Non-Invasive Examination of Plant Surfaces by Opto-Electronic Means—Using Russet as a Prime Example
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Fruit Sourcing
2.2. Technique of Luster Measurement
2.3. Non-Invasive 3D Color Microscopy
2.4. Data Processing
3. Results and Discussion
3.1. Fine Structure, Geometry and Roughness of Russet Peel
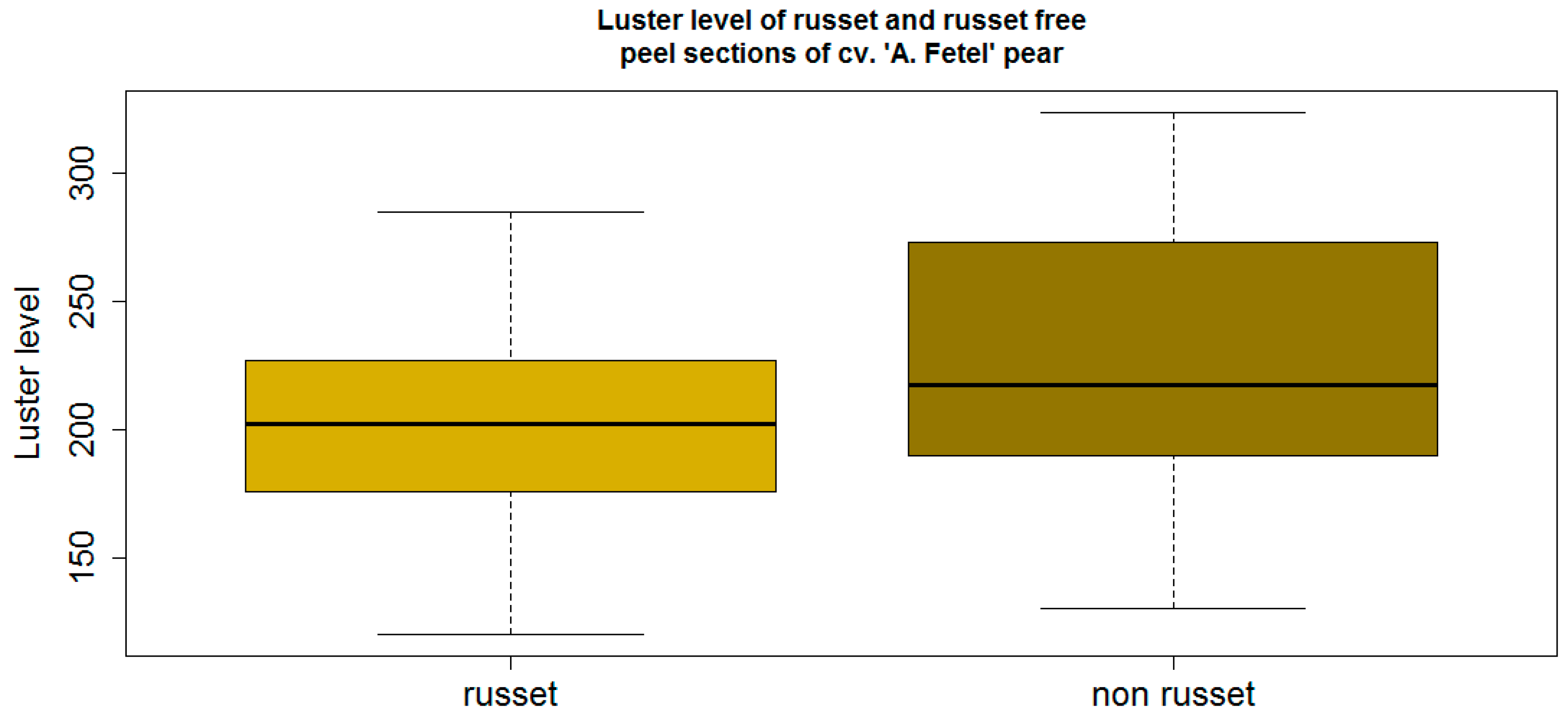
3.2. Luster
3.3. Detection of Russet on Pome Fruits
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teviotdale, B.L.; Viveros, M.; Grant, G.A. Apple russetting influenced by more than copper sprays. Calif. Agric. 1997, 51, 10–11. [Google Scholar] [CrossRef]
- Gill, G.F.; Urquiza, D.A.; Bofarull, J.A.; Montenegro, G.; Zoffoli, J.P. Russet development in the “Beurre Bosc” pear. Acta Hortic. 1994, 367, 239–247. [Google Scholar] [CrossRef]
- Jones, K.M.; Bound, S.A.; Summers, C.R.; Oakford, M.J. Crop regulation and skin finish with Fuji apples. Compact Fruit Trees 1991, 31, 1–5. [Google Scholar]
- Reil, W.O.; Beutel, S.A.; Moller, W.J. Effects of contol sprays on ruessting of Bartlett pears. Calif. Agric. 1973, 27, 5–6. [Google Scholar]
- Maas, F. Evaluation of yield efficiency and winter hardiness of quince rootstocks for cv. “Conference” pear. Acta Horti. 2015, 1094, 93–100. [Google Scholar] [CrossRef]
- Kirby, A.H.M.; Bennett, M. Susceptibility of apple and pear varieties to damage by certain organic fungicides. J. Hortic. Sci. 1967, 42, 117–132. [Google Scholar] [CrossRef]
- Reid, W.S. Optical detection of apple skin, bruise, flesh, stem and calyx. J. Agric. Eng. Res. 1976, 21, 291–295. [Google Scholar] [CrossRef]
- Ward, G.; Nussinovitch, A. Peel gloss as a potential indicator of banana ripeness. LWT Food Sci. Technol. 1986, 29, 288–294. [Google Scholar] [CrossRef]
- Blanke, M.M.; Olzem, S.; Köhler, H. Glossyness of cherries—[Glanz der Kirschen]. J. Appl. Bot. 2001, 75, 30–41. [Google Scholar]
- Bohlin, E. Optics of Coated Paperboards. Aspects of surface treatment on porous structures. Karlstad University Studies 2011-1. Available online: http://www.diva-portal.org/smash/get/diva2:380890/FULLTEXT01.pdf (accessed on 10 January 2016).
