Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of m-SnO2 Powders
2.2. Fabrication of SnO2 Sensors
2.3. Measurement of Sensing Performance
3. Results and Discussion
3.1. Characterization of the c-SnO2 Powders and the m-SnO2 Powders
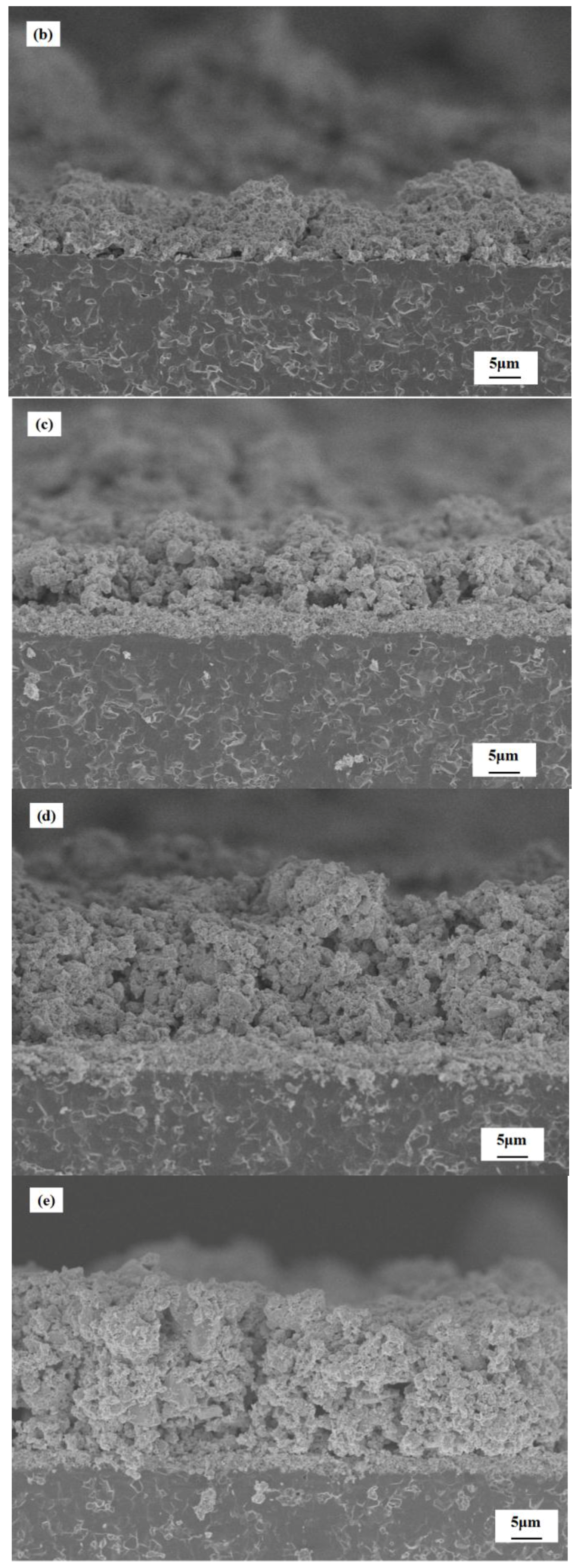
3.2. Characterization of Gas Sensors
3.3. The Resistance of the Gas Sensors in Air
3.4. Sensing Responses to the Testing Gas
3.5. The Response and Recovery Times of the Gas Sensors
3.6. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, S.; Klein, K.; Herkel, S.; Sirén, K. Approaches to enhance the energy performance of a zero-energy building integrated with a commercial-scale hydrogen fueled zero-energy vehicle under finnish and german conditions. Energy Convers. Manag. 2017, 142, 153–175. [Google Scholar] [CrossRef]
- Andronov, D.Y.; Arseniev, D.G.; Polyanskiy, A.M.; Polyanskiy, V.A.; Yakovlev, Y.A. Application of multichannel diffusion model to analysis of hydrogen measurements in solid. Int. J. Hydrogen Energy 2017, 42, 699–710. [Google Scholar] [CrossRef]
- Bockris, J.O.M. On hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2003, 28, 131–133. [Google Scholar] [CrossRef]
- Cardozacontrera, M.N.; Romoherrera, J.M.; Ríos, L.A.; Garcíagutiérrez, R.; Zepeda, T.A.; Contreras, O.E. Single ZnO Nanowire-Based Gas Sensors to Detect Low Concentrations of Hydrogen. Sensors 2015, 15, 30539–30544. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.; Jung, M.; Joo, S.; Kim, S. Silicon carbide-based hydrogen gas sensors for high-temperature application. Sensors 2013, 13, 13575–13583. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Wongchoosuk, C.; Wisitsoraat, A.; Phokharatkul, D.; Tuantranont, A.; Kerdcharoen, T. Multi-Walled Carbon Nanotube-Doped Tungsten Oxide Thin Films for Hydrogen Gas Sensing. Sensors 2010, 10, 7705–7715. [Google Scholar] [CrossRef] [PubMed]
- Krško, O.; Plecenik, T.; Roch, T.; Grančič, B.; Satrapinskyy, L.; Truchlý, M.; Ďurina, P.; Gregor, M.; Kúš, P.; Plecenik, A. Flexible highly sensitive hydrogen gas sensor based on a TiO2 thin film on polyimide foil. Sens. Actuators B Chem. 2017, 240, 1058–1065. [Google Scholar] [CrossRef]
- Kimura, Y.; Kimura, S.; Kojima, R.; Bitoh, M.; Abe, M.; Niwano, M. Micro-scaled hydrogen gas sensors with patterned anodic titanium oxide nanotube film. Sens. Actuators B Chem. 2013, 177, 1156–1160. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Umar, A.; Tomar, M.; Gupta, V. Custom designed metal anchored SnO2 sensor for H2 detection. Int. J. Hydrogen Energy 2017, 42, 4597–4609. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, V.C.; Nguyen, V.D.; Hoang, S.H.; Hugo, N.; Nguyen, D.H.; Nguyen, V.H. Fabrication of highly sensitive and selective H2 gas sensor based on SnO2 thin film sensitized with microsized Pd islands. J. Hazard. Mater. 2016, 301, 433–442. [Google Scholar] [PubMed]
- Ammar, A.H.; Abo-Ghazala, M.S.; Farag, A.A.M.; Abdel-Moniem, N.M.; Farag, E.-S.M. Effect of gas type, pressure and temperature on the electrical characteristics of Al-doped SnO2 thin films deposited by RGTO method for gas sensor application. Vacuum 2013, 94, 30–40. [Google Scholar] [CrossRef]
