Fabrication of a Textile-Based Wearable Blood Leakage Sensor Using Screen-Offset Printing
Abstract
1. Introduction
2. Materials and Methods
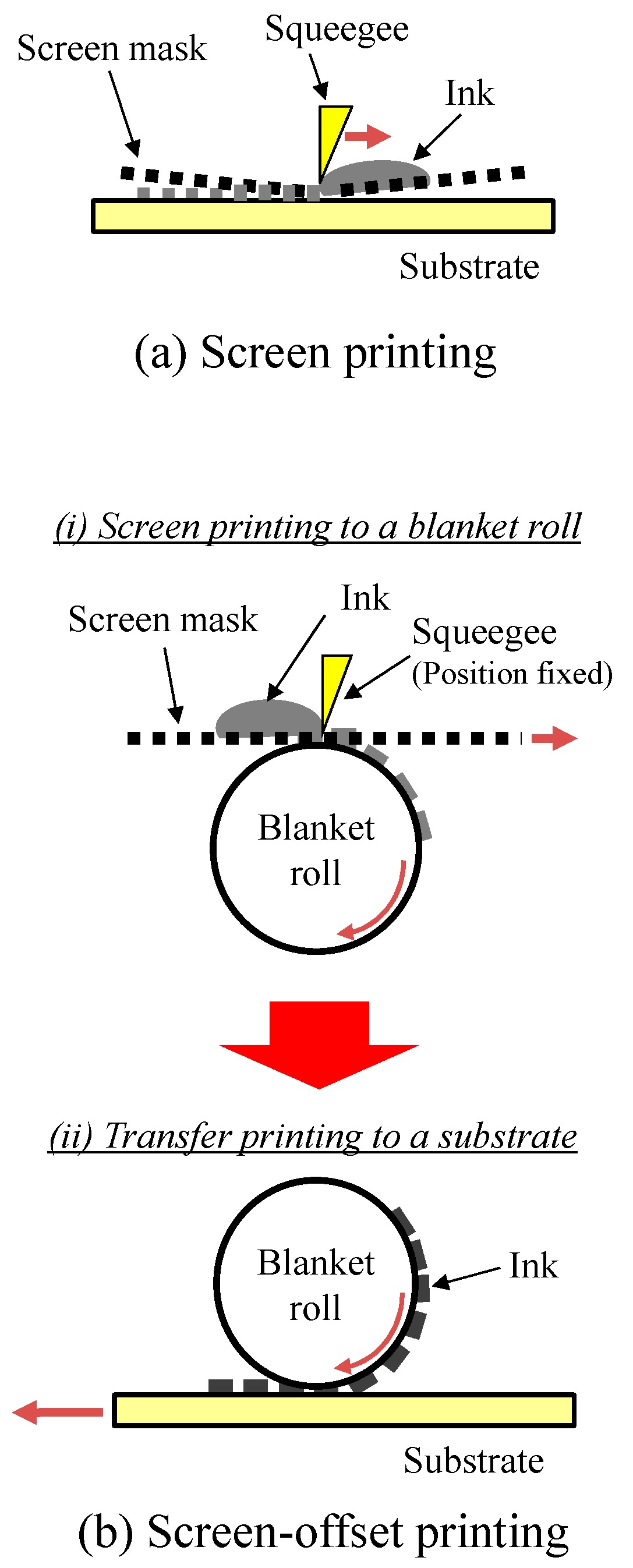
2.1. Formation of Interdigitated Electrodes on Textiles
2.2. Detection of Blood Permeating the Textile
3. Results and Discussion
4. Conclusions
5. Patent
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Ueda, M.; Ishikawa, K.; Jie, C.; Mizuno, S.; Touma, Y. Highly sensitive optical sensor system for blood leakage detection. Opt. Lasers Eng. 1994, 21, 307–316. [Google Scholar] [CrossRef]
- Ahlmén, J.; Gydell, K.-H.; Hadimeri, H.; Hernandez, I.; Rogland, B.; Strömbom, U. A new safety device for hemodialysis. Hemodial. Int. 2008, 12, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-C.; Shih, C.-Y.; Chou, C.-H.; Huang, J.-T.; Wu, C.-J. The development of a blood leakage monitoring system for the applications in hemodialysis therapy. IEEE Sens. J. 2015, 15, 1515–1522. [Google Scholar] [CrossRef]
- Du, Y.-C.; Lim, B.-Y.; Ciou, W.-S.; Wu, M.-J. Novel wearable device for blood leakage detection during hemodialysis using an array sensing patch. Sensors 2016, 16, 849. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, W.-L.; Li, C.-M.; Wu, M.-J.; Huang, P.-T.; Chen, Y.-S. Assistive technology using integrated flexible sensor and virtual alarm unit for blood leakage detection during dialysis therapy. Healthc. Tech. Lett. 2016, 3, 290–296. [Google Scholar] [CrossRef]
- Huang, P.-T.; Jong, T.-L.; Li, C.-M.; Chen, W.-L.; Lin, C.-H. Integrating flexible sensor and virtual self-organizing DC grid model with cloud computing for blood leakage detection during hemodialysis. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ushijima, H.; Mitsui, R.; Takahashi, S.; Nakajima, S. Screen-offset printing for fine conductive patterns. Microelectron. Eng. 2014, 123, 58–61. [Google Scholar] [CrossRef]
- Nomura, K.; Kusaka, Y.; Ushijima, H.; Nagase, K.; Ikedo, H.; Mitsui, R.; Takahashi, S.; Nakajima, S.; Iwata, S. Continuous fine pattern formation by screen-offset printing using a silicone blanket. J. Micromech. Microeng. 2014, 24, 095021. [Google Scholar] [CrossRef]
- Nomura, K.; Kaji, R.; Iwata, S.; Otao, S.; Imawaka, N.; Yoshino, K.; Mitsui, R.; Sato, J.; Takahashi, S.; Nakajima, S.; et al. A flexible proximity sensor formed by duplex screen/screen-offset printing and its application to non-contact detection of human breathing. Sci. Rep. 2016, 6, 19947. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ushijima, H.; Nagase, K.; Ikedo, H.; Mitsui, R.; Sato, J.; Takahashi, S.; Nakajima, S.; Arai, M.; Kurata, Y.; et al. Simultaneous formation of fine and large-area electrode patterns using screen-offset printing and its application to the patterning on adhesive materials. Jpn. J. Appl. Phys. 2016, 55, 03DD01. [Google Scholar] [CrossRef]
- Deganello, D.; Cherry, J.A.; Gethin, D.T.; Claypole, T.C. Impact of metered ink volume on reel-to-reel flexographic printed conductive networks for enhanced thin film conductivity. Thin Solid Films 2012, 520, 2233–2237. [Google Scholar] [CrossRef]
- Lloyd, J.S.; Fung, C.M.; Deganello, D.; Wang, R.J.; Maffeis, T.G.G.; Lau, S.P.; Teng, K.S. Flexographic printing-assisted fabrication of ZnO nanowire devices. Nanotechnology 2013, 24, 195602. [Google Scholar] [CrossRef] [PubMed]
