Detection of Stress Levels from Biosignals Measured in Virtual Reality Environments Using a Kernel-Based Extreme Learning Machine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.1.1. Participants
2.1.2. Stress-Inducing Task in VR Environments
2.1.3. Experimental Procedure
2.2. Feature Preparation
2.2.1. Heart-Rate Variability (HRV)
2.2.2. Skin Conductance (SC)
2.2.3. Skin Temperature (SKT)
2.3. Kernel-Based Extreme-Learning Machine (K-ELM)
2.4. Cross-Validation
2.5. Statistical Test
3. Evaluation
3.1. Correlations between Stress Levels and VR Videos
3.2. Classification Performance
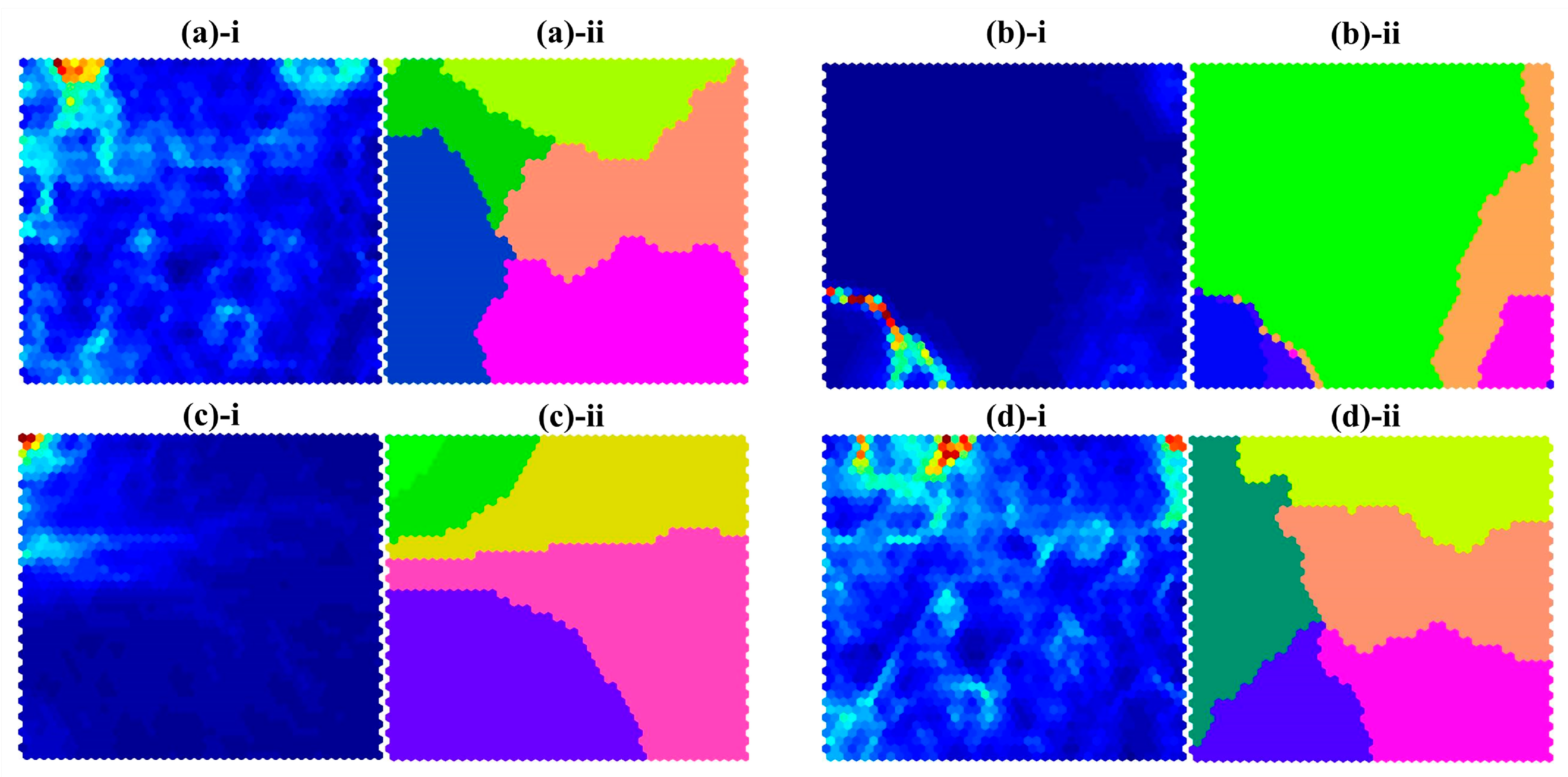
3.3. Self-Organizing Map (SOM) Analysis
3.4. Statistical Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blascovich, J.; Loomis, J.; Beall, A.C.; Swinth, K.R.; Hoyt, C.L.; Bailenson, J.N. Immersive virtual environment technology as a methodological tool for social psychology. Psychol. Inq. 2002, 13, 103–124. [Google Scholar] [CrossRef]
- Rothbaum, B.; Hodges, L.; Ready, D.; Graap, K.; Alarcon, R. Virtual reality exposure therapy for Vietnam veterans with posttraumatic stress disorder. J. Clin. Psychiatry 2001, 62, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Bullinger, A.H.; Hemmeter, U.M.; Stefani, O.; Angehrn, I.; Mueller-Spahn, F.; Bekiaris, E.; Wiederhold, B.K.; Sulzenbacher, H.; Mager, R. Stimulation of cortisol during mental task performance in a provocative virtual environment. Appl. Psychophysiol. Biofeedback 2005, 30, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Crifaci, G.; Tartarisco, G.; Billeci, L.; Pioggia, G.; Gaggioli, A. Innovative technologies and methodologies based on integration of virtual reality and wearable systems for psychological stress treatment. Int. J. Psychophysiol. 2012, 85, 402. [Google Scholar] [CrossRef]
- Jonsson, P.; Wallergard, M.; Osterberg, K.; Hansen, A.M.; Johansson, G.; Karlson, B. Cardiovascular and cortisol reactivity and habituation to a virtual reality version of the Trier Social Stress Test: A pilot study. Psychoneuroendocrinology 2010, 35, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-T.; Chung, I.-F.; Ko, L.-W.; Chen, Y.-C.; Liang, S.-F.; Duann, J.-R. EEG-based assessment of driver cognitive responses in a dynamic virtual-reality driving environment. IEEE Trans. Biomed. Eng. 2007, 54, 1349–1352. [Google Scholar] [PubMed]
- Villani, D.; Rotasperti, C.; Cipresso, P.; Triberti, S. Assessing the Emotional State of Job Applicants Through a Virtual Reality Simulation: A Psycho-Physiological Study. eHealth 360° 2017, 181, 119–126. [Google Scholar]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Rage; D. Appleton and Company: Oxford, UK, 1929. [Google Scholar]
