Supramolecular Recognition of Escherichia coli Bacteria by Fluorescent Oligo(Phenyleneethynylene)s with Mannopyranoside Termini Groups
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Methods
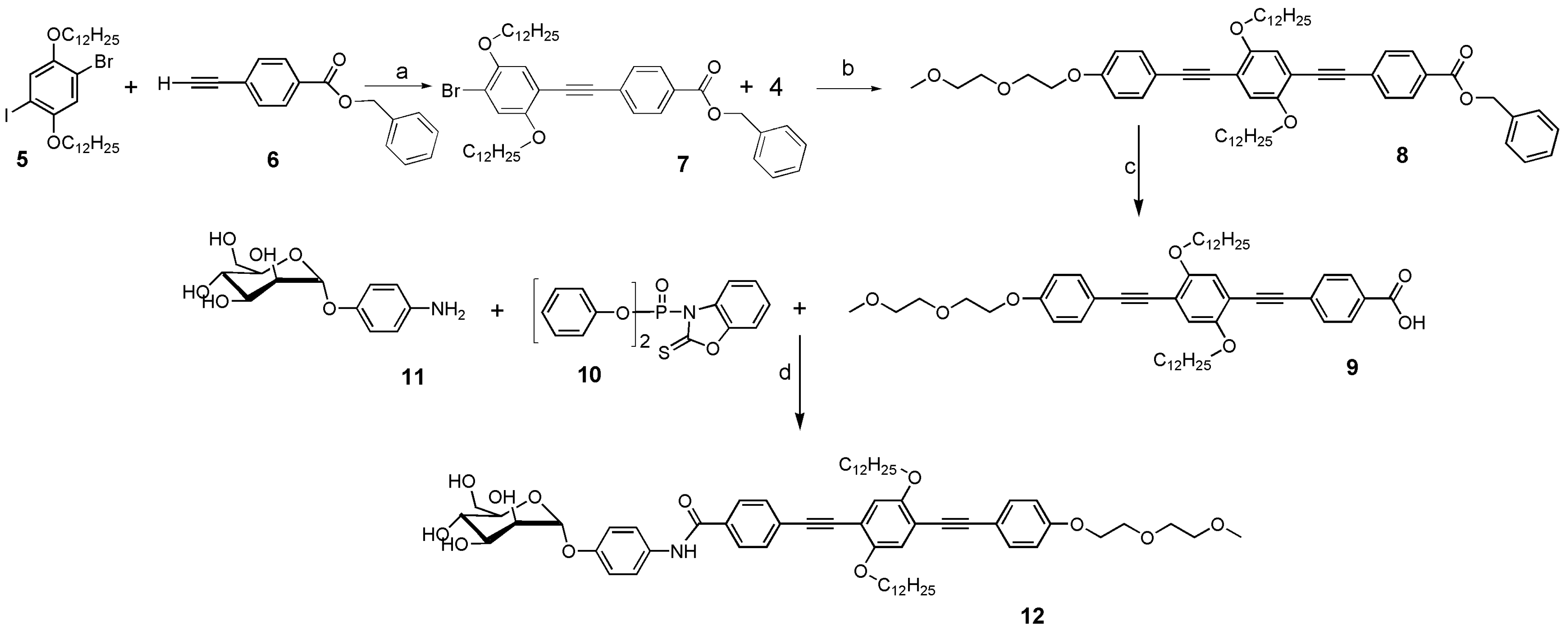
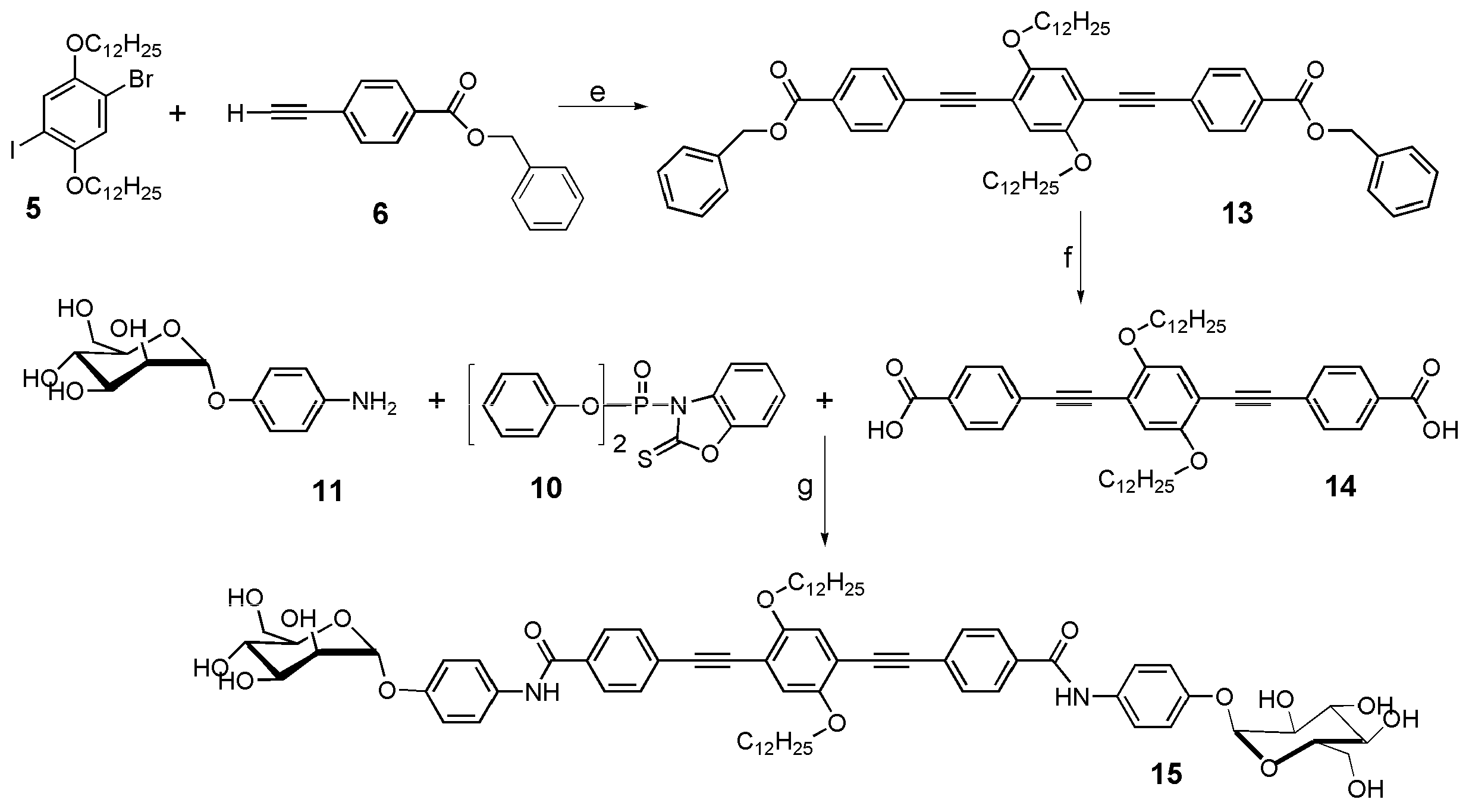
2.2. Synthesis
2.3. Bacteria Microscopic Characterization and Agglutination Assays
2.4. Recognition Assays By LSCM
2.5. SPR Tests
3. Results and Discussion
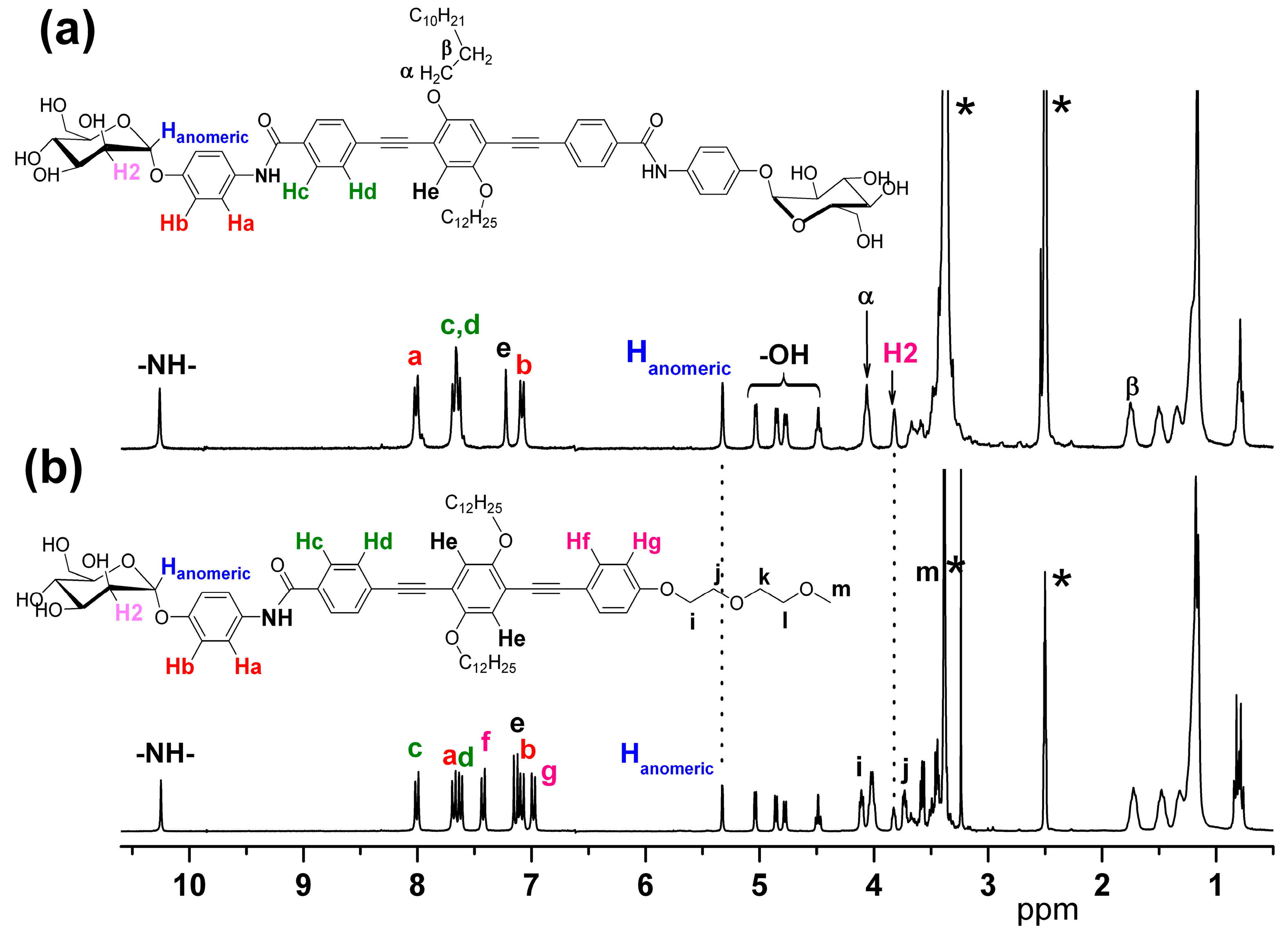
3.1. Synthesis and Characterization of the α-D-Mannosides Substituted Phenyleneethynylenes
3.2. Optical Properties in DMF
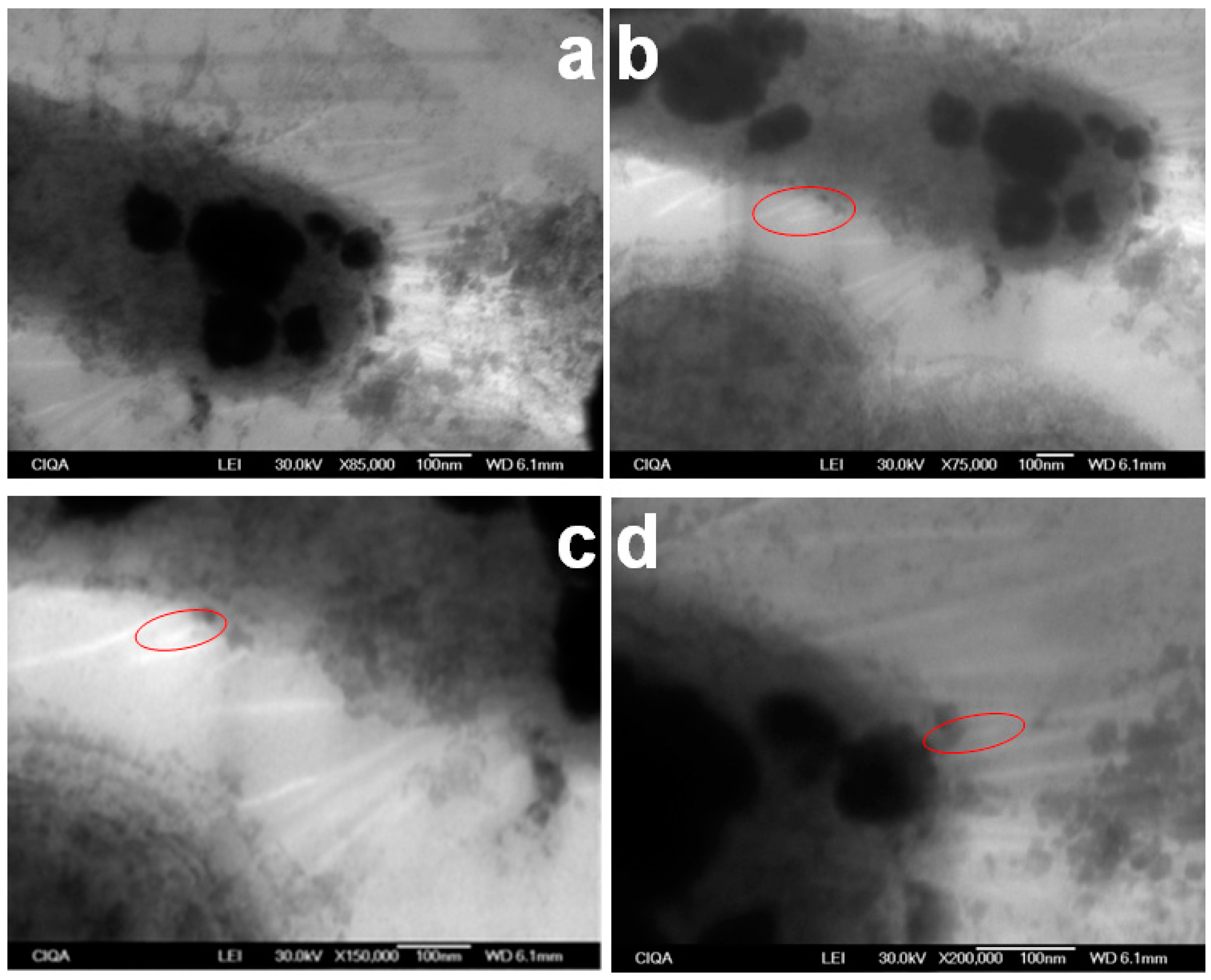
3.3. E. coli Propagation and Fimbriae I Characterization
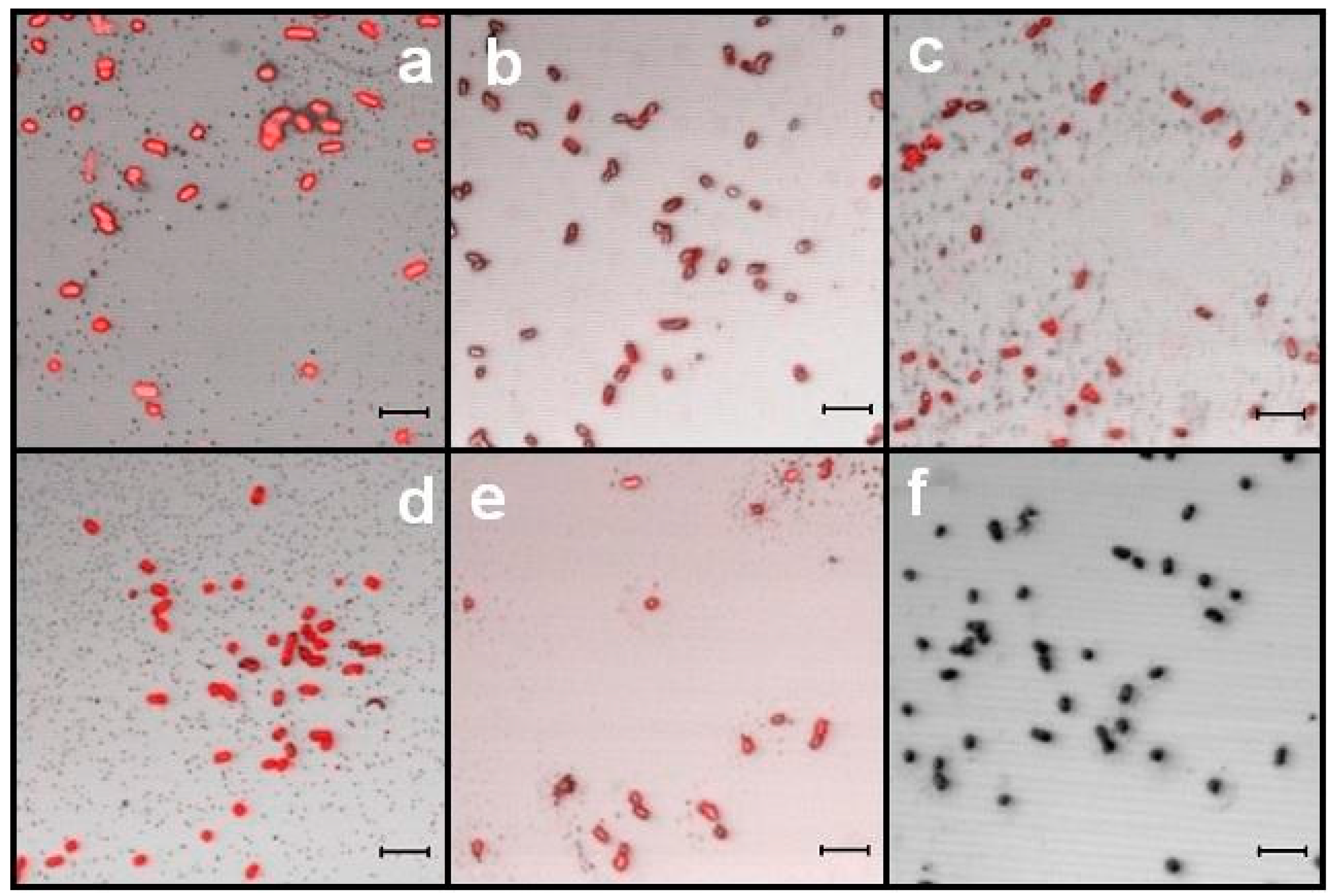
3.4. Recognition Assays by LSCM
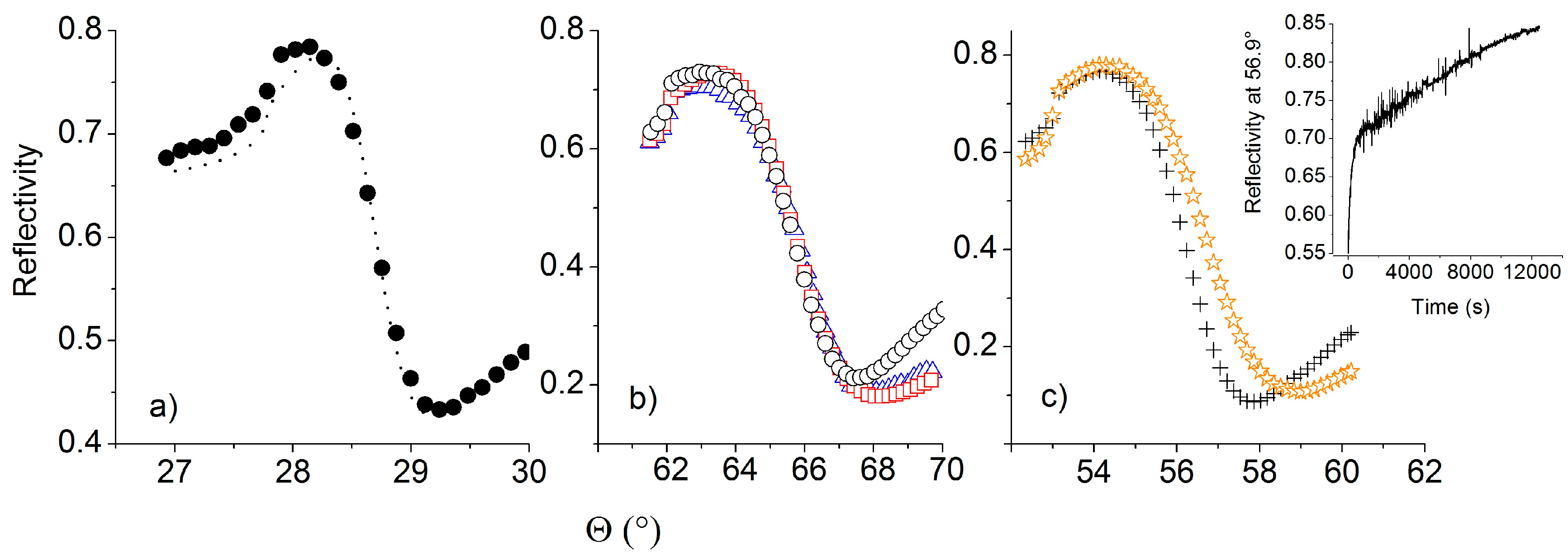
3.5. SPR Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Remaut, H.; Waksman, G. Structural biology of bacterial pathogenesis. Curr. Opin. Struct. Biol. 2004, 14, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A.; Lopez-Boado, Y.S.; Wilson, C.L.; Roth, R.; Parks, W.C.; Heuser, J.; Hultgren, S.J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998, 282, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Ronald, A.R.; Nicolle, L.E.; Stamm, E.; Krieger, J.; Warren, J.; Schaeffer, A.; Naber, K.G.; Hooton, T.M.; Johnson, J.; Chambers, S.; et al. Urinary tract infection in adults: Research priorities and strategies. Int. J. Antimicrob. Agents 2001, 17, 343–348. [Google Scholar] [CrossRef]
- Bouckaert, J.; Berglund, J.; Schembri, M.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.-S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesion. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar]
