New Frontiers for Applications of Thermal Infrared Imaging Devices: Computational Psychopshysiology in the Neurosciences
Abstract
:1. Introduction
2. Infrared Sensors Technology
2.1. Modern IR Thermal Detectors
2.2. Theory of Thermographic Measurement
- Emission from the object, i.e., :
- Reflected emission from ambient source, i.e., , where (1 − ε) is the reflectance of the object (it is assumed that the temperature is the same for all emitting surfaces within the half sphere seen from a point on the object’s surface);
- Emission from the atmosphere, i.e., , where (1 − τ) is the emissivity of the atmosphere.
3. Applications in Psychophysiology
3.1. General Procedures for Thermal Imaging on Human Body
- The usage of vasomotor substances (i.e., coffee, tea, alchool, drugs, tobacco) has to be avoided by the subjects the day of the experimental session [41]. The effects of the intake of these substances would influence the cutaneous thermal pattern.
- The region of interset for the measurement has to be depilated at least 4 h before the examination and the usage of moisturizing cream, make up or nail polish (in case of measurement on hands or feet) has to be avoided [41].
- When executing a thermal imaging measurement, it is mandatory to control the tempearture and humidity of the experimetal room. International Academy of Thermology (IACT) guidelines [41] suggest a temperature range of 18–23 °C and a controlled humidity range. A humidity range between 40% and 70% is reported in [40]. It is adviceble to execute the measurement in a large room (minimal room size is 2 × 3 m [40]) with no direct ventilation on the subject and no direct sunlight (no windows or with curtains or blinds).
- The distance between the subject and the camera should be enough to fill the viewable image area as to maintain adequate spatial resolution and interpretation accuracy, and the camera has to be as much as possible orthogonal to the plane of the region of investigation, to maximize the flux of thermal energy revealed by the camera [41].
- Moreover, it is necessary an acclimatization phase of the subject within the 15 min before the experimental session [41]. This phase is usefull both for thermal acclimatization and equilibrium with the experimetal room and stabilization of the emotianal status of the subjects.