- Ekman, P.; Levenson, R.W.; Friesen, W.V. Autonomic nervous system activity distinguishes among emotions. Science 1983, 221, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Reisine, T.D. Stress hormones: Their interaction and regulation. Science 1984, 224, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Kuo, C.; Cheng, H.; Buthpitiya, S.; Collins, P.; Griss, M. Activity-aware Mental Stress Detection Using Physiological Sensors. In International Conference on Mobile Computing Applications, and Services; Springer: Berlin/Heidelberg, Germany, 2010; pp. 211–230. [Google Scholar]
- Verkuil, B.; Brosschot, J.F.; de Beurs, D.P.; Thayer, J.F. Effects of explicit and implicit perseverative cognition on cardiac recovery after cognitive stress. Int. J. Psychophysiol. 2009, 74, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.G. Medical Instrumentation: Application and Design; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Yoon, Y.; Cho, J.; Yoon, G. Non-constrained blood pressure monitoring using ECG and PPG for personal healthcare. J. Med. Syst. 2009, 33, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Malik, M. Heart rate variability. Ann. Noninvasive Electrocardiol. 1996, 1, 151–181. [Google Scholar] [CrossRef]
- Von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Pechenizkiy, M.; Sidorova, N. What’s your current stress level? Detection of stress patterns from GSR sensor data. In Proceedings of the 2011 IEEE 11th International Conference on Data Mining Workshops (ICDMW), Washington, DC, USA, 11 December 2011; pp. 573–580. [Google Scholar]
- Lim, C.L.; Rennie, C.; Barry, R.J.; Bahramali, H.; Lazzaro, I.; Manor, B.; Gordon, E. Decomposing skin conductance into tonic and phasic components. Int. J. Psychophysiol. 1997, 25, 97–109. [Google Scholar] [CrossRef]
- Roelands, B.; Goekint, M.; Heyman, E.; Piacentini, M.F.; Watson, P.; Hasegawa, H.; Buyse, L.; Pauwels, F.; de Schutter, G.; Meeusen, R. Acute norepinephrine reuptake inhibition decreases performance in normal and high ambient temperature. J. Appl. Physiol. 2008, 105, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Johannes, S.; Rüdiger, P.; Marc, S.; Manfred, R. Towards Flexible Mobile Data Collection in Healthcare. In Proceedings of the 29th IEEE International Symposium on Computer-Based Medical Systems (CBMS 2016), Dublin, Ireland, 20–24 June 2016; pp. 181–182. [Google Scholar]
- Mazomenos, E.B. A low-complexity ECG feature extraction algorithm for mobile healthcare applications. IEEE J. Biomed. Health Inform. 2013, 17, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Thyagaraju, P.; Varma, R. Design and Development of a Physiological Stress Monitoring/Alert System Using a Wristband; Florida Institute Technology: Melbourne, FL, USA, 2016. [Google Scholar]