- Xue, C.; Jog, S.P.; Murthy, P.; Liu, H. Synthesis of Highly Water-Soluble Fluorescent Conjugated Glycopoly(p-phenylene)s for Lectin and Escherichia coli. Biomacromolecules 2006, 7, 2470–2474. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Velayudham, S.; Johnson, S.; Saha, R.; Smith, A.; Brewer, W.; Murthy, P.; Bagley, S.T.; Liu, H. Highly Water-Soluble, Fluorescent, Conjugated Fluorene-Based Glycopolymers with Poly(ethylene glycol)-Tethered Spacers for Sensitive Detection of Escherichia coli. Chem. Eur. J. 2009, 15, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Disney, M.D.; Zheng, J.; Swager, T.M.; Seeberger, P.H. Detection of Bacteria with Carbohydrate-Functionalized Fluorescent Polymers. J. Am. Chem. Soc. 2004, 126, 13343–13346. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.L.; Kim, I.-B.; Carson, B.L.; Tidbeck, B.; Bai, Y.; Lowary, T.L.; Tolbert, L.M.; Bunz, U.H.F. Sugar-Substituted Poly(p-phenyleneethynylene)s: Sensitivity Enhancement toward Lectins and Bacteria. Macromolecules 2008, 41, 7316–7320. [Google Scholar] [CrossRef]
- Phillips, R.L.; Kim, I.-B.; Tolbert, L.M.; Bunz, U.H.F. Fluorescence Self-Quenching of a Mannosylated Poly (p-phenyleneethynylene) Induced by Concanavalin A. J. Am. Chem. Soc. 2008, 130, 6952–6954. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.L.; Miranda, O.R.; You, C.