Highly Sensitive Humidity Sensor Based on Oblique Carbon Nanoplumes
Abstract
:1. Introduction
2. Experiment Details
2.1. Preparations of the Carbon Nanostructures
2.2. Characterizations
2.3. Measurement of Sensing Properties
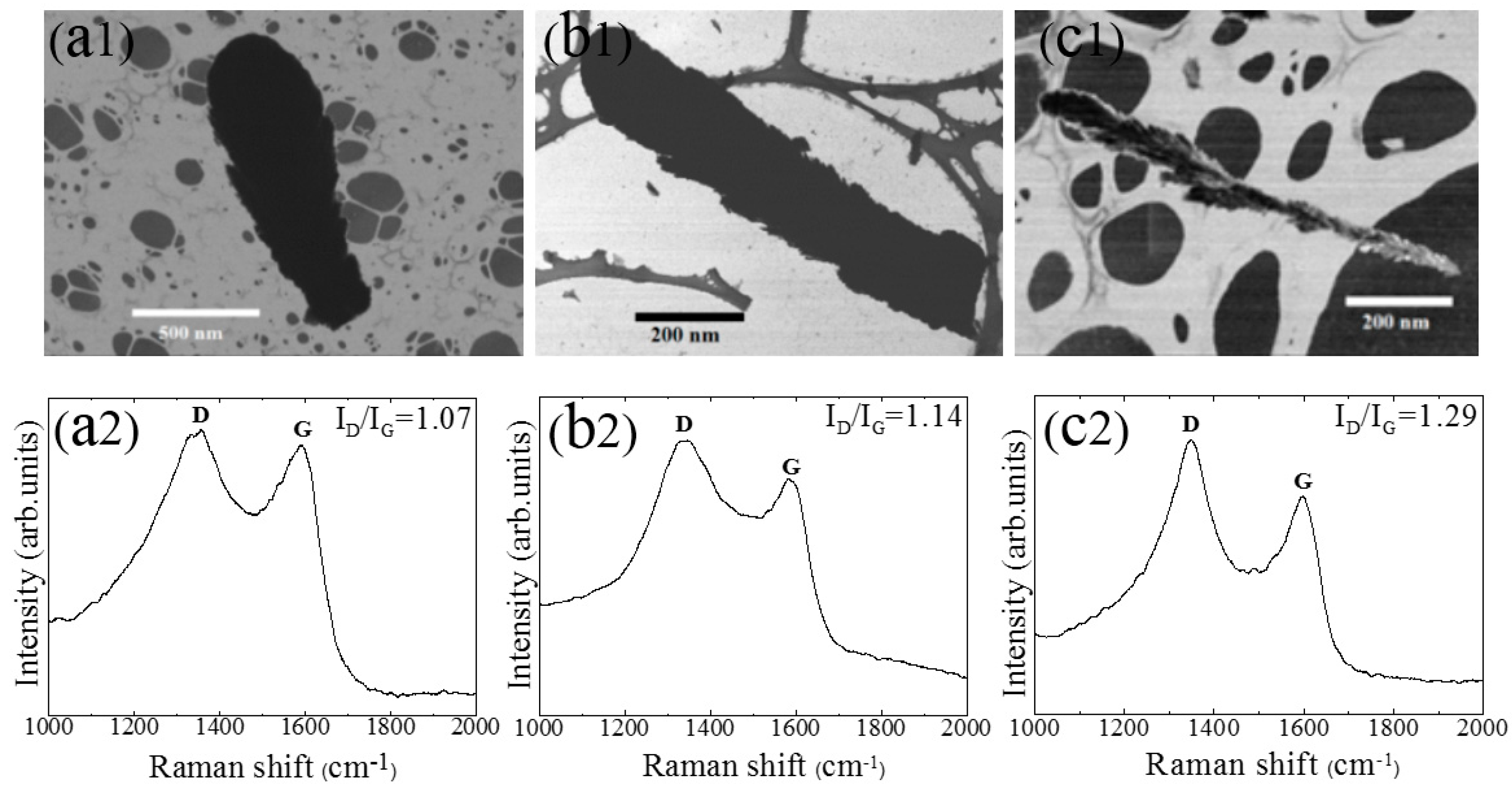
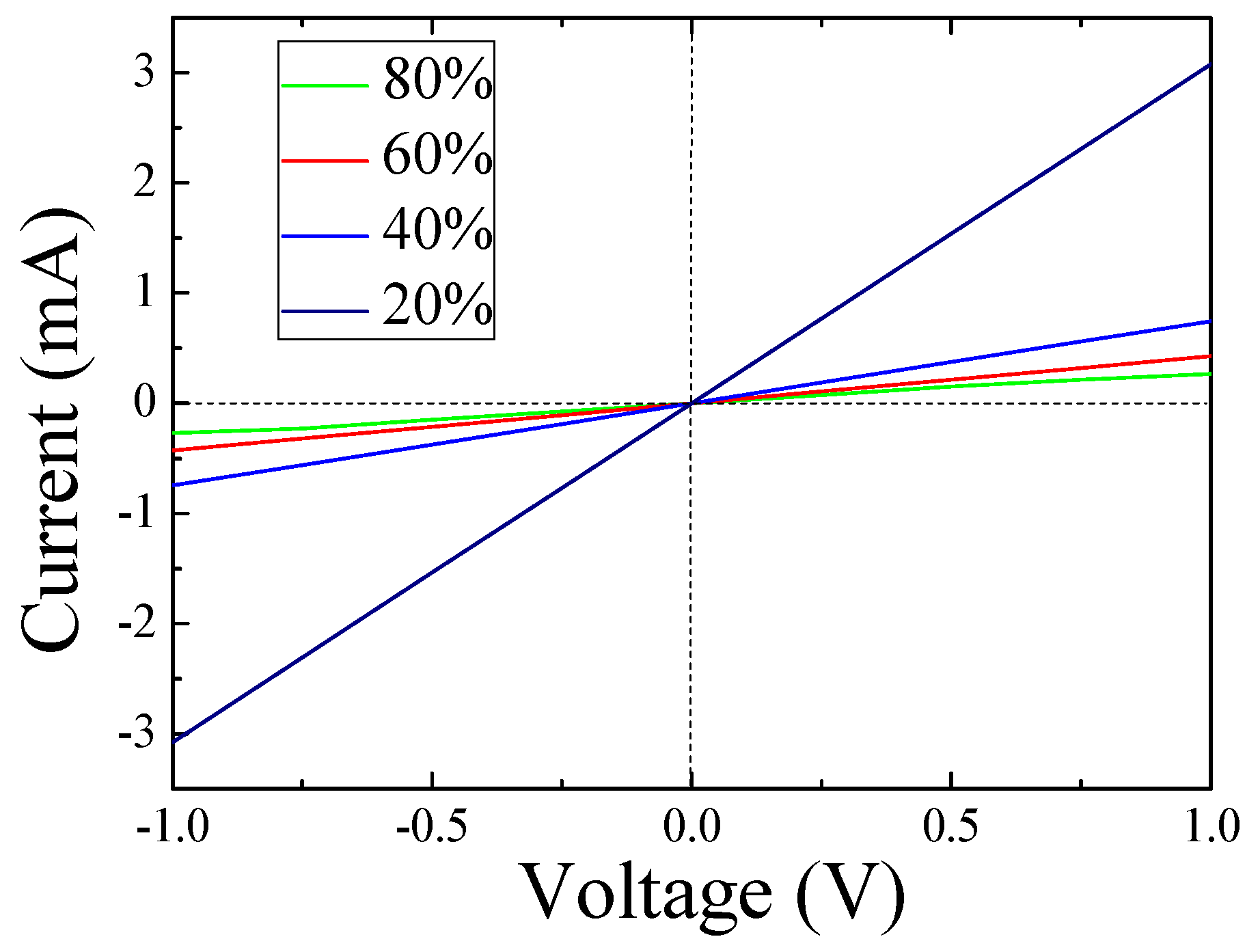
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nela, L.; Tang, J.S.; Cao, Q.; Tulevski, G.; Han, S.J. Large-area high-performance flexible pressure sensor with carbon nanotube active matrix for electronic skin. Nano Lett. 2018, 18, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Bahoumina, P.; Hallil, H.; Lachaud, J.L.; Abdelghani, A.; Frigui, K.; Bila, S.; Baillargeat, D.; Ravichandran, A.; Coquet, P.; Paragua, C.; et al. Microwave flexible gas sensor based on polymer multi wall carbon nanotubes sensitive layer. Sens. Actuators B Chem. 2017, 249, 708–714. [Google Scholar] [CrossRef]
- Rudreshappa, G.E.; Samal, S.S.; Manohara, S.R. Humidity sensing properties of multiwalled carbon nanotube/polyvinyl alcohol nanocomposite films. Nanosci. Nanotechnol. 2016, 6, 127–134. [Google Scholar]
- Liu, Y.Z.; Lai, W.P.; Yu, T.; Kang, Y.; Ge, Z.X. Interactions of carbon nanotubes with the nitromethane-water mixture governing selective adsorption of energetic molecules from aqueous solution. Phys. Chem. Chem. Phys. 2015, 17, 6995–7001. [Google Scholar] [CrossRef] [PubMed]
- Cantalini, C.; Valentini, L.; Lozzi, L.; Armentano, I.; Kenny, J.M.; Santucci, S. NO2 gas sensitivity of carbon nanotubes obtained by plasma enhanced chemical vapor deposition. Sens. Actuators B Chem. 2003, 93, 333–337. [Google Scholar] [CrossRef]