3.2. Computational Physiology
3.2.1. Cardiac Pulse
3.2.2. Breathing Rate
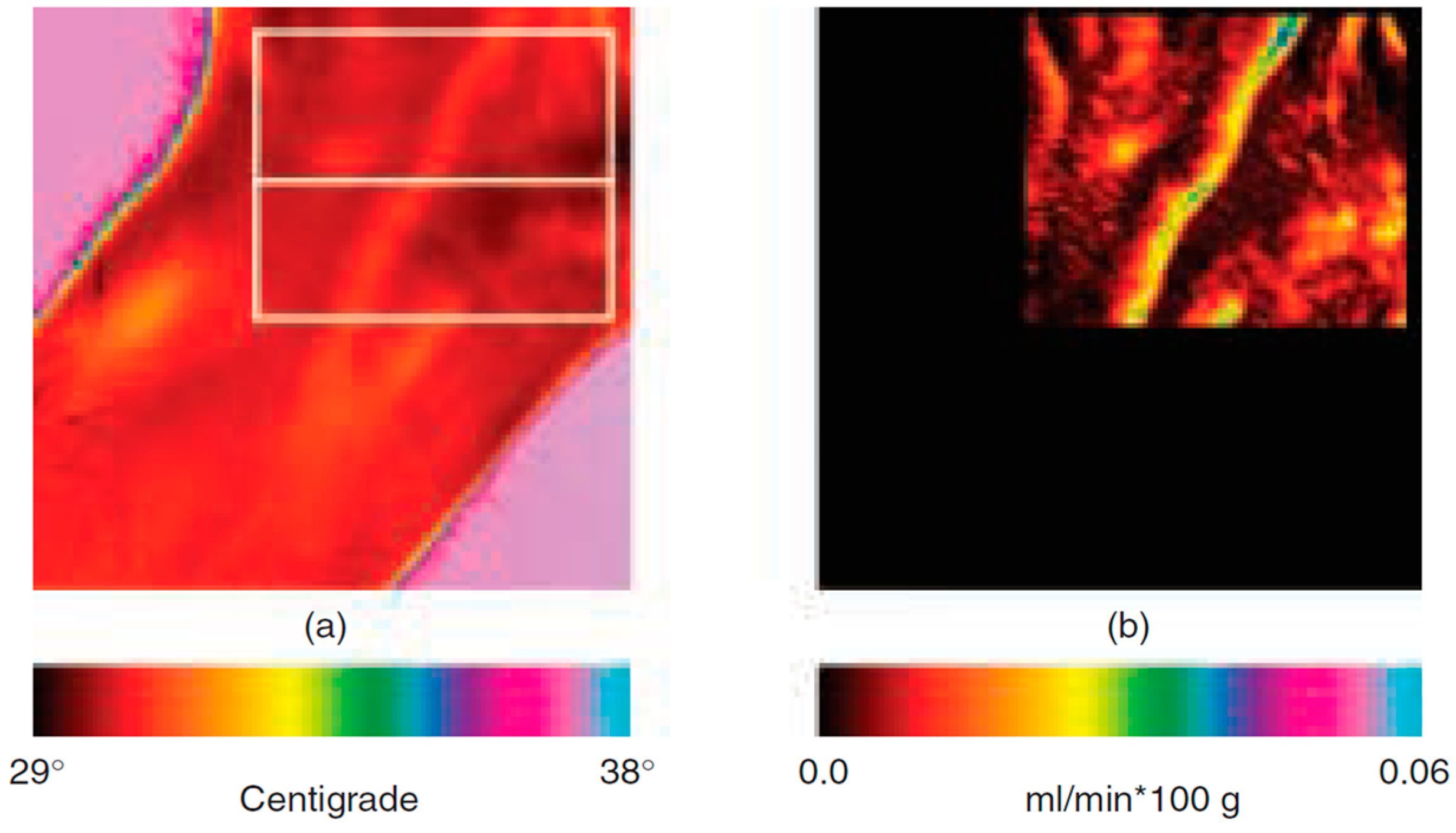
3.2.3. Cutaneous Blood Perfusion
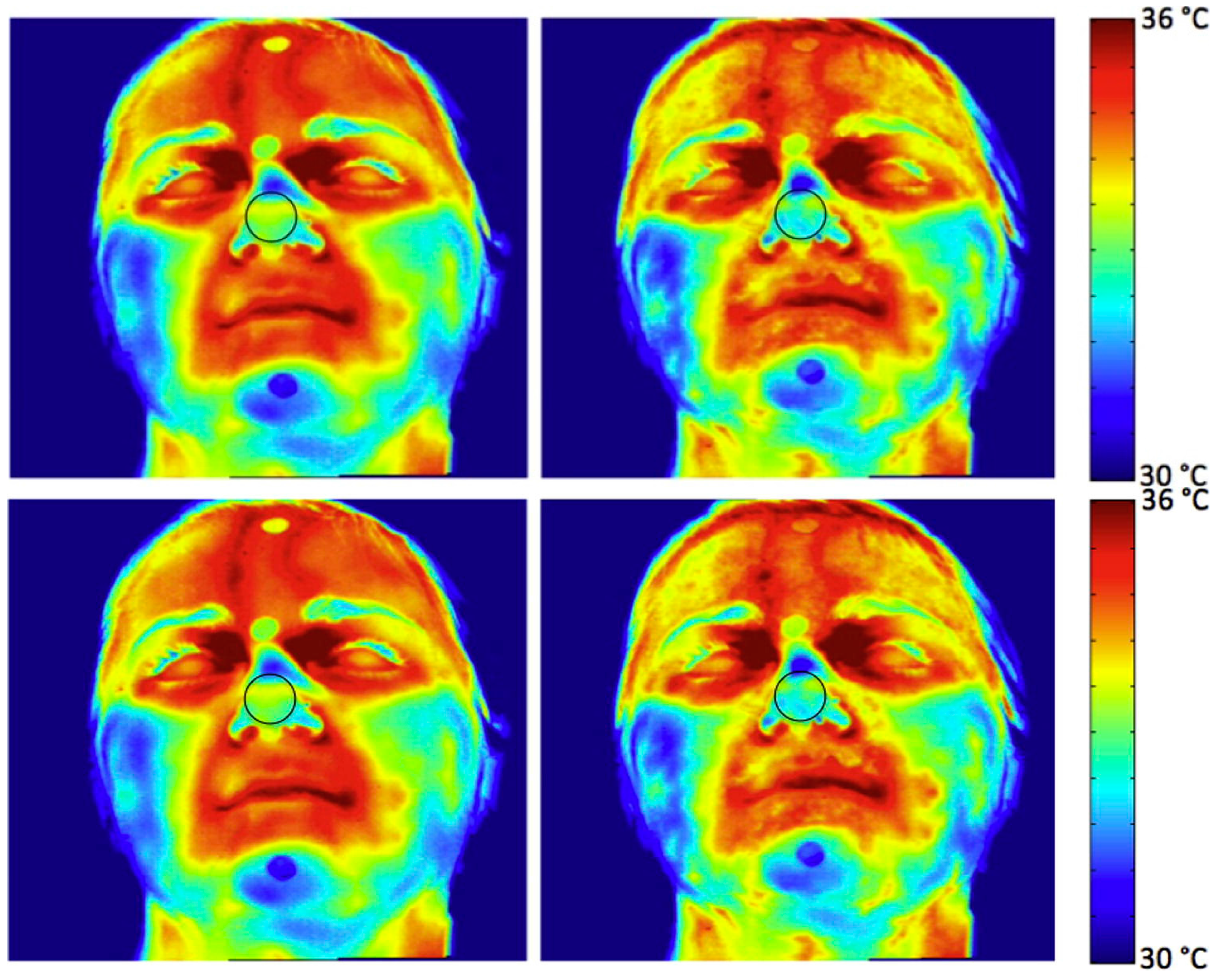
3.2.4. Sudomotor Response
3.3. Computational Psychophysiology
3.3.1. Stress Response
3.3.2. Social Interactions
4. Discussion
5. Conclusions
Conflicts of Interest
Appendix A. Measurement Properties and Specification of IR Thermal Camera
References
- Ayata, D.; Yaslan, Y.; Kamasak, M. Emotion Recognition via Galvanic Skin Response: Comparison of Machine Learning Algorithms and Feature Extraction Methods. In Proceedings of the Medical Technologies National Congress (TIPTEKNO), Antalya, Turkey, 27–29 October 2016; Volume 3093, p. 3129. [Google Scholar]
- Merla, A.; Romani, G.L. Thermal signatures of emotional arousal: a functional infrared imaging study. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; Available online: http://ieeexplore.ieee.org/abstract/document/4352270/ (accessed on 26 February 2017).
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural correlates of heart rate variability during emotion. Neuroimage 2009, 44, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Iani, C.; Gopher, D.; Lavie, P. Effects of task difficulty and invested mental effort on peripheral vasoconstriction. Psychophysiology 2004, 41, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Merla, A.; Di Donato, L.; Rossini, P.M.; Romani, G.L. Emotion detection through functional infrared imaging: preliminary results. Biomed. Tech. 2004, 48, 284–286. [Google Scholar]
- Shrivastava, D.; Vaughan, J.T. A generic bioheat transfer thermal model for a perfused tissue. J. Biomech. Eng. 2009, 131, 074506. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Singh, R.; Repaka, R.; Mishra, S.C. Conventional and newly developed bioheat transport models in vascularized tissues: A review. J. Therm. Biol. 2013, 38, 107–125. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [PubMed]