- Kim, J.; Cho, D.; Lee, K.J.; Lee, B. A real-time pinch-to-zoom motion detection by means of a surface EMG-based human-computer interface. Sensors 2014, 15, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Krakovská, A.; Mezeiová, K. Automatic sleep scoring: A search for an optimal combination of measures. Artif. Intell. Med. 2011, 53, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Min, B.; Kim, J.; Lee, B. EEG-based Prediction of Epileptic Seizures Using Phase Synchronization Elicited from Noise-Assisted Multivariate Empirical Mode Decomposition. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 25, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-B.; Wang, D.H.; Lan, Y. Extreme learning machines: A survey. Int. J. Mach. Learn. Cybern. 2011, 2, 107–122. [Google Scholar] [CrossRef]
- Huang, G.B.; Zhu, Q.Y.; Siew, C.K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef]
- Muschalla, B.; Linden, M.; Olbrich, D. The relationship between job-anxiety and trait-anxiety-A differential diagnostic investigation with the Job-Anxiety-Scale and the State-Trait-Anxiety-Inventory. J. Anxiety Disord. 2010, 24, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.X.; Berjot, S.; Deschamps, F. Clinical stress assessment using a visual analogue scale. Occup. Med. 2012, 62, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, C.; Lee, M. Adaptive threshold method for the peak detection of photoplethysmographic waveform. Comput. Biol. Med. 2009, 39, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Almeida, P.R.; Cunha, J.P.S.; Aguiar, A. Heart rate variability metrics for fine-grained stress level assessment. Comput. Methods Programs Biomed. 2017, 148, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Almeida, P.R.; Cunha, J.P.S.; Aguiar, A. Validation of a low intrusiveness heart rate sensor for stress assessment. Biomed. Phys. Eng. Express 2017, 3, 017004. [Google Scholar] [CrossRef]
- Clifford, G. Signal Processing Methods for Heart Rate Variability; University of Oxford: Oxford, UK, 2002. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, 2039–2049. [Google Scholar]
- Al-Angari, H.M.; Sahakian, A.V. Use of sample entropy approach to study heart rate variability in obstructive sleep apnea syndrome. IEEE Trans. Biomed. Eng. 2007, 54, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Melillo, P.; Bracale, M.; Pecchia, L. Nonlinear Heart Rate Variability features for real-life stress detection. Case study: Students under stress due to university examination. Biomed. Eng. Online 2011, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.M.; Trengove, C.; Johnston, P.; Cooper, T.; August, J.P.; Gordon, E. Separating individual skin conductance responses in a short interstimulus-interval paradigm. J. Neurosci. Methods 2005, 146, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Benedek, M.; Kaernbach, C. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 2010, 190, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Vinkers, C.H.; Penning, R.; Hellhammer, J.; Verster, J.C.; Klaessens, J.H.; Olivier, B.; Kalkman, C.J. The effect of stress on core and peripheral body temperature in humans. Stress 2013, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Haykin, S. Neural Networks and Learning Machines; Prentice-Hall: Englewood Cliffs, NJ, USA, 2009. [Google Scholar]
