Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances
Abstract
:1. Introduction
1.1. Characteristic of Human MMP-9
1.2. Biological Function of MMP-9
1.3. MMP-9 and Cancers
2. MMP-9 as a Potential Marker for Cancer: Recent Discoveries
2.1. MMP-9 as a Potential Cancer Biomarker in Giant Cell Tumor of Bone (GCTB)
2.2. MMP-9 as a Potential Cancer Biomarker in Non-Small Cell Lung Cancer (NSCLC)
2.3. MMP-9 as a Potential Cancer Biomarker in Cervical Cancer
2.4. MMP-9 as a Potential Cancer Biomarker in Ovarian Cancer
2.5. MMP-9 as a Potential Cancer Biomarker in Pancreatic Cancer
2.6. MMP-9 as a Potential Cancer Biomarker in Osteosarcoma
2.7. MMP-9 as a Potential Cancer Biomarker in Breast Cancer
2.8. MMP-9/MMP-2 Ratio as a Potential Cancer Biomarker in Hepatitis B Virus-Related Hepatocellular Carcinoma
2.9. MMP-9/NGAL Complex as a Potential Cancer Biomarker
3. Recent Advances in MMP-9 Biosensor Research
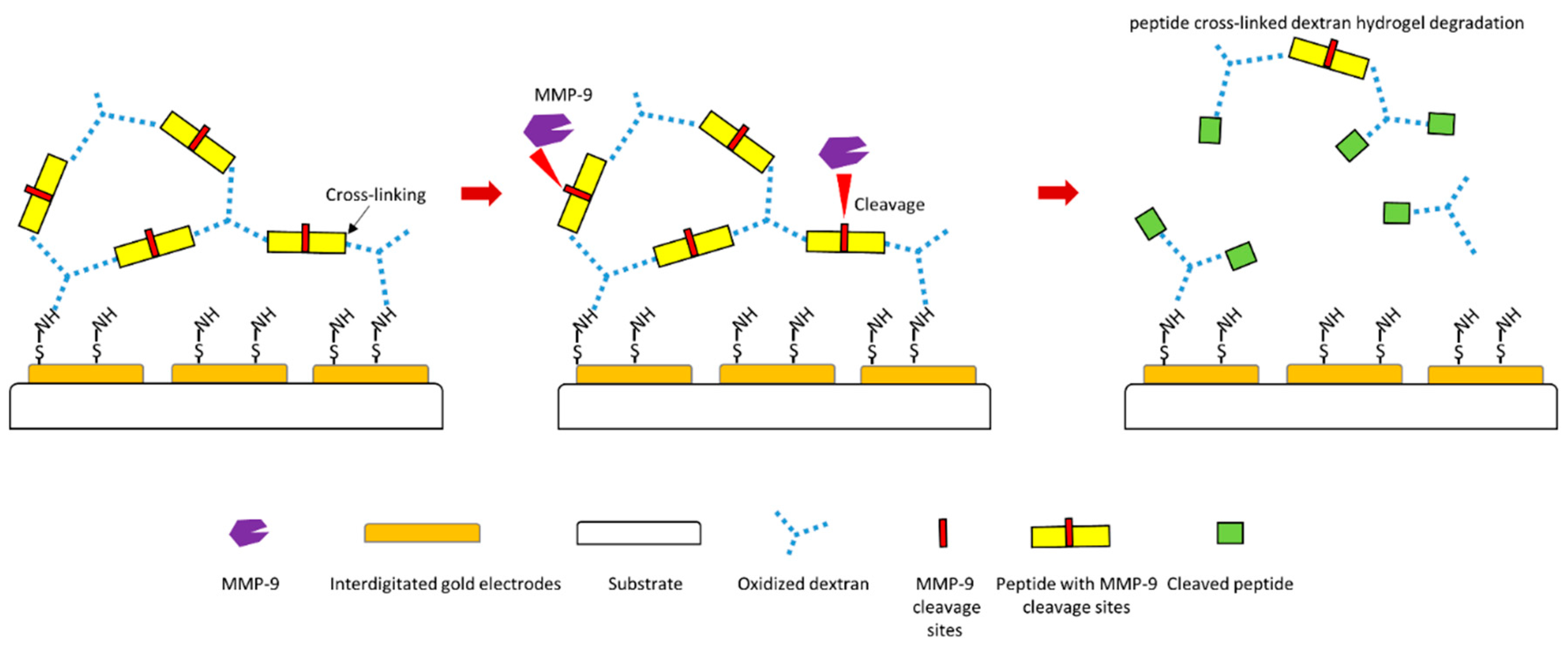
3.1. Cleavage-Based MMP-9 Biosensors
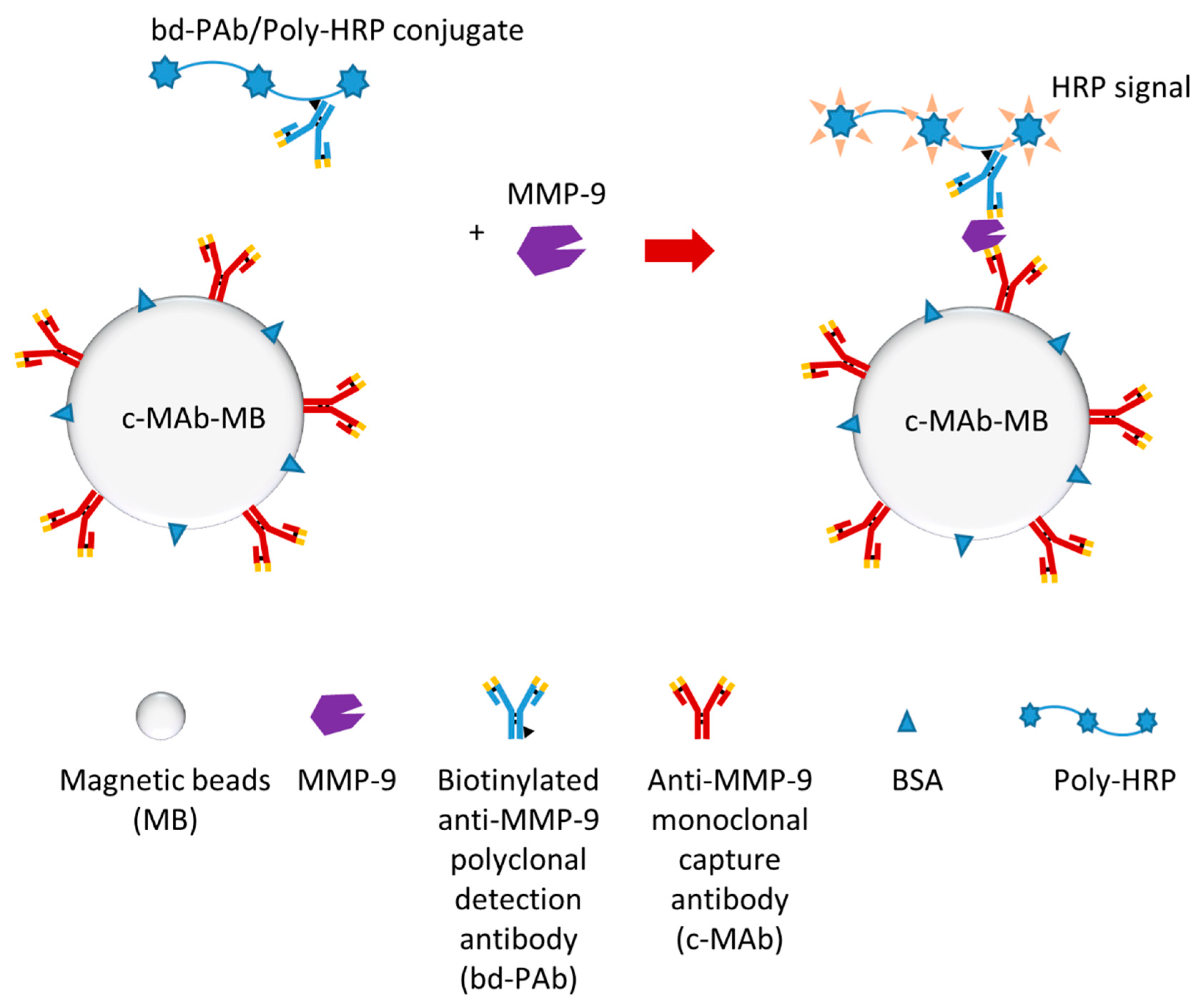
3.2. Non-Cleavage-Based MMP-9 Biosensors
4. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and timps. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Pinto, B. The significance of matrix metalloproteinases in parasitic infections involving the central nervous system. Pathogens 2013, 2, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Romero-Perez, D.; Jacobsen, J.A.; Villarreal, F.J.; Cohen, S.M. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem 2008, 3, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; de Souza, A.P.; Gerlach, R.F.; Trevilatto, P.C.; Scarel-Caminaga, R.M.; Line, S.R. Inhibition of human pulpal gelatinases (MMP-2 and MMP-9) by zinc oxide cements. J. Oral Rehabil. 2004, 31, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Opdenakker, G.; Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Van Coillie, E.; Masure, S.; Proost, P.; Van Damme, J. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 2001, 69, 851–859. [Google Scholar] [PubMed]
- Rosenblum, G.; Van den Steen, P.E.; Cohen, S.R.; Grossmann, J.G.; Frenkel, J.; Sertchook, R.; Slack, N.; Strange, R.W.; Opdenakker, G.; Sagi, I. Insights into the structure and domain flexibility of full-length pro-matrix metalloproteinase-9/gelatinase B. Structure 2007, 15, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Butler, G.S. Protease yoga: Extreme flexibility of a matrix metalloproteinase. Structure 2007, 15, 1159–1161. [Google Scholar] [CrossRef] [PubMed]
- Shipley, J.M.; Doyle, G.A.; Fliszar, C.J.; Ye, Q.Z.; Johnson, L.L.; Shapiro, S.D.; Welgus, H.G.; Senior, R.M. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type ii-like repeats. J. Biol. Chem. 1996, 271, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, T.J.; Pourmotabbed, T. The fibronectin-like domain is required for the type V and XI collagenolytic activity of gelatinase B. Arch. Biochem. Biophys. 1998, 354, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Lauer-Fields, J.L.; Whitehead, J.K.; Li, S.; Hammer, R.P.; Brew, K.; Fields, G.B. Selective modulation of matrix metalloproteinase 9 (MMP-9) functions via exosite inhibition. J. Biol. Chem. 2008, 283, 20087–20095. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E.; Schleinkofer, K.; Kernebeck, T.; Potsch, S.; Jansen, B.; Behrmann, I.; Matern, S.; Grotzinger, J. The matrix metalloproteinase 9 (MMP-9) hemopexin domain is a novel gelatin binding domain and acts as an antagonist. J. Biol. Chem. 2002, 277, 50326–50332. [Google Scholar] [CrossRef] [PubMed]