- Murthy, R.; Pavlidis, I. Noncontact measurement of breathing function. IEEE Eng. Med. Biol. Mag. 2006, 25, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Garbey, M.; Sun, N.; Merla, A.; Pavlidis, I. Contact-free measurement of cardiac pulse based on the analysis of thermal imagery. IEEE Trans. Biomed. Eng. 2007, 54, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.; Dowdall, J.; Sun, N.; Puri, C.; Fei, J.; Garbey, M. Interacting with human physiology. Comp. Vis. Image Underst. 2007, 108, 150–170. [Google Scholar] [CrossRef]
- Shastri, D.; Merla, A.; Tsiamyrtzis, P.; Pavlidis, I. Imaging facial signs of neurophysiological responses. IEEE Trans. Biomed. Eng. 2009, 56, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.; Tsiamyrtzis, P.; Shastri, D.; Wesley, A.; Zhou, Y.; Lindner, P.; Buddharaju, P.; Joseph, R.; Mandapati, A.; Dunkin, B.; et al. Fast by nature-how stress patterns define human experience and performance in dexterous tasks. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Merla, A. Method and System for the Control of the Residual Efficiency of the Interaction Man-Vehicle. European Patent EP13425145, 6 November 2013. [Google Scholar]
- Engert, V.; Merla, A.; Grant, J.A.; Cardone, D.; Tusche, A.; Singer, T. Exploring the use of thermal infrared imaging in human stress research. PLoS ONE 2014, 9, e90782. [Google Scholar] [CrossRef] [PubMed]
- Krzywicki, A.T.; Berntson, G.G.; O’Kane, B.L. A non-contact technique for measuring eccrine sweat gland activity using passive thermal imaging. Int. J. Psychophysiol. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.B.; Skipper, J.A.; Petkie, D. Thermal imaging to detect physiological indicators of stress in humans. Proc. SPIE 2013. [Google Scholar] [CrossRef]
- Dowdall, J.; Pavlidis, I.T.; Tsiamyrtzis, P. Coalitional tracking in facial infrared imaging and beyond. In Proceedings of the Conference on Computer Vision and Pattern Recognition Workshop, New York, NY, USA, 17–22 June 2006; p. 134. [Google Scholar]
- Zhou, Y.; Tsiamyrtzis, P.; Pavlidis, I.T. Tissue tracking in thermo-physiological imagery through spatio-temporal smoothing. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1092–1099. Available online: http://link.springer.com/chapter/10.1007/978-3-642-04271-3_132 (accessed on 26 February 2017).
- Merla, A.; Manini, B. Assessing emotions and arousal in developmental psychophysiology studies with thermal infrared imaging. In Discoveries about Infant Language Learning and “Readiness to Learn” from Integrated fNIRS, Thermal IR, Robot, and Avatar Sciences; Petitto, L.A., Ed.; Society for Research on Child Development: Austin, TX, USA, 2017. [Google Scholar]