- Leppäniemi, J.; Huttunen, O.-H.; Majumdar, H.; Alastalo, A. Flexography-printed In2O3 semiconductor layers for high-mobility thin-film transistors on flexible plastic substrate. Adv. Mater. 2015, 27, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Kina, O.; Koutake, M.; Matsuoka, K.; Yase, K. Organic thin-film transistors fabricated by microcontact printing. Jpn. J. Appl. Phys. 2010, 49, 01AB07. [Google Scholar] [CrossRef]
- Pudas, M.; Halonen, N.; Granat, P.; Vähäkangas, J. Gravure printing of conductive particulate polymer inks on flexible substrates. Prog. Org. Coat. 2005, 54, 310–316. [Google Scholar] [CrossRef]
- Pudas, M.; Hagberg, J.; Leppävuori, S. Printing parameters and ink components affecting ultra-fine-line gravure-offset printing for electronics applications. J. Eur. Ceram. Soc. 2004, 24, 2943–2950. [Google Scholar] [CrossRef]
- Lee, T.-M.; Noh, J.-H.; Kim, C.H.; Jo, J.; Kim, D.-S. Development of a gravure offset printing system for the printing electrodes of flat panel display. Thin Solid Films 2010, 518, 3355–3359. [Google Scholar] [CrossRef]
- Izumi, K.; Yoshida, Y.; Tokito, S. Soft blanket gravure printing technology for finely patterned conductive layers on three-dimensional or curved surfaces. Jpn. J. Appl. Phys. 2017, 56, 05EA03. [Google Scholar] [CrossRef]
- Kim, M.; You, I.-K.; Han, H.; Jung, S.-W.; Kim, T.-Y.; Ju, B.-K.; Koo, J.B. Organic thin-film transistors with short channel length fabricated by reverse offset printing. Electrochem. Solid-State Lett. 2011, 14, H333–H336. [Google Scholar] [CrossRef]
- Kusaka, Y.; Sugihara, K.; Koutake, M.; Ushijima, H. Overlay of semi-dried functional layers in offset printing for rapid and high-precision fabrication of flexible TFTs. J. Micromech. Microeng. 2014, 24, 035020. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Lee, E.-S.; Lee, T.-M.; Kim, K.-Y. Optimization of a reverse-offset printing process and its application to a metal mesh touch screen sensor. Microelectron. Eng. 2015, 134, 1–6. [Google Scholar] [CrossRef]
- Fukuda, K.; Yoshimura, Y.; Okamoto, T.; Takeda, Y.; Kumaki, D.; Katayama, Y.; Tokito, S. Reverse-offset printing optimized for scalable organic thin-film transistors with submicrometer channel lengths. Adv. Electron. Mater. 2015, 1, 1500145. [Google Scholar] [CrossRef]
- Kusaka, Y.; Koutake, M.; Ushijima, H. High-resolution patterning of silver conductive lines by adhesion contrast planography. J. Micromech. Microeng. 2015, 25, 095002. [Google Scholar] [CrossRef]
- Newman, J.D.; Tumer, A.P.F. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Teng, Y.; Yuan, H.; Lan, M. Direct electrochemistry of glucose oxidase on screen-printed electrodes through one-step enzyme immobilization process with silica sol–gel/polyvinyl alcohol hybrid film. Sens. Actuators B Chem. 2008, 133, 555–560. [Google Scholar] [CrossRef]
- Müller, M.; Lottspeich, F. Evaluation of determination methods of the Si/Al contact resistance of screen-printed passivated emitter and rear solar cells. J. Appl. Phys. 2014, 115, 084505. [Google Scholar] [CrossRef]