-C.; Rotello, V.M.; Bunz, U.H.F. Rapid and Efficient Identification of Bacteria Using Gold-Nanoparticle—Poly (para-phenyleneethynylene) constructs. Angew. Chem. Int. Ed. 2008, 47, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.L.; Lam, M.C.W.; Wolf, M.O. Carbohydrate-Labeled Fluorescent Microparticles and Their Binding to Lectins. Bioconjugate Chem. 2006, 17, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Luo, F.-T.; Liu, H. Post-Polymerization Functionalization Approach for Highly Water-Soluble Well-Defined Regioregular Head-to-Tail Glycopolythiophenes. Macromolecules 2007, 40, 6863–6870. [Google Scholar] [CrossRef]
- Takasu, A.; Iso, K.; Dohmae, T.; Hirabayashi, T. Synthesis of Sugar-Substituted Poly (phenylenevinylene)s. Biomacromolecules 2006, 7, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Xiaowei, G.J. Surface plasmon resonance based biosensor technique: A review. Biophotonics 2012, 1–19. [Google Scholar] [CrossRef]
- Waswa, J.; Irudayaraj, J.; DebRoy, C. Direct detection of E. Coli O157:H7 in selected food systems by a surface plasmon resonance biosensor. LWT 2007, 40, 187–192. [Google Scholar] [CrossRef]
- Salminen, A.; Loimaranta, V.; Joosten, J.A.F.; Salam Khan, A.; Hacker, J.; Pieters, R.J.; Finne, J. Inhibition of P-fimbriated Escherichia coli adhesion by multivalent galabiose derivatives studied by a live-bacteria application of surface plasmon resonance. J. Antimicrob. Chemother. 2007, 60, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Zagorodko, O.; Bouckaert, J.; Dumych, T.; Bilyy, R.; Larroulet, I.; Yanguas Serrano, A.; Alvarez Dorta, D.; Gouin, S.G.; Dima Florin Oancea, S.