Classification of Fusarium-Infected Korean Hulled Barley Using Near-Infrared Reflectance Spectroscopy and Partial Least Squares Discriminant Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Near-Infrared Measurement System
2.3. Medium Preparation for Fusarium Culture of Hulled Barley
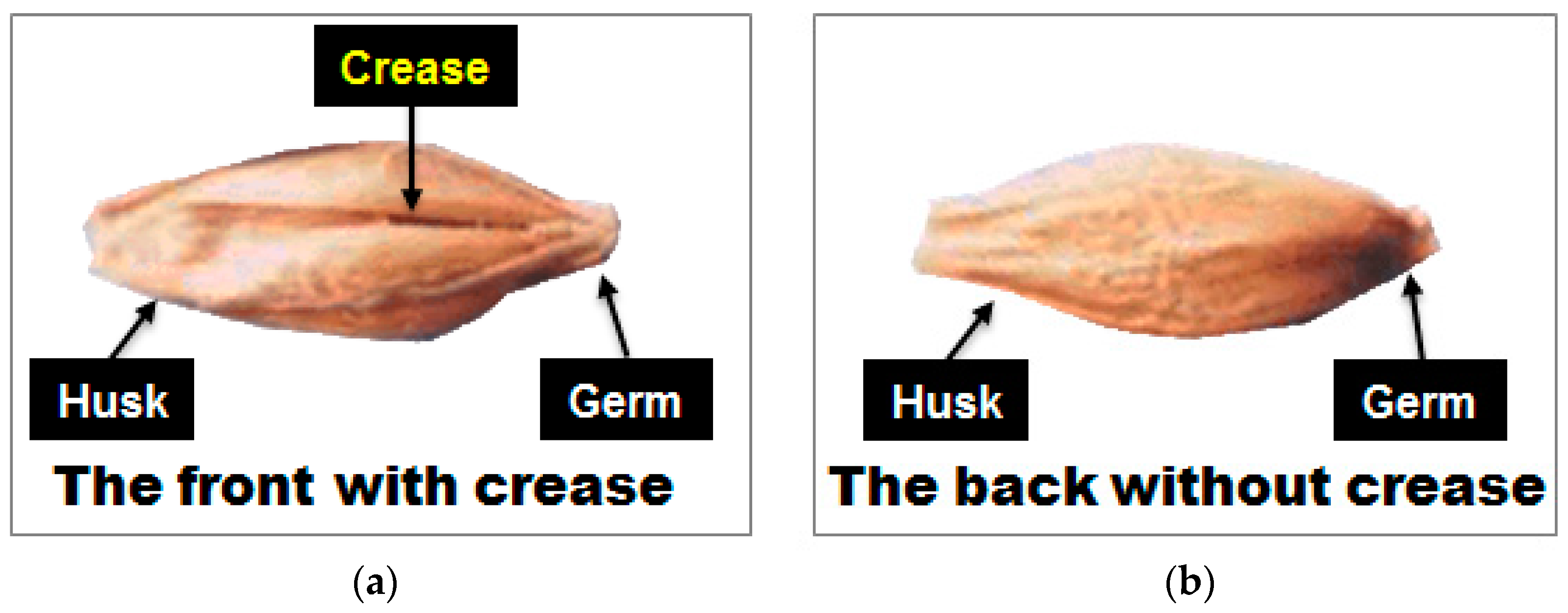
2.4. Acquisition and Pretreatment of NIR Reflectance Spectra
2.5. Fusarium Discrimination Prediction Model Development
3. Results
3.1. Culture Results of Hulled Barley
3.1.1. Culture Results of Hulled Barley Classified as Control Group
3.1.2. Culture Results of Hulled Barley Classified as Experimental Group
3.2. Spectral Characteristics of Hulled Barley
3.3. Prediction Results of PLS-DA Model for Fusarium-Infected Huske Barley
3.3.1. Prediction Results of PLS-DA Model Using Reflectance Spectra Obtained from Front Side and Back Side of Hulled Barley
3.3.2. Prediction Results of PLS-DA Model Using Reflectance Spectra Obtained from Front Side of Hulled Barley
3.3.3. Prediction Results of PLS-DA Model Using Reflectance Spectra Obtained from the Back Side of the Hulled Barley
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jansen, C.; Wettsteint, D.V.; Schafer, W.; Kogel, K.H.; Felk, A.; Maier, F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef] [PubMed]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop. Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Del Ponte, E.M.D.; Fernandes, J.M.C.; Bergstrom, G.C. Influence of Growth Stage on Fusarium Head Blight Deoxynivalenol Production in Wheat. J. Phytopathol. 2007, 155, 577–581. [Google Scholar] [CrossRef]
- Miedaner, T. Review Breeding wheat and rye for resistance to Fusarium diseases. Plant Breed. 1997, 116, 210–220. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Girolamo, A.D.; Lippolis, V.; Nordkvist, E.; Visconti, A. Rapid and non-invasive analysis of deoxynivalenol in durum and common wheat by Fourier-Transform Near Infrared (FT-NIR) spectroscopy. Food Addit. Contam. Part A 2009, 26, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Koppen, R.; Koch, M.; Siegel, D.; Merkel, S.; Maul, R.; Nehls, I. Determination of mycotoxins in foods: Current state of analytical methods and limitations. Appl. Microbiol. Biotechnol. 2010, 86, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- Norris, K.H. Reports on the design and development of a new moisture meter. Agric. Eng. 1964, 45, 370–372. [Google Scholar]