- Ethell, I.M.; Ethell, D.W. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Roderfeld, M.; Graf, J.; Giese, B.; Salguero-Palacios, R.; Tschuschner, A.; Muller-Newen, G.; Roeb, E. Latent MMP-9 is bound to TIMP-1 before secretion. Biol. Chem. 2007, 388, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Massova, I.; Kotra, L.P.; Fridman, R.; Mobashery, S. Matrix metalloproteinases: Structures, evolution, and diversification. FASEB J. 1998, 12, 1075–1095. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Sampson, N.S.; Li, J.; Kuscu, C.; Rizzo, R.C.; Deleon, J.L.; Zhi, J.; Jaber, N.; Liu, E.; Zucker, S.; et al. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Res. 2011, 71, 4977–4988. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Enghild, J.J.; Nagase, H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J. Biol. Chem. 1992, 267, 3581–3584. [Google Scholar] [PubMed]
- Fridman, R.; Toth, M.; Pena, D.; Mobashery, S. Activation of progelatinase b (MMP-9) by gelatinase a (MMP-2). Cancer Res. 1995, 55, 2548–2555. [Google Scholar] [PubMed]
- Imai, K.; Yokohama, Y.; Nakanishi, I.; Ohuchi, E.; Fujii, Y.; Nakai, N.; Okada, Y. Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J. Biol. Chem. 1995, 270, 6691–6697. [Google Scholar] [CrossRef] [PubMed]
- Knauper, V.; Smith, B.; Lopez-Otin, C.; Murphy, G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur. J. Biochem. 1997, 248, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science 2002, 297, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Paquette, B.; Bisson, M.; Therriault, H.; Lemay, R.; Pare, M.; Banville, P.; Cantin, A.M. Activation of matrix metalloproteinase-2 and -9 by 2- and 4-hydroxyestradiol. J. Steroid Biochem. Mol. Biol. 2003, 87, 65–73. [Google Scholar] [CrossRef]
- Manabe, S.; Gu, Z.; Lipton, S.A. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4747–4753. [Google Scholar] [CrossRef] [PubMed]
- Ridnour, L.A.; Windhausen, A.N.; Isenberg, J.S.; Yeung, N.; Thomas, D.D.; Vitek, M.P.; Roberts, D.D.; Wink, D.A. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 16898–16903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kridel, S.J.; Chen, E.; Kotra, L.P.; Howard, E.W.; Mobashery, S.; Smith, J.W. Substrate hydrolysis by matrix metalloproteinase-9. J. Biol. Chem. 2001, 276, 20572–20578. [Google Scholar] [CrossRef] [PubMed]
- Prudova, A.; Auf dem Keller, U.; Butler, G.S.; Overall, C.M. Multiplex n-terminome analysis of MMP-2 and MMP-9 substrate degradomes by itraq-tails quantitative proteomics. Mol. Cell. Proteom. 2010, 9, 894–911. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, Y.; Qi, P.; Chen, Y.; Xu, P.; Yang, X.; Jin, X.; Tian, X. Microrna-183 functions as the tumor suppressor via inhibiting cellular invasion and metastasis by targeting MMP-9 in cervical cancer. Gynecol. Oncol. 2016, 141, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Aung, L.L.; Mouradian, M.M.; Dhib-Jalbut, S.; Balashov, K.E. MMP-9 expression is increased in b lymphocytes during multiple sclerosis exacerbation and is regulated by microrna-320a. J. Neuroimmunol. 2015, 278, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Muschel, R.J. Regulation of matrix metalloproteinase-9 (MMP-9) by translational efficiency in murine prostate carcinoma cells. Cancer Res. 2002, 62, 1910–1914. [Google Scholar] [PubMed]