-O.; Boukherroub, R.; et al. Surface Plasmon Resonance (SPR) for the Evaluation of Shear-Force-Dependent Bacterial Adhesion. Biosensors 2015, 5, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Wellens, A.; Shiao, T.C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. Mannosylated G(0) dendrimers with nanomolar affinities to Escherichia coli FimH. Chem. Med. Chem. 2007, 2, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.-F.; Taganna, J.; Bouckaert, J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH—Towards novel bacterial antiadhesives. Chem. Commun. 2011, 47, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative Fluorescence Quantum Yields Using a Computer-controlled Luminescence Spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Sacconi, A.; Moncelli, M.R.; Margheri, G.; Tadini-Buoninsegni, F. Enhanced Adsorption of Ca-ATPase Containing Vesicles on a Negatively Charged Solid-Supported-Membrane for the Investigation of Membrane Transporters. Langmuir 2013, 29, 13883–13889. [Google Scholar] [CrossRef] [PubMed]
- Dondoni, A.; Marra, A.; Zampolli, M.G. Synthesis of All Carbon Linked Glycoside Clusters Round Benzene Scaffold via Sonogashira-Heck-Cassar Cross-Coupling of Iodobenzenes with Ethynyl C-Glycosides. Synlett 2002, 11, 1850–1854. [Google Scholar] [CrossRef]
- Ratner, D.M.; Plante, O.J.; Seeberger, P.H. A Linear Synthesis of Branched High-Mannose Oligosaccharides from the HIV-1 Viral Surface Envelope Glycoprotein gp120. Eur. J. Org. Chem. 2002, 5, 826–833. [Google Scholar] [CrossRef]
- Alzeer, J.; Vasella, A. Oligosaccharide Analogues of Polysaccharides. Part 2. Regioselective deprotection of monosaccharide-derived monomers and dimers. Helv. Chim. Acta 1995, 78, 177–193. [Google Scholar] [CrossRef]