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for scanning electron microscopy of plant surfaces—Horses for courses. Mircon 2008, 39, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, P.; Fernández, V.; Khayet, M.; García, M.L.; Fernández, A.; Gil, L. Ultrastructure of plant leaf cuticles in relation to sample preparation as observed by transmission electron microscopy. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Blanke, M.M. Fine structure and elemental composition of segment membranes of cv. Valencia orange fruit. J. Appl. Bot. 2005, 77, 28–31. [Google Scholar]
- Blanke, M.M.; Pring, R.J.; Baker, E.A. Structure and elemental composition of grape berry stomata. J. Plant Physiol. 1999, 154, 477–481. [Google Scholar] [CrossRef]
- Blanke, M.M.; Höfer, M.A.; Pring, R.J. Stomata and structure of tetraploid apple leaves cultered in vitro. Ann. Bot. 1994, 73, 651–654. [Google Scholar] [CrossRef]
- Blanke, M.M. Non-destructive measurement of light reflection of pressure-injured, russeting and scab lesions of mature apple fruit using portable instrumentation. In Proceedings of the Deutsche Gesellschaft fur Qualitätsforschung. Pflanzliche Nahrungsmittel DGQ eV, XXXIV Vortragstagung, Zerstörungsfreie Qualitätsanalyse, Freising-Weihenstephan, Germany, 22–23 March 1999; pp. 171–180.




| Traditional SEM/TEM | New Generation 3D Color Microscopy | |
|---|---|---|
| Sample size | small, e.g., 1 cm × 1 cm | n.a. |
| Sample preparation | Large effort and timely | Not necessary |
| Sample (gold) coating in high vacuum | Often necessary for better results | Not necessary |
| Vacuum can lead to | Sample dehydration and tissue collapse | Specimen remains intact and unaffected |
| Non-invasive examination | Invasive | Non-invasive; allows repetition of the same samples |
| Examination | 2D | 3D |
| Microscope dimension | Occupies most of an air-conditioned room | Large but portable |
| Feature | Value |
|---|---|
| LED source (red) | 665 nm |
| Luster values | 0 to 4000 a.u. |
| Spot size | 3 or 5 mm diameter (optional) |
| Distance measurement | 10–20 mm (center 15 mm) |
| Power | 14.8 V (ca. 1 watt) |
| Response time | <8 ms |
| Weight | ca. 50 g |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klemm, M.; Röttger, O.; Damerow, L.; Blanke, M. Non-Invasive Examination of Plant Surfaces by Opto-Electronic Means—Using Russet as a Prime Example. Sensors 2016, 16, 452. https://doi.org/10.3390/s16040452
Klemm M, Röttger O, Damerow L, Blanke M. Non-Invasive Examination of Plant Surfaces by Opto-Electronic Means—Using Russet as a Prime Example. Sensors. 2016; 16(4):452. https://doi.org/10.3390/s16040452
Chicago/Turabian StyleKlemm, Matthias, Olga Röttger, Lutz Damerow, and Michael Blanke. 2016. "Non-Invasive Examination of Plant Surfaces by Opto-Electronic Means—Using Russet as a Prime Example" Sensors 16, no. 4: 452. https://doi.org/10.3390/s16040452
APA StyleKlemm, M., Röttger, O., Damerow, L., & Blanke, M. (2016). Non-Invasive Examination of Plant Surfaces by Opto-Electronic Means—Using Russet as a Prime Example. Sensors, 16(4), 452. https://doi.org/10.3390/s16040452