- Melamed, D.; Messika, O.; Glass-Marmor, L.; Miller, A. Modulation of matrix metalloproteinase-9 (MMP-9) secretion in B lymphopoiesis. Int. Immunol. 2006, 18, 1355–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, C.W.; Pabisiak, P.J.; Brilha, S.; Singh, P.; Roncaroli, F.; Elkington, P.T.; Friedland, J.S. Complex regulation of neutrophil-derived MMP-9 secretion in central nervous system tuberculosis. J. Neuroinflamm. 2017, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H. Role of microrna-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol. Ther. 2013, 14, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Zariffard, M.R.; Anastos, K.; French, A.L.; Munyazesa, E.; Cohen, M.; Landay, A.L.; Spear, G.T. Cleavage/alteration of interleukin-8 by matrix metalloproteinase-9 in the female lower genital tract. PLoS ONE 2015, 10, e0116911. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, J.R.; Lim, G.P.; Cullen, M.J.; Tokes, Z.A. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40). J. Neurosci. 1996, 16, 7910–7919. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V.; et al. Matrix metalloproteinase gelatinase b (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, M.G.; Baiza-Gutman, L.A.; Castillo-Trapala, A.; Armant, D.R. Regulation of proteinases during mouse peri-implantation development: Urokinase-type plasminogen activator expression and cross talk with matrix metalloproteinase 9. Reproduction 2011, 141, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Dziembowska, M.; Wlodarczyk, J. MMP9: A novel function in synaptic plasticity. Int. J. Biochem. Cell Biol. 2012, 44, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.M.; Lau, L.; Yong, V.W. MMPs in the central nervous system: Where the good guys go bad. Semin. Cell Dev. Biol. 2008, 19, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.R.; Mackay, A.R. Gelatinase b/MMP-9 in tumour pathogenesis and progression. Cancers 2014, 6, 240–296. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Fusco, C.; Romero, P.; Stamenkovic, I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene 2002, 21, 5213–5223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaisar, T.; Kassim, S.Y.; Gomez, I.G.; Green, P.S.; Hargarten, S.; Gough, P.J.; Parks, W.C.; Wilson, C.L.; Raines, E.W.; Heinecke, J.W. MMP-9 sheds the beta2 integrin subunit (CD18) from macrophages. Mol. Cell. Proteom. MCP 2009, 8, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Cauwe, B.; Opdenakker, G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 351–423. [Google Scholar] [CrossRef] [PubMed]
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta 2017, 1864, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Amorosa, L.F.; Coyle, S.M.; Macor, M.A.; Lubitz, S.E.; Carson, J.L.; Birnbaum, M.J.; Lee, L.Y.; Haimovich, B. Proteolytic cleavage of ampkalpha and intracellular MMP9 expression are both required for tlr4-mediated mtorc1 activation and hif-1alpha expression in leukocytes. J. Immunol. 