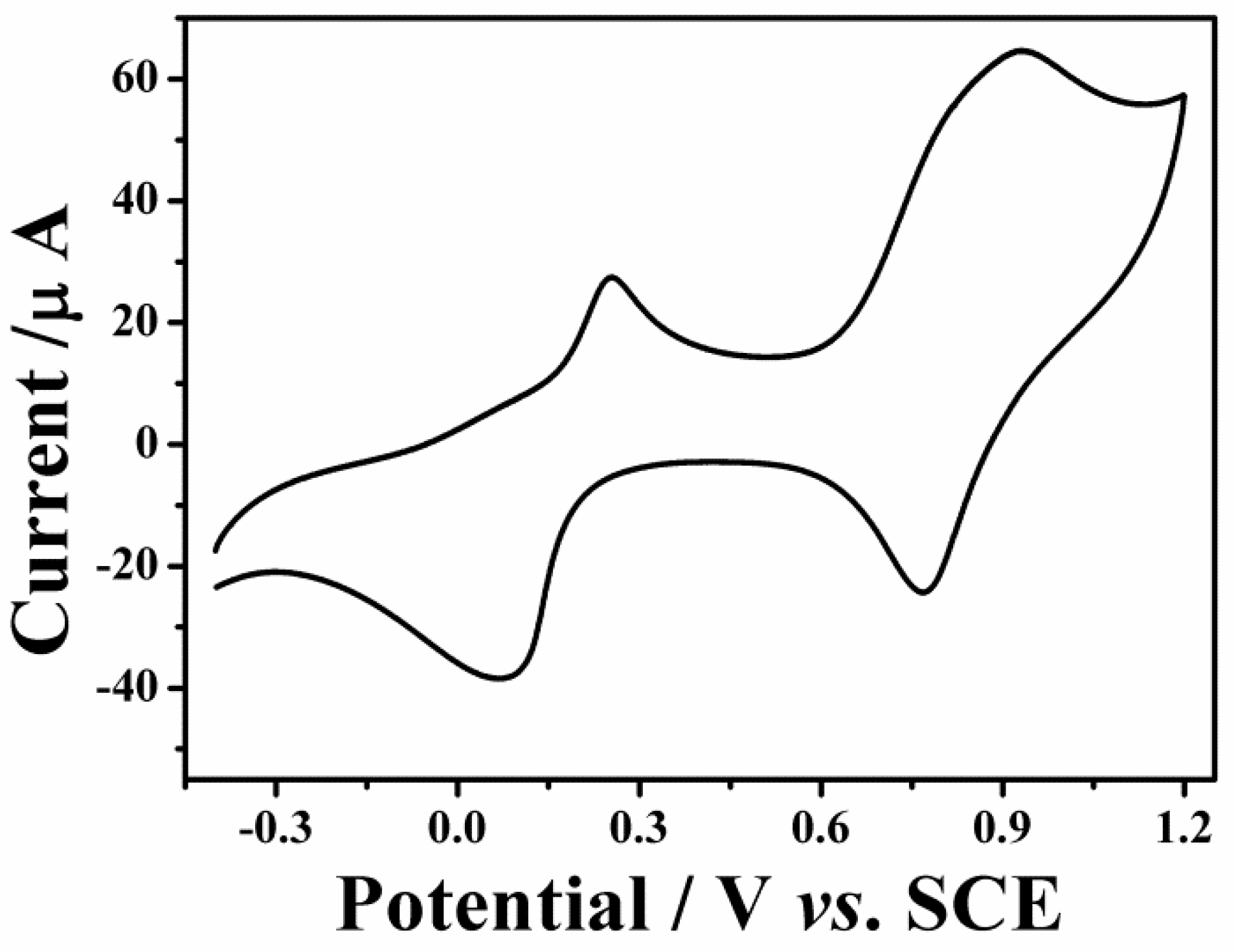
3.3. Electrochemical Behaviors of Fe3O4-PB and GOD-BSA/Fe3O4-PB/GCE
3.4. Detection of Glucose
3.5. Storage Stability of the Biosensor
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Wang, C.; Chen, S.; Xiang, Y.; Li, W.; Zhong, X.; Che, X.; Li, J. Glucose biosensor based on the highly efficient immobilization of glucose oxidase on Prussian blue-gold nanocomposite films. J. Mol. Catal. B Enzym. 2011, 69, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Yuan, Y. Synthesis of magnetic nanoparticles composed by Prussian blue and glucose oxidase for preparing highly sensitive and selective glucose biosensor. Sens. Actuators B Chem. 2009, 139, 400–406. [Google Scholar] [CrossRef]
- Fu, G.; Liu, W.; Feng, S.; Yue, X. Prussian blue nanoparticles operate as a new generation of photothermal ablation agents for cancer therapy. Chem. Commun. 2012, 48, 11567–11569. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.C.; Pandey, A.K. Tetrahydrofuran hydroperoxide mediated synthesis of Prussian blue nanoparticles: A study of their electrocatalytic activity and intrinsic peroxidase-like behavior. Electrochim. Acta 2014, 125, 465–472. [Google Scholar] [CrossRef]
- Wu, J.; Yin, F. Sensitive enzymatic glucose biosensor fabricated by electrospinning composite nanofibers and electrodepositing Prussian blue film. J. Electroanal. Chem. 2013, 694, 1–5. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Zhou, X.; Xue, Z.; Liu, X.; Sun, K.; Lu, X. Improved sensing in physiological buffers by controlling the nanostructure of Prussian blue film. J. Phys. Chem. C 2010, 114, 14786–14793. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; O’Neill, R.D.; Roche, R.; González-Mora, J.L. Improvement and characterization of surfactant-modified Prussian blue screen-printed carbon electrodes for selective H2O2 detection at low applied potentials. J. Electroanal. Chem. 2012, 674, 48–56. [Google Scholar] [CrossRef]
- Thammawong, C.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16. [Google Scholar] [CrossRef]
- Zhao, G.; Feng, J.; Zhang, Q.; Li, S.; Chen, H.-Y. Synthesis and characterization of Prussian blue modified magnetite nanoparticles and its application to the electrocatalytic reduction of H2O2. Chem. Mater. 2005, 17, 3154–3159. [Google Scholar] [CrossRef]
- Fu, G.; Liu, W.; Li, Y.; Jin, Y.; Jiang, L.; Liang, X.; Feng, S.; Dai, Z. Magnetic Prussian blue nanoparticles for targeted photothermal therapy under magnetic resonance imaging guidance. Bioconjugate Chem. 2014, 25, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, S.; Zhang, Y.; Yang, T.; Wang, C.; Gu, N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Massimiliano, M.; Davide, B.; Gabriella, S.; Katerin, P.; Giorgio, Z.; Jiri, T.; Josef, K.; Radek, Z.; Fabio, V. Core-shell hybrid nanomaterial based on Prussian blue and surface active maghemite nanoparticles as stable electrocatalyst. Biosens. Bioelectron. 2014, 52, 159–165. [Google Scholar]