- Ok, Y.-W.; Upadhyaya, A.D.; Tao, Y.; Zimbardi, F.; Ryu, K.; Kang, M.-H.; Rohatgi, A. Ion-implanted and screen-printed large area 20% efficient N-type front junction Si solar cells. Sol. Energy Mater. Sol. Cells 2014, 123, 92–96. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Polymer thermoelectric modules screen-printed on paper. RSC Adv. 2014, 4, 28802–28806. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Jiang, Z.; Yu, D. Low temperature sintering nano-silver conductive ink printed on cotton fabric as printed electronics. Prog. Org. Coat. 2016, 101, 604–611. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S.; Kusaka, Y.; Yamamoto, N.; Ushijima, H. Novel printing process for the fabrication of cantilever structures by the partially controlled sintering of ink. Jpn. J. Appl. Phys. 2017, 56, 010313. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kusaka, Y.; Yamamoto, N.; Ushijima, H. One-batch transfer process for the additive manufacturing of a cantilever with a weight. Jpn. J. Appl. Phys. 2017, 56, 06GN03. [Google Scholar] [CrossRef]
- Lisin, R.; Ginzburg, B.Z.; Schlesinger, M.; Feldman, Y. Time domain dielectric spectroscopy study of human cells. I. Erythrocytes and ghosts. Biochim. Biophys. Acta 1996, 1280, 34–40. [Google Scholar] [CrossRef]
- Chelidze, T. Dielectric spectroscopy of blood. J. Non Cryst. Solids 2002, 305, 285–294. [Google Scholar] [CrossRef]
- Kudo, H.; Sawada, T.; Kazawa, E.; Yoshida, H.; Iwasaki, Y.; Mitsubayashi, K. A flexible and wearable glucose sensor based on functional polymers with Soft-MEMS techniques. Biosens. Bioelectron. 2006, 22, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A Disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 2016, 6, 27582. [Google Scholar] [CrossRef] [PubMed]







| Type | General Feature | General Problems | Other Information |
|---|---|---|---|
| Electrical sensor (Conductivity/Impedance change) | Simple structure, cheap equipment | Low accuracy, impermeable to air | Limit of detection: 10 μL−1 mL [4] |
| Optical sensor | High accuracy | Impermeable to air, causes physical discomfort | Sensitivity: 4 × 10−6 blood concentration in volume [2] |
| Camera (visual and IR) | High accuracy | Psychologically uncomfortable | |
| Pressure-sensitive sheet | Simple structure, cheap equipment | Impermeable to air | |
| Blood-flow rate sensor | Simple structure, cheap equipment | Delayed detection |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, K.-i.; Horii, Y.; Kanazawa, S.; Kusaka, Y.; Ushijima, H. Fabrication of a Textile-Based Wearable Blood Leakage Sensor Using Screen-Offset Printing. Sensors 2018, 18, 240. https://doi.org/10.3390/s18010240
Nomura K-i, Horii Y, Kanazawa S, Kusaka Y, Ushijima H. Fabrication of a Textile-Based Wearable Blood Leakage Sensor Using Screen-Offset Printing. Sensors. 2018; 18(1):240. https://doi.org/10.3390/s18010240
Chicago/Turabian StyleNomura, Ken-ichi, Yoshinori Horii, Shusuke Kanazawa, Yasuyuki Kusaka, and Hirobumi Ushijima. 2018. "Fabrication of a Textile-Based Wearable Blood Leakage Sensor Using Screen-Offset Printing" Sensors 18, no. 1: 240. https://doi.org/10.3390/s18010240
APA StyleNomura, K.-i., Horii, Y., Kanazawa, S., Kusaka, Y., & Ushijima, H. (2018). Fabrication of a Textile-Based Wearable Blood Leakage Sensor Using Screen-Offset Printing. Sensors, 18(1), 240. https://doi.org/10.3390/s18010240