- Ziener, U.; Godt, A. Synthesis and Characterization of Monodisperse Oligo (phenyleneethynylene)s. J. Org. Chem. 1997, 62, 6137–6141. [Google Scholar]
- Castruita, G.; Arias, E.; Moggio, I.; Pérez, F.; Medellin, D.; Torres, R.; Ziolo, R.; Olivas, A.; Giorgetti, E.; Muniz-Miranda, M. Synthesis, optical properties and supramolecular order of p-conjugated 2, 5-di (alcoxy) phenyleneethynylene oligomers. J. Mol. Struct. 2009, 936, 177–186. [Google Scholar] [CrossRef]
- García, L.A.; Arias, E.; Moggio, I.; Romero, J.; Ledezma, A.; Ponce, A.; Pérez, O. Fluorescent core-sheath fibers by electrospinning of a phenyleneethynylene/poly(styrene-co-maleimide) blend. Polymer 2011, 52, 5326–5334. [Google Scholar] [CrossRef]
- Sudeep, P.K.; James, P.V.; Thomas, K.G.; Kamat, P.V. Singlet and Triplet Excited-State Interactions and Photochemical Reactivity of Phenyleneethynylene Oligomers. J. Phys. Chem. A 2006, 110, 5642–5649. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.L.; Spears, P.A.; Havell, E.A.; Hamrick, T.S.; Horton, J.R.; Orndorff, P.E. Characterization of Escherichia coli type 1 pilus mutants with altered binding specificities. J. Bacteriol. 2001, 183, 4099–4102. [Google Scholar] [CrossRef] [PubMed]
- Krogfelt, K.A.; Bergmans, H.; Klemm, P. Direct Evidence that the FimH Protein is the Mannose-Specific Adhesin of Escherichia coli Type 1 Fimbriae. Infect. Immun. 1990, 58, 1995–1998. [Google Scholar] [PubMed]
- Salit, I.E.; Gotschlich, E.C. Hemagglutination by purified type I Escherichia coli pili. J. Exp. Med. 1977, 146, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Eshdat, Y.; Speth, V.; Jann, K. Participation of Pili and Cell Wall Adhesin in the Yeast Agglutination Activity of Escherichia coli. Infect. Immun. 1981, 34, 980–986. [Google Scholar] [PubMed]