- Valentini, L.; Mercuri, F.; Armentano, I.; Cantalini, C.; Picozzi, S.; Lozzi, L.; Santucci, S.; Sgamellotti, A.; Kenny, J.M. Role of defects on the gas sensing properties of carbon nanotubes thin films: Experiment and theory. Chem. Phys. Lett. 2004, 387, 356–361. [Google Scholar] [CrossRef]
- Kombarakkaran, J.; Clewett, C.F.M.; Pietrass, T. Ammonia adsorption on multi-walled carbon nanotubes. Chem. Phys. Lett. 2007, 441, 282–285. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.Q.; Kim, J.K.; Schubert, E.F. Silica nanorod-array films with very low refractive indices. Nano Lett. 2005, 5, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, S.; Nagaraja, K.S. Electrical and humidity sensing properties of chromium(III) oxide–tungsten(VI) oxide composites. Sens. Actuators B Chem. 2003, 92, 144–150. [Google Scholar] [CrossRef]
- Zhao, J.; Bulduml, A.; Han, J.; Lu, J.P. Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.; Phan, H.P.; Nguyen, T.K.; Qamar, A.; Woodfeld, P.; Zhu, Y.; Nguyen, N.T.; Dao, D.V. Solvent-free fabrication of biodegradable hot-film flow sensor for noninvasive respiratory monitoring. J. Phys. D Appl. Phys. 2017, 50, 215401. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.Y.; Aldalbahi, A.; Panhuis, M.; Velazquez, R.; Feng, P.X. A simple route to carbon micro- and nanorod hybrid structures by physical vapour deposition. J. Phys. D Appl. Phys. 2012, 45, 395102. [Google Scholar] [CrossRef]
- Feng, P.X.; Zhang, H.X.; Peng, X.Y.; Sajjad, M.; Chu, J. A novel compact design of calibration equipment for gas and thermal sensors. Rev. Sci. Instrum. 2011, 82, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Chan, Y.C.; Zhang, K.L. Fast response resistive humidity sensitivity ofpolyimide/multiwall carbon nanotube composite films. Sens. Actuators B Chem. 2011, 152, 99–106. [Google Scholar] [CrossRef]