2015, 195, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Arkell, J.; Jackson, C.J. Active and tissue inhibitor of matrix metalloproteinase-free gelatinase b accumulates within human microvascular endothelial vesicles. J. Biol. Chem. 1998, 273, 5400–5404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Xiao, A.Z.; Newcomer, R.G.; Park, H.I.; Kang, T.; Chung, L.W.; Swanson, M.G.; Zhau, H.E.; Kurhanewicz, J.; Sang, Q.X. Activation of pro-gelatinase b by endometase/matrilysin-2 promotes invasion of human prostate cancer cells. J. Biol. Chem. 2003, 278, 15056–15064. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mohammad, G.; dos Santos, J.M.; Zhong, Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 2011, 60, 3023–3033. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Poddar, R.; Thompson, J.F.; Rosenberg, G.A.; Yang, Y. Intranuclear matrix metalloproteinases promote DNA damage and apoptosis induced by oxygen-glucose deprivation in neurons. Neuroscience 2012, 220, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Candelario-Jalil, E.; Thompson, J.F.; Cuadrado, E.; Estrada, E.Y.; Rosell, A.; Montaner, J.; Rosenberg, G.A. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J. Neurochem. 2010, 112, 134–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.; Zhang, G.; Wang, H.; Gong, H.; Wang, C.; Zhang, X. High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction. Neural Regen. Res. 2014, 9, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Misko, A.; Ferguson, T.; Notterpek, L. Matrix metalloproteinase mediated degradation of basement membrane proteins in trembler j neuropathy nerves. J. Neurochem. 2002, 83, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, E.; Kakehi, Y.; Okuno, H.; Yoshida, O. Role of matrix metalloproteinase-9 in the basement membrane destruction of superficial urothelial carcinomas. J. Urol. 1999, 161, 1359–1363. [Google Scholar] [CrossRef]
- Hsu, C.C.; Huang, S.F.; Wang, J.S.; Chu, W.K.; Nien, J.E.; Chen, W.S.; Chow, S.E. Interplay of n-cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carcinoma cell invasion. BMC Cancer 2016, 16, 800. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kwon, H.J.; Kim, D.S. Matrix metalloproteinase 9 (MMP-9)-dependent processing of betaig-h3 protein regulates cell migration, invasion, and adhesion. J. Biol. Chem. 2012, 287, 38957–38969. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.; Slater, S.C.; George, S.J. MMP-9 and -12 cause n-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc. Res. 2009, 81, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Behonick, D.J.; Colnot, C.; Cooper, D.N.; Werb, Z. Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation. Mol. Biol. Cell 2005, 16, 3028–3039. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; McKee, C.M.; Cao, Y.; Ding, Y.; Kessler, B.M.; Muschel, R.J. Matrix metalloproteinase-9 regulates tumor cell invasion through cleavage of protease nexin-1. Cancer Res. 2010, 70, 6988–6998. [Google Scholar] [CrossRef] [PubMed]
- Pego, E.R.; Fernandez, I.; Nunez, M.J. Molecular basis of the effect of MMP-9 on the prostate bone metastasis: A review. Urol. Oncol. 2018, 36, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Tanioka, M.; Matsuda, H.; Nishimoto, H.; Yoshioka, T.; Suzuki, R.; Uehira, M. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin. Exp. Metast. 1999, 17, 177–181. [Google Scholar] [CrossRef]