- Li, B.; Rong, X.; Li, Y. An improved kernel based extreme learning machine for robot execution failures. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006. [Google Scholar]
- Rao, C.R.; Mitra, S.K. Generalized Inverse of Matrices and Its Applications; Wiley: New York, NY, USA, 1972. [Google Scholar]






| Measure | Description | Section |
|---|---|---|
| PPG | ||
| Average heart rate | Time-domain measures | |
| Average NN intervals | ||
| SDNN | Standard deviation of NN intervals | |
| SDSD | Standard deviation of difference between adjacent NN intervals | |
| RMSSD | Square root of the mean of the sum of the squares of the difference between adjacent NN intervals | |
| pNN20 | (Number of pairs of adjacent NN intervals differing by more than 20 ms)/(total number of NN intervals) | |
| pNN50 | (Number of pairs of adjacent NN intervals differing by more than 50 ms)/(total number of NN intervals) | |
| Average of normalized low-frequency component (0.04–0.15 Hz) power | Frequency-domain measures | |
| Average of normalized high-frequency component (0.15–0.4 Hz) power | ||
| LF/HF | Ratio between averages of low-frequency and high-frequency powers | |
| ApEn (2, 0.2) | Approximate entropy of NN intervals (m = 2 r = 0.2 × SDNN) | Nonlinear measures |
| SampEn (2, 0.2) | Sample entropy of NN intervals (m = 2 r = 0.2 × SDNN) | |
| SD1 | Standard deviation of data perpendicular to the axis of line-of-identity in Poincaré plot | |
| SD2 | Standard deviation of data along the axis of line-of-identity in Poincaré plot | |
| SD1/SD2 | Ratio between SD1 and SD2 | |
| EDA | ||
| Average of total skin conductance (SC) signal | SC measure | |
| Average of total SC level (SCL) signal | SCL measures | |
| Difference between maximum SCL and minimum SCL | ||
| Average of total SC response (SCR) signal | SCR measures | |
| Maximum SCR signal | ||
| Number of peaks in the SCR signal | ||
| SKT | ||
| Average of total SKT signal | SKT measures | |
| Difference between maximum SKT and minimum SKT | ||
| Standard deviation of total SKT signal |
| Subject | Age | Sex | MIS-S | MOS-S | SES-S | |||
|---|---|---|---|---|---|---|---|---|
| STAI | VAS | STAI | VAS | STAI | VAS | |||
| BJY | 32 | M | 34 | 2 | 48 | 4 | 58 | 10 |
| KKH | 25 | M | 25 | 2 | 60 | 6 | 67 | 8 |
| NES | 25 | F | 25 | 2 | 48 | 6 | 65 | 10 |
| KSH | 28 | M | 28 | 2 | 64 | 8 | 71 | 6 |
| LJK | 30 | M | 30 | 2 | 52 | 4 | 68 | 6 |