- Petitto, L.A. Discoveries about Infant Language Learning and “Readiness to Learn” from Integrated fNIRS, Thermal IR, Robot, and Avatar Sciences; Society for Research on Child Development: Austin, TX, USA, 2017. [Google Scholar]
- Kozhevnikova, I.S.; Pankov, M.N.; Gribanov, A.V.; Startseva, L.F.; Ermoshina, N.A. The use of infrared thermography in modern medicine (Literature Review). Ekologiya Cheloveka/Hum. Ecol. 2017, 2, 39–46. [Google Scholar]
- Vardasca, R.; Mendes, J.G. Innovative Research in Thermal Imaging for Biology and Medicine; IGI Global: Hershey, PA, USA, 2017; pp. 1–340. [Google Scholar]
- Clay-Warner, J.; Robinson, D.T. Infrared thermography as a measure of emotion response. Emot. Rev. 2015, 7, 157–162. [Google Scholar] [CrossRef]
- Salazar-López, E.; Domínguez, E.; Ramos, V.J.; de la Fuente, J.; Meins, A.; Iborra, O.; Gálvez, G.; Rodríguez-Artacho, M.A.; Gómez-Milán, E. The mental and subjective skin: Emotion, empathy, feelings and thermography. Conscious. Cogn. 2015, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.H.; Sidek, S.N.I.; Rusli, N.; Fatai, S. Emotion detection from thermal facial imprint based on GLCM features. ARPN J. Eng. Appl. Sci. 2016, 11, 345–350. [Google Scholar]
- Cho, S.Y.; Wang, L.; Ong, W.J. Thermal imprint feature analysis for face recognition. In Proceedings of the IEEE International Symposium on Industrial Electronics, Seoul, Korea, 5–8 July 2009; pp. 1875–1880. [Google Scholar]
- Ghiass, R.S.; Arandjelović, O.; Bendada, A.; Maldague, X. Infrared face recognition: A comprehensive review of methodologies and databases. Pattern Recognit. 2014, 47, 2807–2824. [Google Scholar] [CrossRef]
- Hermosilla, G.; Gallardo, F.; Farias, G.; Martin, C.S. Fusion of visible and thermal descriptors using genetic algorithms for face recognition systems. Sensors 2015, 15, 17944–17962. [Google Scholar] [CrossRef] [PubMed]
- Bronzino, J.D. Medical Devices and Systems; CRC Press: Boca Raton, FL, USA, 2006; Available online: https://books.google.it/books?hl=it&lr=&id=sITMBQAAQBAJ&oi=fnd&pg=PP1&dq=Medical+Devices+and+Systems&ots=UoI0ig31gu&sig=Zymh_hD-yRiRXdExcYngIUPzi6M (accessed on 26 February 2017).