- Adams, M.J. Chemometrics in Analytical Spectroscopy, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2004. [Google Scholar] [CrossRef]
- Lorente, D.; Aleixos, N.; Gómez-Sanchis, J.; Cubero, S.; García-Navarrete, O.L.; Blasco, J. Recent Advances and Applications of Hyperspectral Imaging for Fruit and Vegetable Quality Assessment. Food Bioprocess. Technol. 2011, 5, 1121–1142. [Google Scholar] [CrossRef]
- Cen, H.; Yong, H. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Giacomo, D.R.; Stefania, D.Z.A. A multivariate regression model for detection of fumonisins content in maize from near infrared spectra. Food Chem. 2013, 141, 4289–4294. [Google Scholar] [CrossRef] [PubMed]
- Sirisomboon, C.D.; Putthang, R.; Sirisomboon, P. Application of near infrared spectroscopy to detect aflatoxigenic fungal contamination in rice. Food Control 2013, 33, 207–217. [Google Scholar] [CrossRef]
- Delwiche, S.R. Classification of scab- and other mold-damaged wheat kernels by near-infrared reflectance spectroscopy. Trans. ASAE 2003, 46, 731–738. [Google Scholar] [CrossRef]
- Polder, G.; Van Der Heijden, G.W.A.M.; Waalwijk, C.; Young, A.I.T. Detection of Fusarium in single wheat kernels using spectral imaging. Seed Sci. Technol. 2005, 33, 655–668. [Google Scholar] [CrossRef]
- Liu, Y.; Delwiche, S.R.; Dong, Y. Feasibility of FT–Raman spectroscopy for rapid screening for DON toxin in ground wheat and barley. Food Addit. Contam. Part A 2009, 26, 1396–1401. [Google Scholar] [CrossRef]
- Gaspardo, B.; Del Zotto, S.; Torelli, E.; Cividino, S.R.; Firrao, G.; Della Riccia, G.; Stefanon, B. A rapid method for detection of fumonisins B1 and B2 in corn meal using Fourier transform near infrared (FT-NIR) spectroscopy implemented with integrating sphere. Food Chem. 2012, 135, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.Y.; Kim, G.Y.; Lee, K.J.; Kim, M.S.; Cho, B.K.; Lim, J.G.; Kang, S.W. Non-Destructive Quality Evaluation of Pepper (Capsicum annuum L.) Seeds Using LED-Induced Hyperspectral Reflectance Imaging. Sensors 2014, 14, 7489–7504. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.G.; Kim, G.Y.; Mo, C.Y.; Kim, M.S. Design and Fabrication of a Real-Time Measurement System for the Capsaicinoid Content of Korean Red Pepper (Capsicum annuum L.) Powder by Visible and Near-Infrared Spectroscopy. Sensors 2015, 15, 27420–27435. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Hruschka, W.R.; Abbott, J.A.; Noh, S.H.; Park, B.S. Predicting the soluble solids of apples by near infrared spectroscopy(II)—PLS and ANN models. J. Biosyst. Eng. 1998, 23, 571–582. [Google Scholar]
- Son, J.R.; Lee, K.J.; Kang, S.W.; Yang, G.M.; Seo, Y.M. Development of prediction model for sugar content of strawberry using NIR spectroscopy. Food Eng. Prog. 2009, 13, 297–301. [Google Scholar]
- Alexandrakis, D.; Downey, G.; Scannell, A.G.M. Detection and identification of bacteria in an isolated system with near-infrared spectroscopy and multivariate analysis. J. Agric. Food Chem. 2008, 56, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.G.; Mo, C.Y.; Kim, G.Y.; Kang, S.W.; Lee, K.J.; Kim, M.S.; Moon, J.H. Non-destructive and Rapid Prediction of Moisture Content in Red Pepper (Capsicum annuum L.) Powder Using Near-infrared Spectroscopy and a Partial Least Squares Regression Model. J. Biosyst. Eng. 2014, 39, 184–193. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]














| Province | Groups | Sample Group Based on Region | Number of Kernels |
|---|---|---|---|
| Jeonnam | Control group | JN151 | 127 |
| Gyeongnam | Experimental group | GN121 | 80 |
| Jeonbuk | JB021 | 95 | |
| JB061 | 108 | ||
| JB094 | 105 | ||
| Total | 5 | 515 |
| Control Group | Experimental Groups | ||||
|---|---|---|---|---|---|
| JN151 (127) | GN121 (80) | JB021 (95) | JB061 (108) | JB094 (105) | |
 |  |  |  |  |  |
| Model (Manufacturer) | Ava Spec-NIR256-2.2TEC (Avantes BV, Apeldoorn, The Netherlands) |
|---|---|
| Appearance |  |
| Spectral range | 1175–2170 nm |
| Detection sensor | InGaAs linear array |
| Pixel pitch | 3.4 nm |
| Pixel size | 50 × 500 μm |