- Wang, X.; Nagase, H.; Watanabe, T.; Nobusue, H.; Suzuki, T.; Asami, Y.; Shinojima, Y.; Kawashima, H.; Takagi, K.; Mishra, R.; et al. Inhibition of MMP-9 transcription and suppression of tumor metastasis by pyrrole-imidazole polyamide. Cancer Sci. 2010, 101, 759–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, C.H.; Teng, C.M.; Tzen, K.Y.; Chang, Y.C.; Chen, J.H.; Cheng, J.C. MMP-9 from sublethally irradiated tumor promotes lewis lung carcinoma cell invasiveness and pulmonary metastasis. Oncogene 2012, 31, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Hawinkels, L.J.; Zuidwijk, K.; Verspaget, H.W.; de Jonge-Muller, E.S.; van Duijn, W.; Ferreira, V.; Fontijn, R.D.; David, G.; Hommes, D.W.; Lamers, C.B.; et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur. J. Cancer 2008, 44, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Leifler, K.S.; Svensson, S.; Abrahamsson, A.; Bendrik, C.; Robertson, J.; Gauldie, J.; Olsson, A.K.; Dabrosin, C. Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. J. Immunol. 2013, 190, 4420–4430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q. Relationship between matrix metalloproteinases and the occurrence and development of ovarian cancer. Braz. J. Med. Biol. Res. 2017, 50, e6104. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Bruni-Cardoso, A.; Johnson, L.C.; Vessella, R.L.; Peterson, T.E.; Lynch, C.C. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Mol. Cancer Res. 2010, 8, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Abrams, S.L.; Steelman, L.S.; Lertpiriyapong, K.; Fitzgerald, T.L.; Martelli, A.M.; Cocco, L.; Montalto, G.; Cervello, M.; Polesel, J.; et al. Roles of ngal and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy. Biochim. Biophys. Acta 2016, 1863, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Sarma, D.; Jeppsson, S.; Patel, N.R.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res. 2010, 70, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Pujada, A.; Walter, L.; Patel, A.; Bui, T.A.; Zhang, Z.; Zhang, Y.; Denning, T.L.; Garg, P. Matrix metalloproteinase MMP9 maintains epithelial barrier function and preserves mucosal lining in colitis associated cancer. Oncotarget 2017, 8, 94650–94665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, L.; Pujada, A.; Bhatnagar, N.; Bialkowska, A.B.; Yang, V.W.; Laroui, H.; Garg, P. Epithelial derived-matrix metalloproteinase (MMP9) exhibits a novel defensive role of tumor suppressor in colitis associated cancer by activating MMP9-Notch1-ARF-p53 axis. Oncotarget 2017, 8, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, Z.; Abdan, Z.; Rahimi, Z.; Razazian, N.; Shiri, H.; Vaisi-Raygani, A.; Shakiba, E.; Vessal, M.; Moradi, M.T. Functional promoter polymorphisms of MMP-2 C-735T and MMP-9 C-1562T and their synergism with MMP-7 A-181G in multiple sclerosis. Immunol. Investig. 2016, 45, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Sherer, Y.; Shoenfeld, Y. Matrix metalloproteinase-9 and autoimmune diseases. J. Clin. Immunol. 2006, 26, 299–307. [Google Scholar] [CrossRef] [PubMed]
- De Rooy, D.P.; Zhernakova, A.; Tsonaka, R.; Willemze, A.; Kurreeman, B.A.; Trynka, G.; van Toorn, L.; Toes, R.E.; Huizinga, T.W.; Houwing-Duistermaat, J.J.; et al. A genetic variant in the region of MMP-9 is associated with serum levels and progression of joint damage in rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.Y.; Jackson, C.J. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naouali, A.; Kaabachi, W.; Tizaoui, K.; Amor, A.B.; Hamzaoui, A.; Hamzaoui, K. Association of MMP-9 gene polymorphisms with behcet’s disease risk. Immunol. Lett. 2015, 164, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Chang, L. Serum matrix metalloproteinase-9 level as a biomarker for colorectal cancer: A diagnostic meta-analysis. Biomark. Med. 2018, 12, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wang, W.; Xiong, X.G.; Cao, C.; Yan, T.D.; Chen, G.; Chen, H.; Yin, W.; Liu, J.; Gu, Y.; et al. Prognostic impact of MMP-2 and MMP-9 expression in pathologic stage ia non-small cell lung cancer. J. Surg. Oncol. 2011, 104, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Zhou, X.; Gu, Y.; Yan, J. Prognostic value of MMP-9 in ovarian cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, D.; Zhang, W.; Zhou, J.; Tang, B.; Li, L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch. Gynecol. Obstet. 2012, 286, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; He, Y.; Ding, X.Y. Matrix metalloproteinase-9 expression of gctsc in peripheral tissue and central tissue of gctb. J. Cell. Biochem. 2018, 119, 5805–5812. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Thomas, A.; Subramaniam, D.; Giaccone, G.; Rajan, A. Biomarkers in early-stage non-small-cell lung cancer: Current concepts and future directions. J. Thorac. Oncol. 2014, 9, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Korpanty, G.J.; Graham, D.M.; Vincent, M.D.; Leighl, N.B. Biomarkers that currently affect clinical practice in lung cancer: Egfr, alk, met, ros-1, and kras. Front. Oncol. 2014, 4, 204. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Prieto, S.; Barcia-Castro, L.; Paez de la Cadena, M.; Rodriguez-Berrocal, F.J.; Vazquez-Iglesias, L.; Botana-Rial, M.I.; Fernandez-Villar, A.; De Chiara, L. Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. BMC Cancer 2017, 17, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, T.; Zhang, B.; Yao, Y.; Yin, G. Matrix metalloproteinase-9 is a prognostic marker for patients with cervical cancer. Med. Oncol. 2012, 29, 3394–3399. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, M.; Zbucka-Kretowska, M.; Sidorkiewicz, I.; Lubowicka, E.; Bedkowska, G.E.; Gacuta, E.; Szmitkowski, M.; Lawicki, S. Human Plasma Levels of Vascular Endothelial Growth Factor, Matrix Metalloproteinase 9, and Tissue Inhibitor of Matrix Metalloproteinase 1 and Their Applicability as Tumor Markers in Diagnoses of Cervical Cancer Based on ROC Analysis. Cancer Control J. Moffitt Cancer Cent. 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, M.; Zbucka-Kretowska, M.; Sidorkiewicz, I.; Lubowicka, E.; Gacuta, E.; Szmitkowski, M.; Chrostek, L.; Lawicki, S. Plasma levels and diagnostic utility of macrophage-colony stimulating factor, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 as tumor markers in cervical cancer patients. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2018, 40. [Google Scholar] [CrossRef] [PubMed]
- Lubowicka, E.; Gacuta, E.; Zajkowska, M.; Glazewska, E.K.; Przylipiak, A.; Chrostek, L.; Zbucka-Kretowska, M.; Lawicki, S. [The plasma levels and diagnostic utility of matrix metalloproteinase-9 and CA 125 in cervical cancer patients]. Pol. Merkur. Lekarski 2017, 43, 10–14. [Google Scholar] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed]