- Diakides, M.; Bronzino, J.D.; Peterson, D.R. Medical Infrared Imaging: Principles and Practices; CRC Press: Boca Raton, FL, USA, 2012; Available online: http://www.crcnetbase.com/doi/pdf/10.1201/b12938-1 (accessed on 26 February 2017).
- Hasinoff, S.W. Saturation (Imaging). In Computer Vision; Springer: Berlin/Heidelberg, Germany, 2014; pp. 699–701. Available online: http://link.springer.com/10.1007/978-0-387-31439-6_483 (accessed on 26 February 2017).
- Rogalski, A. Infrared Detectors; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Rogalski, A. Infrared detectors: An overview. Infrared Phys. Technol. 2002, 43, 187–210. [Google Scholar] [CrossRef]
- Rogalski, A. Infrared detectors for the future. Acta Phys. Pol. Ser. A Gen. Phys. 2009, 116, 389. [Google Scholar] [CrossRef]
- Rogalski, A. History of infrared detectors. Opto-Electron. Rev. 2012, 20, 279–308. [Google Scholar] [CrossRef]
- The Ultimate Infrared Handbook for R & D Professionals. Available online: http://www.flir.com/science/display/?id=69528 (accessed on 26 February 2017).
- Holst, G.C. Common Sense Approach to Thermal Imaging; SPIE Optical Engineering Press: Bellingham, WA, USA, 2000. [Google Scholar]
- Budzier, H.; Gerlach, G. Calibration of uncooled thermal infrared cameras. J. Sens. Sens. Syst. 2015, 4, 187–197. [Google Scholar] [CrossRef]
- Fernández-Cuevas, I.; Marins, J.C.B.; Lastras, J.A.; Carmona, P.M.G.; Cano, S.P.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of factors influencing the use of infrared thermography in humans: A review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- Thermography Guidelines (TG), Standards and Protocols in Clinical Thermographic Imaging. 2002. Available online: http://www.iact-org.org/professionals/thermog-guidelines.html. (accessed on 26 February 2017).
- Sun, N.; Pavlidis, I.; Garbey, M.; Fei, J. Harvesting the thermal cardiac pulse signal. Proceedings of International Conference on Medical Image Computing and Computer-Assisted Intervention, Copenhagen, Denmark, 1–6 October 2016; Springer: Berlin/Heidelberg, Germany, 2016; pp. 569–576. Available online: http://link.springer.com/10.1007%2F11866763_70 (accessed on 26 February 2017).
- Bourlai, T.; Buddharaju, P.; Pavlidis, I.; Bass, B. On enhancing cardiac pulse measurements through thermal imaging. In Proceedings of the 9th International Conference on Information Technology and Applications in Biomedicine, Larnaca, Cyprus, 4–7 November 2009; pp. 1–4. Available online: http://ieeexplore.ieee.org/abstract/document/5394334/ (accessed on 26 February 2017).