- Inyawilert, K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Ultra-sensitive and highly selective H2 sensors based on FSP-made Rh-substituted SnO2 sensing films. Sens. Actuators B Chem. 2017, 240, 1141–1152. [Google Scholar] [CrossRef]
- Bianchetti, M.F.; Arrieta, C.; Walsöe de Reca, N.E. Microstructural study of nanocrystalline pure and doped tin dioxide to be used for resistive gas sensors. Sens. Actuators B Chem. 2015, 217, 113–118. [Google Scholar] [CrossRef]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Ultra-sensitive H2 sensors based on flame-spray-made Pd-loaded SnO2 sensing films. Sens. Actuators B Chem. 2013, 176, 893–905. [Google Scholar] [CrossRef]
- Jang, B.-H.; Landau, O.; Choi, S.-J.; Shin, J.; Rothschild, A.; Kim, I.-D. Selectivity enhancement of SnO2 nanofiber gas sensors by functionalization with Pt nanocatalysts and manipulation of the operation temperature. Sens. Actuators B Chem. 2013, 188, 156–168. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Anggraini, S.A.; Fujio, Y.; Sato, T.; Breedon, M.; Miura, N. Stabilized zirconia-based sensor utilizing SnO2-based sensing electrode with an integrated Cr2O3 catalyst layer for sensitive and selective detection of hydrogen. Int. J. Hydrogen Energy 2013, 38, 305–312. [Google Scholar] [CrossRef]
- Tournier, G.; Pijolat, C. Selective filter for SnO-based gas sensor: Application to hydrogen trace detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Masuzawa, S.; Okazaki, S.; Maru, Y.; Mizutani, T. Catalyst-type-an optical fiber sensor for hydrogen leakage based on fiber bragg gratings. Sens. Actuators B Chem. 2015, 217, 151–157. [Google Scholar] [CrossRef]
- Yaqoob, U.; Uddin, A.S.M.I.; Chung, G.-S. Foldable hydrogen sensor using Pd nanocubes dispersed into multiwall carbon nanotubes-reduced graphene oxide network assembled on nylon filter membrane. Sens. Actuators B Chem. 2016, 229, 355–361. [Google Scholar] [CrossRef]
- Hyodo, T.; Abe, S.; Shimizu, Y.; Egashira, M. Gas-sensing properties of ordered mesoporous SnO2 and effects of coatings thereof. Sens. Actuators B Chem. 2003, 93, 590–600. [Google Scholar] [CrossRef]
- Pijolat, C.; Viricelle, J.P.; Tournier, G.; Montmeat, P. Application of membranes and filtering films for gas sensors improvements. Thin Solid Films 2005, 490, 7–16. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Lokhande, C.D. Chemical route to synthesis of mesoporous ZnO thin films and their liquefied petroleum gas sensor performance. J. Alloys Compd. 2011, 509, 10092–10097. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Manjula, P.; Satyanarayana, L.; Swarnalatha, Y.; Manorama, S.V. Raman and MASNMR studies to support the mechanism of low temperature hydrogen sensing by Pd doped mesoporous SnO2. Sens. Actuators B Chem. 2009, 138, 28–34. [Google Scholar] [CrossRef]
- Seftel, E.M.; Cool, P.; Lloyd-Spetz, A.; Lutic, D. Pt-doped semiconductive oxides loaded on mesoporous SBA-15 for gas sensing. Comptes Rendus Chim. 2014, 17, 717–724. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, W.; Fan, A.; Wei, D.; Liu, W.; Han, C.; Shen, Y.; Meng, D.; San, X. SnO2: Highly sensitive hydrogen sensors based on SnO2 nanomaterials with different morphologies. Int. J. Hydrogen Energy 2015, 40, 15773–15779. [Google Scholar] [CrossRef]