| Total pixel count | 256 |
| Minimum exposure time | 1 ms |
| Signal to noise ratio | 4100:1 |
| PC interface | USB 2.0 |
| Dimensions | 315 × 235 × 135 mm |
| Weight | 5.1 kg |
| Experimental Group | Number of Grains (Sample Group) | Culture Results | |
|---|---|---|---|
| a NIF | b IF | ||
| Hulled barley group classified as not infected with Fusarium | 127 (JN151) | 127 | 0 |
| Hulled barley group classified as infected with Fusarium | 80 (GN121) | 34 | 46 |
| 95 (JB021) | 13 | 82 | |
| 108 (JB061) | 26 | 82 | |
| 105 (JB094) | 17 | 88 | |
| Pretreatment | a F | b PC | c PV | ||||
|---|---|---|---|---|---|---|---|
| RC2 | SEC | RV2 | SEP | d CCR | |||
| NIF | IF | ||||||
| The front and back measurement results of hulled barley | |||||||
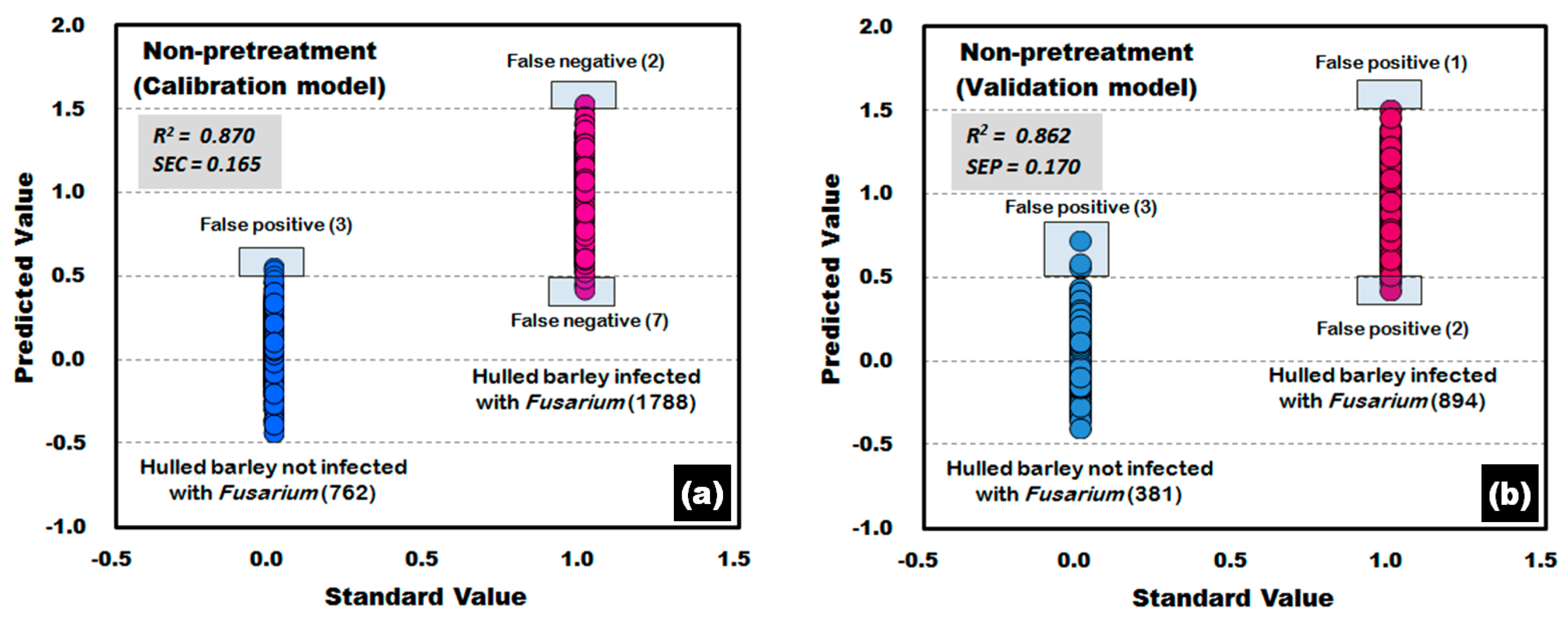
| Non-pretreatment | 17 | 0.870 | 0.165 | 0.865 | 0.168 | 99.61 | 99.50 |
| 1st order Derivative | 14 | 0.924 | 0.126 | 0.919 | 0.131 | 99.87 | 100.00 |
| 2nd order Derivative | 10 | 0.950 | 0.103 | 0.948 | 0.105 | 100.00 | 100.00 |
| 3rd order Derivative | 9 | 0.942 | 0.110 | 0.941 | 0.112 | 100.00 | 100.00 |
| Mean Normalization | 17 | 0.868 | 0.166 | 0.863 | 0.170 | 99.87 | 99.55 |
| Maximum Normalization | 17 | 0.865 | 0.168 | 0.860 | 0.172 | 99.74 | 99.38 |
| Range Normalization | 17 | 0.860 | 0.171 | 0.855 | 0.175 | 99.87 | 99.44 |
| MSC | 16 | 0.852 | 0.176 | 0.846 | 0.180 | 99.87 | 99.22 |
| Baseline | 14 | 0.838 | 0.184 | 0.835 | 0.186 | 99.48 | 98.99 |
| SNV | 17 | 0.853 | 0.176 | 0.847 | 0.179 | 99.87 | 99.16 |
| The front measurement results with crease of hulled barley | |||||||
| Non-pretreatment | 17 | 0.872 | 0.164 | 0.862 | 0.170 | 99.21 | 99.66 |
| 1st order Derivative | 15 | 0.938 | 0.114 | 0.929 | 0.122 | 99.74 | 100.00 |
| 2nd order Derivative | 10 | 0.948 | 0.104 | 0.944 | 0.108 | 99.74 | 100.00 |
| 3rd order Derivative | 9 | 0.943 | 0.109 | 0.939 | 0.113 | 100.00 | 100.00 |
| Mean Normalization | 16 | 0.858 | 0.172 | 0.847 | 0.179 | 99.48 | 99.78 |
| Maximum Normalization | 16 | 0.854 | 0.175 | 0.843 | 0.182 | 99.48 | 99.66 |
| Range Normalization | 17 | 0.858 | 0.173 | 0.847 | 0.179 | 99.48 | 99.33 |
| MSC | 16 | 0.845 | 0.180 | 0.833 | 0.187 | 99.21 | 99.22 |