- Farag, A.A.; Chekmenev, S.Y. Non-Contact and Passive Measurement of Arterial Pulse through Thermal Ir Imaging, and Analysis of Thermal IR Imagery. U.S. Patent US20130109976 A1, 19 December 2012. [Google Scholar]
- Chekmenev, S.Y.; Farag, A.A.; Essock, E.A. Thermal imaging of the superficial temporal artery: An arterial pulse recovery model. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Minneapolis, MN, USA, 17–22 June 2007; pp. 1–6. Available online: http://ieeexplore.ieee.org/abstract/document/4270441/ (accessed on 26 February 2017).
- Kamal, A. Assessment of autonomic function in patients with rheumatoid arthritis using spectral analysis and approximate entropy method. Neurosciences 2007, 12, 136–139. [Google Scholar] [PubMed]
- Murthy, J.N.; van Jaarsveld, J.; Fei, J.; Pavlidis, I.; Harrykissoon, R.I.; Lucke, J.F.; Faiz, S.; Castriotta, R.J. Thermal infrared imaging: a novel method to monitor airflow during polysomnography. Sleep 2009, 32, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Gatto, R.G.; Porges, S.W. A novel method for extracting respiration rate and relative tidal volume from infrared thermography. Psychophysiology 2011, 48, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.; Pavlidis, I.; Tsiamyrtzis, P. Touchless monitoring of breathing function. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; Volume 1, pp. 1196–1199. Available online: http://ieeexplore.ieee.org/abstract/document/1403382/ (accessed on 26 February 2017).
- Chekmenev, S.Y.; Rara, H.; Farag, A.A. Non-contact, wavelet-based measurement of vital signs using thermal imaging. In Proceedings of the First International Conference on Graphics Vision, and Image Processing, Cairo, Egypt, 19–21 December 2005; pp. 107–112. Available online: http://www.cvip.louisville.edu/wwwcvip/research/publications/Pub_Pdf/2005_2/GVIP_2005.pdf (accessed on 26 February 2017).
- Fei, J.; Pavlidis, I. Thermistor at a distance: Unobtrusive measurement of breathing. IEEE Trans. Biomed. Eng. 2010, 57, 988–998. [Google Scholar] [PubMed]
- Goldman, L.J. Nasal airflow and thoracoabdominal motion in children using infrared thermographic video processing. Pediatr. Pulmonol. 2012, 47, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Pavlidis, I.; Murthy, J. Thermal vision for sleep apnea monitoring. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, London, UK, 20–24 September 2009; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1084–1091. Available online: http://link.springer.com/chapter/10.1007/978-3-642-04271-3_131 (accessed on 26 February 2017).
- Hu, M.H.; Zhai, G.T.; Li, D.; Fan, Y.Z.; Chen, X.H.; Yang, X.K. Synergetic use of thermal and visible imaging techniques for contactless and unobtrusive breathing measurement. J. Biomed. Opt. 2017, 22, 036006. [Google Scholar] [CrossRef] [PubMed]
- Merla, A.; Di Donato, L.; Romani, G.L.; Proietti, M.; Salsano, F. Comparison of thermal infrared and laser doppler imaging in the assessment of cutaneous tissue perfusion in scleroderma patients and healthy controls. Int. J. Immunopathol. Pharmacol. 2008, 21, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.; Levine, J. Thermal image analysis for polygraph testing. IEEE Eng. Med. Biol. Mag. 2002, 21, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Fujimasa, I.; Chinzei, T.; Saito, I. Converting far infrared image information to other physiological data. IEEE Eng. Med. Biol. Mag. 2000, 19, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, A.M.; Wang, H.; Wiedenbeck, B.; Liu, W.; Smith, P.D.; Elster, E. Functional assessment of hand vasculature using infrared and laser speckle imaging. In SPIE BiOS: Biomedical Optics; International Society for Optics and Photonics: Bellingham, WA, USA, 2009; p. 716919. [Google Scholar]
- Fontanella, L.; Ippoliti, L.; Merla, A. Multiresolution Karhunen Loéve analysis of galvanic skin response for psycho-physiological studies. Metrika 2012, 75, 287–309. [Google Scholar] [CrossRef]
- Merla, A. Computational physiology in a thermal image setting. Proceedings of 5th Conference on Complex Models and Computational Intensive Methods for Estimation and Prediction (S.Co. ’07), Venice, Italy, 6–8 September 2007; pp. 338–343. [Google Scholar]
- Di Giacinto, A.; Brunetti, M.; Sepede, G.; Ferretti, A.; Merla, A. Thermal signature of fear conditioning in mild post traumatic stress disorder. Neuroscience 2014, 266, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Or, C.K.; Duffy, V.G. Development of a facial skin temperature-based methodology for non-intrusive mental workload measurement. Occup. Ergon. 2007, 7, 83–94. [Google Scholar]
- Pavlidis, I.; Dcosta, M.; Taamneh, S.; Manser, M.; Ferris, T.; Wunderlich, R.; Akleman, E.; Tsiamyrtzis, P. Dissecting Driver Behaviors under Cognitive, Emotional, Sensorimotor, and Mixed Stressors. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Puri, C.; Olson, L.; Pavlidis, I.; Levine, J.; Starren, J. StressCam: Non-contact measurement of users’ emotional states through thermal imaging. In Proceedings of the Extended Abstracts on Human Factors in Computing Systems, Portland, OR, USA, 2–7 April 2005; pp. 1725–1728. Available online: http://dl.acm.org/citation.cfm?id=1057007 (accessed on 26 February 2017).