- Yeow, S.C.; Ong, W.L.; Wong, A.S.W.; Ho, G.W. Template-free synthesis and gas sensing properties of well-controlled porous tin oxide nanospheres. Sens. Actuators B Chem. 2009, 143, 295–301. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Liu, Y.; Ma, J.; Li, X.; Du, Y.; Lu, G. Ordered mesoporous Pd/SnO2 synthesized by a nanocasting route for high hydrogen sensing performance. Sens. Actuators B Chem. 2011, 160, 604–608. [Google Scholar] [CrossRef]
- Hayashi, M.; Hyodo, T.; Shimizu, Y.; Egashira, M. Effects of microstructure of mesoporous SnO2 powders on their H2 sensing properties. Sens. Actuators B Chem. 2009, 141, 465–470. [Google Scholar] [CrossRef]
- Gong, J.; Chen, Q.; Fei, W.; Seal, S. Micromachined nanocrystalline SnO2 chenical gas sensors for electronic nose. Sens. Actuators B Chem. 2004, 102, 117–125. [Google Scholar] [CrossRef]
















| Sample | Basic Layer | Modified Layer | Description |
|---|---|---|---|
| Material | Material | ||
| S(c) | c-SnO2 | / | one layer of c-SnO2 |
| S(m) | m-SnO2 | / | one layer of m-SnO2 |
| S(c/m1) | c-SnO2 | m-SnO2 | one layer of c-SnO2 and one layer of m-SnO2 |
| S(c/m2) | c-SnO2 | m-SnO2 | one layer of c-SnO2 and two layers of m-SnO2 |
| S(c/m3) | c-SnO2 | m-SnO2 | one layer of c-SnO2 and three layers of m-SnO2 |
| Gas | Temperature (°C) | Response Time (s) | Recovery Time (s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S(c) | S(m) | S(c/m1) | S(c/m2) | S(c/m3) | S(c) | S(m) | S(c/m1) | S(c/m2) | S(c/m3) | ||
| Ethanol | 200 | - | - | 286 | 496 | 549 | - | - | 292 | 519 | >600 |
| 250 | - | - | 252 | 277 | 180 | - | - | 281 | 355 | 349 | |
| 300 | 126 | 19 | 197 | 106 | 87 | 14 | 90 | 178 | 182 | 142 | |
| 350 | 115 | 41 | 127 | 289 | 430 | 26 | 271 | 246 | 235 | 226 | |
| 400 | 54 | 56 | 152 | 210 | 295 | 37 | 201 | 467 | 467 | 462 | |
| Benzene | 200 | - | - | >600 | >600 | >600 | - | - | >600 | >600 | >600 |
| 250 | - | - | 360 | 486 | >600 | - | - | 60 | 158 | >600 | |
| 300 | - | - | 110 | 225 | 403 | - | - | 14 | 12 | 13 | |
| 350 | - | - | 411 | 422 | 478 | - | - | 89 | 63 | 78 | |
| 400 | - | - | 173 | 218 | 293 | - | - | 207 | 103 | 115 | |
| Hydrogen | 200 | - | - | 344 | 343 | 349 | - | - | 84 | 216 | 233 |
| 250 | - | - | 261 | 162 | 146 | - | - | 387 | 406 | 404 | |
| 300 | 83 | 131 | 96 | 82 | 87 | 194 | >600 | 423 | 451 | 442 | |
| 350 | 80 | 144 | 87 | 110 | 121 | 165 | >600 | 568 | >600 | >600 | |
| 400 | 89 | 34 | 74 | 105 | 110 | 139 | >600 | 507 | 519 | 520 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, N.; Zhang, Q.; Zhang, S.; Zong, P.; Yang, F. Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers. Sensors 2017, 17, 2351. https://doi.org/10.3390/s17102351
Xue N, Zhang Q, Zhang S, Zong P, Yang F. Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers. Sensors. 2017; 17(10):2351. https://doi.org/10.3390/s17102351
Chicago/Turabian StyleXue, Niuzi, Qinyi Zhang, Shunping Zhang, Pan Zong, and Feng Yang. 2017. "Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers" Sensors 17, no. 10: 2351. https://doi.org/10.3390/s17102351
APA StyleXue, N., Zhang, Q., Zhang, S., Zong, P., & Yang, F. (2017). Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers. Sensors, 17(10), 2351. https://doi.org/10.3390/s17102351