- Meza, D.; Arias, E.; Moggio, I.; Romero, J.; Mata, J.M.; Jiménez-Barrera, R.M.; Ziolo, R.; Rodríguez, O.; Ottonelli, M. Synthesis and photophysical and supramolecular study of π-conjugated (diethylene glycol methyl ether) benzoateethynylene oligomers and polymers. Polym. Chem. 2015, 6, 1639–1648. [Google Scholar] [CrossRef]
- Pitarke, J.M.; Silkin, V.M.; Chulkov, E.V.; Echenique, P.M. Theory of surface plasmons and surface -plasmon polaritons. Rep. Prog. Phys. 2007, 70, 1–87. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Myszka, D. Surface plasmon resonance. In Optical Biosensors: Present and Future; Ligler, F.S., Taitt, C.A.R., Eds.; Gulf Professional Publishing: Washington, DC, USA, 2002; pp. 207–251. [Google Scholar]
- Guo, X. Surface plasmon resonance based biosensor technique: A review. J. Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Sarkar, A. Immobilization of bio-macromolecules on self-assembled monolayers: Methods and sensor applications. Chem. Soc. Rev. 2011, 40, 2567–2592. [Google Scholar] [CrossRef] [PubMed]
- Agoston, R.; Emad, L.I.; Sivanesan, A.; Lott, W.B.; Sillence, M.; Steel, R. Rapid isolation and detection of erythropoietin in blood plasma by magnetic core gold nanoparticles and portable Raman spectroscopy. Nanomed. NBM 2016, 12, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Rakic, A.D. Algorithm for the determination of intrinsic optical constants of metal films: application to aluminum. Appl. Opt. 1995, 34, 4755–4767. [Google Scholar] [CrossRef] [PubMed]