- Zhu, Z.; Tsiamyrtzis, P.; Pavlidis, I. Forehead thermal signature extraction in lie detection. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; pp. 243–246. Available online: http://ieeexplore.ieee.org/abstract/document/4352269/ (accessed on 26 February 2017).
- Kang, J.; McGinley, J.A.; McFadyen, G.; Babski-Reeves, K. Determining learning level and effective training times using thermography. In Proceedings of the Army Science Conference, Orlando, FL, USA, 27–30 November 2006; Available online: http://insite.cavs.msstate.edu/publications/docs/2006/07/205Student%20paper%20(ASC).pdf (accessed on 26 February 2017).
- Hines, J.; Brown, G.E. A standard stimulant for measuring vasomotor reactions: Its application in the study of hypertension. In Proceedings of the Staff Meetings of the Mayo Clinic; Mayo Clinic: Rochester, MN, USA, 1932; Volume 7, pp. 332–335. [Google Scholar]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The “Trier Social Stress Test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Aureli, T.; Grazia, A.; Cardone, D.; Merla, A. Behavioral and facial thermal variations in 3-to 4-month-old infants during the Still-Face Paradigm. Front. Psychol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Tronick, E.; Als, H.; Adamson, L.; Wise, S.; Brazelton, T.B. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J. Am. Acad. Child Psychiatry 1978, 17, 1–13. [Google Scholar] [CrossRef]
- Ebisch, S.J.; Aureli, T.; Bafunno, D.; Cardone, D.; Romani, G.L.; Merla, A. Mother and child in synchrony: Thermal facial imprints of autonomic contagion. Biol. Psychol. 2012, 89, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Manini, B.; Cardone, D.; Ebisch, S.J.H.; Bafunno, D.; Aureli, T.; Merla, A. Mom feels what her child feels: Thermal signatures of vicarious autonomic response while watching children in a stressful situation. Front. Hum. Neurosci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Güney, Z.O.; Sattel, H.; Cardone, D.; Merla, A. Assessing embodied interpersonal emotion regulation in somatic symptom disorders: A case study. Front. Psychol. 2015, 6. [Google Scholar] [CrossRef]
- Paolini, D.; Alparone, F.R.; Cardone, D.; van Beest, I.; Merla, A. “The face of ostracism”: The impact of the social categorization on the thermal facial responses of the target and the observer. Acta Psychol. 2016, 163, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Buddharaju, P.; Pavlidis, I.T.; Tsiamyrtzis, P. Physiology-based face recognition. In Proceedings of the IEEE Conference on Advanced Video and Signal Based Surveillance, Teatro Sociale Como, Italy, 15–16 September 2005; pp. 354–359. Available online: http://ieeexplore.ieee.org/abstract/document/1577294/ (accessed on 26 February 2017).