- Liu, S.; Xing, R.; Lu, F.; Rana, R.K.; Zhu, J.-J. One-pot template-free fabrication of hollow magnetite nanospheres and their application as potential drug carriers. J. Phys. Chem. C 2009, 113, 21042–21047. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, K.; Qi, Y.; Sheng, J.; Liu, Z. One-step synthesis of superparamagnetic monodisperse porous Fe3O4 hollow and core-shell spheres. J. Mater. Chem. 2010, 20, 1799–1805. [Google Scholar] [CrossRef]
- Arun, T.; Prakash, K.; Kuppusamy, R.; Joseyphus, R.J. Magnetic properties of Prussian blue modified Fe3O4 nanocubes. J. Phys. Chem. Solids 2013, 74, 1761–1768. [Google Scholar] [CrossRef]
- Daou, T.J.; Grenèche, J.M.; Pourroy, G.; Buathong, S.; Derory, A.; Ulhaq-Bouillet, C.; Donnio, B.; Guillon, D.; Begin-Colin, S. Coupling agent effect on magnetic properties of functionalized magnetite-based nanoparticles. Chem. Mater. 2008, 20, 5869–5875. [Google Scholar] [CrossRef]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of Prussian blue nanoparticles with a hollow interior by controlled chemical etching. Angew. Chem. 2012, 51, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, Y. Synthesis of magnetic Prussian blue nanoparticles and the fabrication of chemically modified electrode. Acta Chim. Sin. 2006, 64, 261–265. [Google Scholar]
- Darder, M.; González-Alfaro, Y.; Aranda, P.; Ruiz-Hitzky, E. Silicate-based multifunctional nanostructured materials with magnetite and Prussian blue: Application to cesium uptake. RSC Adv. 2014, 4, 35415–35421. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, X. Electrochemical synthesis of Fe3O4-PB nanoparticles with core-shell structure and its electrocatalytic reduction toward H2O2. J. Solid State Electrochem. 2008, 13, 1273–1278. [Google Scholar]
- Xue, M.-H.; Xu, Q.; Zhou, M.; Zhu, J.-J. In situ immobilization of glucose oxidase in chitosan-gold nanoparticle hybrid film on Prussian blue modified electrode for high-sensitivity glucose detection. Electrochem. Commun. 2006, 8, 1468–1474. [Google Scholar] [CrossRef]
- Albanese, D.; Sannini, A.; Malvano, F.; Pilloton, R.; Di Matteo, M. Optimisation of glucose biosensors based on sol-gel entrapment and Prussian blue-modified screen-printed electrodes for real food analysis. Food Anal. Methods 2013, 7, 1002–1008. [Google Scholar] [CrossRef]