| Baseline | 17 | 0.863 | 0.169 | 0.853 | 0.176 | 99.48 | 99.66 |
| SNV | 17 | 0.846 | 0.180 | 0.834 | 0.187 | 99.21 | 99.22 |
| The back measurement results without crease of hulled barley | |||||||
| Non-pretreatment | 17 | 0.922 | 0.128 | 0.915 | 0.133 | 100.00 | 99.89 |
| 1st order Derivative | 12 | 0.941 | 0.111 | 0.933 | 0.119 | 100.00 | 99.89 |
| 2nd order Derivative | 10 | 0.962 | 0.089 | 0.959 | 0.093 | 100.00 | 100.00 |
| 3rd order Derivative | 9 | 0.954 | 0.098 | 0.951 | 0.102 | 100.00 | 100.00 |
| Mean Normalization | 13 | 0.881 | 0.158 | 0.874 | 0.163 | 100.00 | 99.66 |
| Maximum Normalization | 13 | 0.878 | 0.160 | 0.871 | 0.165 | 100.00 | 99.55 |
| Range Normalization | 13 | 0.877 | 0.160 | 0.871 | 0.165 | 99.74 | 99.55 |
| MSC | 14 | 0.887 | 0.154 | 0.879 | 0.159 | 100.00 | 99.66 |
| Baseline | 14 | 0.897 | 0.147 | 0.890 | 0.152 | 100.00 | 99.78 |
| SNV | 14 | 0.878 | 0.160 | 0.871 | 0.165 | 100.00 | 99.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Kim, G.; Mo, C.; Oh, K.; Yoo, H.; Ham, H.; Kim, M.S. Classification of Fusarium-Infected Korean Hulled Barley Using Near-Infrared Reflectance Spectroscopy and Partial Least Squares Discriminant Analysis. Sensors 2017, 17, 2258. https://doi.org/10.3390/s17102258
Lim J, Kim G, Mo C, Oh K, Yoo H, Ham H, Kim MS. Classification of Fusarium-Infected Korean Hulled Barley Using Near-Infrared Reflectance Spectroscopy and Partial Least Squares Discriminant Analysis. Sensors. 2017; 17(10):2258. https://doi.org/10.3390/s17102258
Chicago/Turabian StyleLim, Jongguk, Giyoung Kim, Changyeun Mo, Kyoungmin Oh, Hyeonchae Yoo, Hyeonheui Ham, and Moon S. Kim. 2017. "Classification of Fusarium-Infected Korean Hulled Barley Using Near-Infrared Reflectance Spectroscopy and Partial Least Squares Discriminant Analysis" Sensors 17, no. 10: 2258. https://doi.org/10.3390/s17102258
APA StyleLim, J., Kim, G., Mo, C., Oh, K., Yoo, H., Ham, H., & Kim, M. S. (2017). Classification of Fusarium-Infected Korean Hulled Barley Using Near-Infrared Reflectance Spectroscopy and Partial Least Squares Discriminant Analysis. Sensors, 17(10), 2258. https://doi.org/10.3390/s17102258