- Khan, M.M.; Ward, R.D.; Ingleby, M. Classifying pretended and evoked facial expressions of positive and negative affective states using infrared measurement of skin temperature. ACM Trans. Appl. Percept. 2009, 6, 6. [Google Scholar] [CrossRef]
- Nhan, B.R.; Chau, T. Classifying affective states using thermal infrared imaging of the human face. IEEE Trans. Biomed. Eng. 2010, 57, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Gallese, V.; Merla, A. Thermal infrared imaging in psychophysiology: potentialities and limits. Psychophysiology 2014, 51, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Ebisch, S.; Aureli, T.; Bafunno, D.; Ioannides, H.A.; Cardone, D.; Manini, B.; Romani, G.L.; Gallese, V.; Merla, A. The autonomic signature of guilt in children: a thermal infrared imaging study. PLoS ONE 2013, 8, e79440. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.C.; Whitehead, R.D.; Albrecht, M.; Lefevre, C.E.; Perrett, D.I. Hot or not? Thermal reactions to social contact. Biol. Lett. 2012, 8, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Pollina, D.A.; Dollins, A.B.; Senter, S.M.; Brown, T.E.; Pavlidis, I.; Levine, J.A.; Ryan, A.H. Facial skin surface temperature changes during a “concealed information” test. Ann. Biomed. Eng. 2006, 34, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Panasiti, M.S.; Cardone, D.; Pavone, E.F.; Mancini, A.; Merla, A.; Aglioti, S.M. Thermal signatures of voluntary deception in ecological conditions. Sci. Rep. 2016, 6, 35174. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Murata, T.; Hamada, T.; Takahashi, T.; Omori, M.; Suganuma, N.; Yoshida, H.; Wada, Y. Interactions among higher trait anxiety, sympathetic activity, and endothelial function in the elderly. J. Psychiatr. Res. 2007, 41, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Mendolia, M. An index of self-regulation of emotion and the study of repression in social contexts that threaten or do not threaten self-concept. Emotion 2002, 2, 215. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Albarrán, I.A.; Benítez-Rangel, J.P.; Osornio-Ríos, R.A.; Morales-Hernández, L.A. Human emotions detection based on a smart-thermal system of thermographic images. Infrared Phys. Technol. 2017, 81, 250–261. Available online: http://www.sciencedirect.com/science/article/pii/S1350449516304182 (accessed on 26 February 2017). [CrossRef]
- Kaplan, H. Practical Applications of Infrared Thermal Sensing and Imaging Equipment; SPIE Press: Bellingham, WA, USA, 2007; Volume 75. [Google Scholar]






| Feature | FLIR One Pro® | FLIR Lepton® Radiometric | Opgal Therm-App® TH |
|---|---|---|---|
| Size (w × h × d) | 68 × 34 × 14 mm | 11.8 × 12.7 × 7.2 mm | 55 × 65 × 40 mm |
| Weight | 36.5 g | 0.9 g | 123 g |
| FPA | 160 × 120 pixels | 80 × 60 pixels | 384 × 288 pixels |
| NEDT | 0.15 °C | <0.05 °C | <0.07 °C |
| Operating temperature range | 0 °C to +35 °C | −10 °C to +80 °C | −10 °C to +50 °C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, D.; Merla, A. New Frontiers for Applications of Thermal Infrared Imaging Devices: Computational Psychopshysiology in the Neurosciences. Sensors 2017, 17, 1042. https://doi.org/10.3390/s17051042
Cardone D, Merla A. New Frontiers for Applications of Thermal Infrared Imaging Devices: Computational Psychopshysiology in the Neurosciences. Sensors. 2017; 17(5):1042. https://doi.org/10.3390/s17051042
Chicago/Turabian StyleCardone, Daniela, and Arcangelo Merla. 2017. "New Frontiers for Applications of Thermal Infrared Imaging Devices: Computational Psychopshysiology in the Neurosciences" Sensors 17, no. 5: 1042. https://doi.org/10.3390/s17051042
APA StyleCardone, D., & Merla, A. (2017). New Frontiers for Applications of Thermal Infrared Imaging Devices: Computational Psychopshysiology in the Neurosciences. Sensors, 17(5), 1042. https://doi.org/10.3390/s17051042