- Palod, P.A.; Singh, V. Improvement in glucose biosensing response of electrochemically grown polypyrrole nanotubes by incorporating crosslinked glucose oxidase. Mater. Sci. Eng. C 2015, 55, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Chu, Z.; Shi, L.; Jin, W. Amperometric glucose biosensor based on direct assembly of Prussian blue film with ionic liquid-chitosan matrix assisted enzyme immobilization. Sens. Actuators B Chem. 2013, 176, 978–984. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, L.; Yin, L.; Guo, L. Electrochemical glucose biosensor with improved performance based on the use of glucose oxidase and Prussian blue incorporated into a thin film of self-polymerized dopamine. Sens. Actuatos B Chem. 2015, 210, 513–518. [Google Scholar] [CrossRef]
- Li, L.; Sheng, Q.; Zheng, J.; Zhang, H. Facile and controllable preparation of glucose biosensor based on Prussian blue nanoparticles hybrid composites. Bioelectrochemistry 2008, 74, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lad, U.; Kale, G.M.; Bryaskova, R. Glucose oxidase encapsulated polyvinyl alcohol-silica hybrid films for an electrochemical glucose sensing electrode. Anal. Chem. 2013, 85, 6349–6355. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-N.; Dan, S.; Xue, H.; Zhu, D.; Cosnier, S. Glucose biosensor immobilized in alginatelayered double hydroxides hybrid membrane and its biosensing application. Anal. Sci. 2009, 25, 1421–1425. [Google Scholar] [CrossRef] [PubMed]








| Working Electrode | LOD (µM) | Linear Range | Sensitivity | Response Time (s) | Reference |
|---|---|---|---|---|---|
| PB/GOD/TEOS-PVA/NAFION | 20 | 5–1000 µM | 2.06 µA∙mM−1∙cm−2 | No data | [25] |
| Anodisc™/Pt/PPy/GOx nanotube arrays | 50 | 0.2–13 mM | 72.1 mA∙M−1∙cm−2 | 13 | [26] |
| Fe3O4/PB/GOD | 0.1 | 0.5–80 µM | No data | 10 | [2] |
| GOx/Chi-IL/PB/Pt | 5 | 0.01–4.2 mM | 37.8 µA∙mM−1∙cm−2 | 3 | [27] |
| GOx–PDA/PB/GCE | 46.2 | 0.2–3.4 mM | 1.59 nA∙µM−1 | 15 | [28] |
| PBNPs-PANI/MWNTs/GCE | 0.6 | 6.7–1900 µM | 6.28 µAmM−1 | 5 | [29] |
| PVA-Au-pphTEOS-GOD | 700 | 1–8 mM | 49 µA∙mM−1∙cm−2 | 6 | [30] |
| Alg/LDHs/GOD/Pt | 4 | 0.016–2 mM | 68.9 mA∙M−1∙cm−2 | 10 | [31] |
| GOD-BSA/GA/Fe3O4-PB/GCE | 0.5 | 5–1200 µM | 32 µA∙mM−1∙cm−2 | 4 | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jomma, E.Y.; Ding, S.-N. One-Pot Hydrothermal Synthesis of Magnetite Prussian Blue Nano-Composites and Their Application to Fabricate Glucose Biosensor. Sensors 2016, 16, 243. https://doi.org/10.3390/s16020243
Jomma EY, Ding S-N. One-Pot Hydrothermal Synthesis of Magnetite Prussian Blue Nano-Composites and Their Application to Fabricate Glucose Biosensor. Sensors. 2016; 16(2):243. https://doi.org/10.3390/s16020243
Chicago/Turabian StyleJomma, Ezzaldeen Younes, and Shou-Nian Ding. 2016. "One-Pot Hydrothermal Synthesis of Magnetite Prussian Blue Nano-Composites and Their Application to Fabricate Glucose Biosensor" Sensors 16, no. 2: 243. https://doi.org/10.3390/s16020243
APA StyleJomma, E. Y., & Ding, S.-N. (2016). One-Pot Hydrothermal Synthesis of Magnetite Prussian Blue Nano-Composites and Their Application to Fabricate Glucose Biosensor. Sensors, 16(2), 243. https://doi.org/10.3390/s16020243