| LSW | 25 | F | 25 | 2 | 68 | 8 | 71 | 6 |
| KHJ | 24 | F | 24 | 2 | 72 | 8 | 72 | 10 |
| SHL | 31 | M | 31 | 2 | 53 | 6 | 71 | 10 |
| KMH | 25 | F | 25 | 2 | 71 | 6 | 69 | 6 |
| JHR | 33 | F | 33 | 2 | 40 | 4 | 60 | 6 |
| JHM | 26 | F | 26 | 2 | 57 | 6 | 67 | 8 |
| JSH | 26 | M | 26 | 2 | 54 | 6 | 74 | 8 |
| Avg | 27.5 | 27.7 | 2.1 | 57.3 | 5.8 | 67.8 | 8 | |
| BA-S | MIS-S | MOS-S | SES-S | RE-S | |
|---|---|---|---|---|---|
| BA-S | 97.55% | 0.74% | 0.49% | 0.98% | 0.25% |
| MIS-S | 0.00% | 92.40% | 3.43% | 2.70% | 1.47% |
| MOS-S | 0.00% | 3.19% | 91.67% | 3.43% | 1.72% |
| SES-S | 0.25% | 1.72% | 4.17% | 91.42% | 2.45% |
| RE-S | 1.23% | 2.21% | 3.19% | 3.68% | 89.71% |
| BA-S | MIS-S | MOS-S | SES-S | RE-S | |
|---|---|---|---|---|---|
| BA-S | 96.32% | 1.23% | 0.74% | 1.23% | 0.49% |
| MIS-S | 1.72% | 93.63% | 2.70% | 1.96% | 0.00% |
| MOS-S | 0.25% | 2.45% | 94.12% | 2.45% | 0.74% |
| SES-S | 0.98% | 1.47% | 3.68% | 90.93% | 2.94% |
| RE-S | 0.25% | 0.00% | 1.47% | 2.94% | 95.34% |
| BA-S | MIS-S | MOS-S | SES-S | RE-S | |
|---|---|---|---|---|---|
| BA-S | 83.09% | 7.11% | 1.96% | 2.70% | 5.15% |
| MIS-S | 4.66% | 74.51% | 12.50% | 5.39% | 2.94% |
| MOS-S | 2.70% | 9.31% | 72.06% | 10.05% | 5.88% |
| SES-S | 1.72% | 5.15% | 12.25% | 73.77% | 7.11% |
| RE-S | 4.66% | 3.92% | 3.68% | 4.41% | 83.33% |
| BA-S | MIS-S | MOS-S | SES-S | RE-S | |
|---|---|---|---|---|---|
| BA-S | 98.53% | 0.98% | 0.00% | 0.25% | 0.25% |
| MIS-S | 0.00% | 95.34% | 1.72% | 1.47% | 1.47% |
| MOS-S | 0.00% | 2.45% | 95.10% | 2.21% | 0.25% |
| SES-S | 0.00% | 0.98% | 3.43% | 93.38% | 2.21% |
| RE-S | 0.25% | 1.72% | 1.23% | 2.70% | 94.12% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, D.; Ham, J.; Oh, J.; Park, J.; Kim, S.; Lee, N.-K.; Lee, B. Detection of Stress Levels from Biosignals Measured in Virtual Reality Environments Using a Kernel-Based Extreme Learning Machine. Sensors 2017, 17, 2435. https://doi.org/10.3390/s17102435
Cho D, Ham J, Oh J, Park J, Kim S, Lee N-K, Lee B. Detection of Stress Levels from Biosignals Measured in Virtual Reality Environments Using a Kernel-Based Extreme Learning Machine. Sensors. 2017; 17(10):2435. https://doi.org/10.3390/s17102435
Chicago/Turabian StyleCho, Dongrae, Jinsil Ham, Jooyoung Oh, Jeanho Park, Sayup Kim, Nak-Kyu Lee, and Boreom Lee. 2017. "Detection of Stress Levels from Biosignals Measured in Virtual Reality Environments Using a Kernel-Based Extreme Learning Machine" Sensors 17, no. 10: 2435. https://doi.org/10.3390/s17102435
APA StyleCho, D., Ham, J., Oh, J., Park, J., Kim, S., Lee, N. -K., & Lee, B. (2017). Detection of Stress Levels from Biosignals Measured in Virtual Reality Environments Using a Kernel-Based Extreme Learning Machine. Sensors, 17(10), 2435. https://doi.org/10.3390/s17102435