- Reiner, A.T.; Tan, S.; Agreiter, C.; Auer, K.; Bachmayr-Heyda, A.; Aust, S.; Pecha, N.; Mandorfer, M.; Pils, D.; Brisson, A.R.; et al. Ev-associated MMP9 in high-grade serous ovarian cancer is preferentially localized to annexin v-binding evs. Dis. Mark. 2017, 2017, 9653194. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Cui, Y.Z.; Song, G.H.; Zong, M.J.; Zhou, X.Y.; Chen, Y.; Han, J.X. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer 2008, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Q.; Yuan, T.X.; Song, Q.L.; Zhang, Y.; Wei, Q.; Zhou, L.; Luo, J.; Zuo, G.; Tang, M.; et al. Matrix metalloproteinase 9 (MMP-9) in osteosarcoma: Review and meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2014, 433, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Teng, Z.; Chen, J.; Li, Y.; Chen, Z.; Li, Z.; Zhang, Z. Matrix metalloproteinase 9 expression and survival of patients with osteosarcoma: A meta-analysis. Eur. J. Cancer Care 2017, 26, e12364. [Google Scholar] [CrossRef] [PubMed]
- Yousef, E.M.; Tahir, M.R.; St-Pierre, Y.; Gaboury, L.A. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer 2014, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Polyak, K.; Halushka, M.K.; Nassar, H.; Kouprina, N.; Iacobuzio-Donahue, C.; Wu, X.; Sukumar, S.; Hicks, J.; De Marzo, A.; et al. Serial analysis of gene expression of lobular carcinoma in situ identifies down regulation of claudin 4 and overexpression of matrix metalloproteinase 9. Breast Cancer Res. 2008, 10, R91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Distinct patterns of matrix metalloproteinase-2 and -9 expression in normal human cell lines. Oncol. Rep. 2009, 21, 821–826. [Google Scholar] [PubMed]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O.; Yeghiazaryan, K.; Abraham, J.A.; Schild, H.H.; Costigliola, V.; Debald, M.; Kuhn, W. Breast cancer risk assessment: A non-invasive multiparametric approach to stratify patients by MMP-9 serum activity and RhoA expression patterns in circulating leucocytes. Amino Acids 2017, 49, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Darlix, A.; Lamy, P.J.; Lopez-Crapez, E.; Braccini, A.L.; Firmin, N.; Romieu, G.; Thezenas, S.; Jacot, W. Serum NSE, MMP-9 and HER2 extracellular domain are associated with brain metastases in metastatic breast cancer patients: Predictive biomarkers for brain metastases? Int. J. Cancer 2016, 139, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.C.; Lin, S.M.; Chen, M.F.; Pan, T.L.; Wang, P.W.; Yeh, C.T. Evaluation of serum matrix metalloproteinase (MMP)-9 to MMP-2 ratio as a biomarker in hepatocellular carcinoma. Hepato-Gastroenterology 2010, 57, 98–102. [Google Scholar] [PubMed]
- Yan, L.; Borregaard, N.; Kjeldsen, L.; Moses, M.A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001, 276, 37258–37265. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A.; NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Review: Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology 2010, 15, 419–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemin, D.; Dworkin, L.D. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for early acute kidney injury. Crit. Care Clin. 2011, 27, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mitsnefes, M.M.; Kathman, T.S.; Mishra, J.; Kartal, J.; Khoury, P.R.; Nickolas, T.L.; Barasch, J.; Devarajan, P. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr. Nephrol. 2007, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.A.; Yan, L.; Louis, G.; Yang, J.; Kutok, J.L.; Moses, M.A. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin. Cancer Res. 2005, 11, 5390–5395. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.F.; Hu, Y.Y.; Jin, T.; Xu, K.; Wang, S.H.; Du, G.Z.; Wu, B.L.; Li, L.Y.; Xu, L.Y.; Li, E.M.; et al. Matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex activity in human glioma samples predicts tumor presence and clinical prognosis. Dis. Mark. 2015, 2015, 138974. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Dagher, A.; Sachdev, M.; Ebi, M.; Yamada, T.; Yamada, T.; Joh, T.; Moses, M.A. Urinary adam12 and MMP-9/NGAL complex detect the presence of gastric cancer. Cancer Prev. Res. 2015, 8, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, J.Y.; Lee, W.C.; Choi, S.; Lim, J.; Jeong, H.; Shin, D.-S.; Park, Y.J. A reference electrode-free electrochemical biosensor for detecting MMP-9 using a concentric electrode device. Sens. Actuators B Chem. 2017, 240, 735–741. [Google Scholar] [CrossRef]
- Biela, A.; Watkinson, M.; Meier, U.C.; Baker, D.; Giovannoni, G.; Becer, C.R.; Krause, S. Disposable MMP-9 sensor based on the degradation of peptide cross-linked hydrogel films using electrochemical impedance spectroscopy. Biosens. Bioelectron. 2015, 68, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Stawarski, M.; Rutkowska-Wlodarczyk, I.; Zeug, A.; Bijata, M.; Madej, H.; Kaczmarek, L.; Wlodarczyk, J. Genetically encoded fret-based biosensor for imaging MMP-9 activity. Biomaterials 2014, 35, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.; Cong, V.T.; Baek, C.; Min, J. Fabrication of peptide stabilized fluorescent gold nanocluster/graphene oxide nanocomplex and its application in turn-on detection of metalloproteinase-9. Biosens. Bioelectron. 2017, 89, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, M.; Toh, T.B.; Abdullah, N.L.B.; Chow, E.K. Stimuli-responsive nanodiamond-based biosensor for enhanced metastatic tumor site detection. SLAS Technol. 2018, 23, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vega, G.; Garcia-Robaina, A.; Ben Ismail, M.; Pasamar, H.; Garcia-Berrocoso, T.; Montaner, J.; Zourob, M.; Othmane, A.; Del Campo, F.J.; Baldrich, E. Detection of plasma MMP-9 within minutes. Unveiling some of the clues to develop fast and simple electrochemical magneto-immunosensors. Biosens. Bioelectron. 2018, 115, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Scarano, S.; Dausse, E.; Crispo, F.; Toulme, J.J.; Minunni, M. Design of a dual aptamer-based recognition strategy for human matrix metalloproteinase 9 protein by piezoelectric biosensors. Anal. Chim. Acta 2015, 897, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohseni, S.; Moghadam, T.T.; Dabirmanesh, B.; Jabbari, S.; Khajeh, K. Development of a label-free spr sensor for detection of matrixmetalloproteinase-9 by antibody immobilization on carboxymethyldextran chip. Biosens. Bioelectron. 2016, 81, 510–516. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. https://doi.org/10.3390/s18103249
Huang H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors. 2018; 18(10):3249. https://doi.org/10.3390/s18103249
Chicago/Turabian StyleHuang, Hao. 2018. "Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances" Sensors 18, no. 10: 3249. https://doi.org/10.3390/s18103249
APA StyleHuang, H. (2018). Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors, 18(10), 3249. https://doi.org/10.3390/s18103249