- Malitson, I.H.; Dodge, M.J. Refractive Index and Birefringence of Synthetic Sapphire. J. Opt. Soc. Am. 1972, 62, 1405A. [Google Scholar]
- Cairns, G.F.; McNeill, D.A.; Dawson, P. Application of surface plasmon polaritons in the laser ablation and characterisation of thin aluminum films. Surf. Sci. 1999, 429, 117–126. [Google Scholar] [CrossRef]
- Tabassum, R.; Gupta, B.D. SPR based fiber-optic sensor with enhanced electric field intensity and figure of merit using different single and bimetallic configurations. Opt. Commun. 2016, 367, 23–34. [Google Scholar] [CrossRef]
- Flanagan, M.T.; Pantell, R.H. Surface Plasmon Resonance and immunosensors. Elect. Lett. 1984, 20, 968–970. [Google Scholar] [CrossRef]
- Taylor, A.D.; Yu, Q.M.; Chen, S.F.; Homola, J.; Jiang, S.Y. Comparison of E. coli O157:H7 preparation methods used for detection with surface plasmon resonance sensor. Sens. Actuators B Chem. 2005, 107, 202–208. [Google Scholar] [CrossRef]
- Taylor, A.D.; Ladd, J.; Yu, Q.M.; Chen, S.F.; Homola, J.; Jiang, S.Y. Quantitative and simultaneous detection of four foodborne bacterial pathogens with a multi-channel SPR sensor. Biosens. Bioelectron. 2006, 22, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Tawil, N.; Sacher, E.; Mandeville, R.; Meunier, M. Surface Plasmon resonance detection of E. coli and methicillin-resistant S. aureus using bacteriophages. Biosens. Bioelectron. 2012, 37, 24–29. [Google Scholar]
- Yu, F.; Persson, B.; Lofås, S.; Knoll, S. Attomolar Sensitivity in Bioassays Based on Surface Plasmon Fluorescence Spectroscopy. J. Am. Chem. Soc. 2004, 126, 8902–8903. [Google Scholar] [CrossRef] [PubMed]
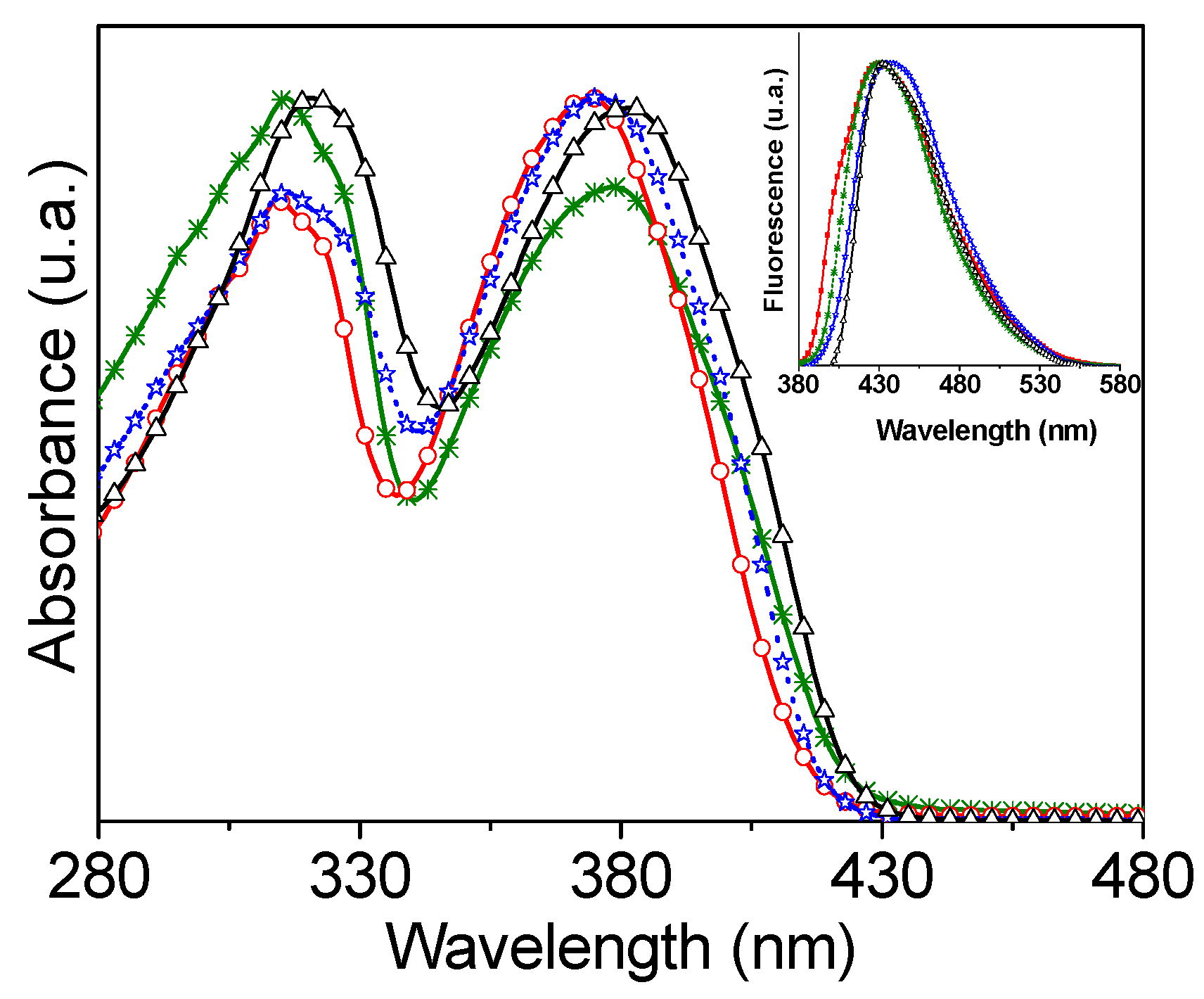


| PEs | λabs (nm) | ε 104 (M−1 cm—1) | λemiss (nm) | φ |
|---|---|---|---|---|
| 8 | 380 | 7.92 | 457 | 0.89 |
| 9 | 314,374 | 2.65 | 430 | 0.94 |
| 12 | 316,376 | 2.14 | 438 | 0.05 |
| 13 | 385 | 4.98 | 450 | 1.00 |
| 14 | 316,378 | 1.84 | 430 | 0.67 |
| 15 | 321,381 | 9.12 | 432 | 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, E.; Méndez, M.T.; Arias, E.; Moggio, I.; Ledezma, A.; Romero, J.; Margheri, G.; Giorgetti, E. Supramolecular Recognition of Escherichia coli Bacteria by Fluorescent Oligo(Phenyleneethynylene)s with Mannopyranoside Termini Groups. Sensors 2017, 17, 1025. https://doi.org/10.3390/s17051025
Arias E, Méndez MT, Arias E, Moggio I, Ledezma A, Romero J, Margheri G, Giorgetti E. Supramolecular Recognition of Escherichia coli Bacteria by Fluorescent Oligo(Phenyleneethynylene)s with Mannopyranoside Termini Groups. Sensors. 2017; 17(5):1025. https://doi.org/10.3390/s17051025
Chicago/Turabian StyleArias, Enrique, Maria Teresa Méndez, Eduardo Arias, Ivana Moggio, Antonio Ledezma, Jorge Romero, Giancarlo Margheri, and Emilia Giorgetti. 2017. "Supramolecular Recognition of Escherichia coli Bacteria by Fluorescent Oligo(Phenyleneethynylene)s with Mannopyranoside Termini Groups" Sensors 17, no. 5: 1025. https://doi.org/10.3390/s17051025
APA StyleArias, E., Méndez, M. T., Arias, E., Moggio, I., Ledezma, A., Romero, J., Margheri, G., & Giorgetti, E. (2017). Supramolecular Recognition of Escherichia coli Bacteria by Fluorescent Oligo(Phenyleneethynylene)s with Mannopyranoside Termini Groups. Sensors, 17(5), 1025. https://doi.org/10.3390/s17051025