- Lukaszewicz, J.P.; Skompska, M. Carbon films for humidity sensors. Sens. Lett. 2006, 113, 970–977. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.Y.; Sajjad, M.; Yang, B.Q.; Feng, P.X. Nanostructures and sensing properties of ZnO prepared using normal and oblique angle deposition techniques. Thin Solid Films 2012, 520, 3493–3498. [Google Scholar] [CrossRef]
- Wang, X.D.; Song, J.H.; Liu, J.; Wang, Z.L. Direct-current nanogenerator driven by ultrasonic waves. Science 2007, 316, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Feng, P.X. Properties of one-dimensional tilted carbon nanorod arrays synthesized by the catalyst-assisted oblique angle deposition technique. J. Phys. D Appl. Phys. 2009, 42, 025406. [Google Scholar] [CrossRef]
- Jia, Y.T.; Yu, H.; Cai, J.; Li, Z.; Dong, F.C. Explore on the quantitative analysis of specific surface area on sensitivity of polyacrylic acid-based QCM ammonia sensor. Sens. Actuators B Chem. 2017, 243, 1042–1045. [Google Scholar] [CrossRef]
- Zhang, H.X.; Feng, P.X. Electrical and structural characterizations of one-dimensional carbon nanostructures synthesized at ambient pressure. J. Phys. D Appl. Phys. 2008, 41, 155425. [Google Scholar] [CrossRef]
- Rossi, M.C.; Salvatori, S.; Ascarelli, P.; Cappelli, E.; Orlando, S. Effect of nanostructure and back contact material on the field emission properties of carbon films. Diam. Relat. Mater. 2002, 11, 819–823. [Google Scholar] [CrossRef]
- Adu, K.W.; Li, Q.; Desai, S.C.; Sidorov, A.N.; Sumanasekera, G.U.; Lueking, A.D. Morphological, structural, and chemical effects in response of novel carbide derived carbon sensor to NH3, N2O, and air. Langmuir 2009, 25, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.P.; Lim, L.T.; Min, N.K.; Lee, M.J.; Lee, C.J.; Park, C.W. Novel resistive-type humidity sensor based on multiwall carbon nanotube/polyimide composite films. Sens. Actuators B Chem. 2010, 145, 120–125. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Liu, X.W.; Chen, W.P.; Li, T. Carbon nanotubes humidity sensor based on high testing frequencies. Sens. Actuators A Phys. 2011, 168, 10–13. [Google Scholar] [CrossRef]
- Liu, L.T.; Ye, X.Y.; Wu, K.; Han, R.; Zhou, Z.Y.; Cui, T.H. Humidity sensitivity of multiwalled carbon nanotube networks deposited by dielectrophoresis. Sensors 2009, 9, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Cao, T.; Zhou, L.D.; Gu, E.D.; Yu, D.S.; Jiang, D.S. Layer-by-layer assembly and humidity sensitive behavior of poly(ethyleneimine)/multiwall carbon nanotube composite films. Sens. Actuators B Chem. 2006, 119, 512–515. [Google Scholar] [CrossRef]
- Chen, H.J.; Xue, Q.Z.; Ma, M.; Zhou, X.Y. Capacitive humidity sensor based on amorphous carbon film/n-Si heterojunctions. Sens. Actuators B Chem. 2010, 150, 487–489. [Google Scholar] [CrossRef]
- Peng, X.Y.; Chu, J.; Aldalbahi, A.; Rivera, M.; Wang, L.D.; Duan, S.K.; Feng, P. A flexible humidity sensor based on KC–MWCNTs composites. Appl. Surf. Sci. 2016, 387, 149–154. [Google Scholar] [CrossRef]
- Tetelin, A.; Pellet, C.; Laville, C.; N’Kaoua, G. Fast response humidity sensors for a medical microsystem. Sens. Actuators B Chem. 2003, 91, 211–218. [Google Scholar] [CrossRef]
- Chu, J.; Peng, X.; Feng, P.; Sheng, Y.; Zhang, J. Study of humidity sensors based on nanostructured carbon films produced by physical vapor deposition. Sens. Actuators B Chem. 2013, 178, 508–513. [Google Scholar] [CrossRef]
- Zahab, A.; Spina, L.; Poncharal, P.; Marliere, C. Water-vapor effect on the electrical conductivity of a single-walled carbon nanotube mat. Phys. Rev. B 2000, 62, 10000–10003. [Google Scholar] [CrossRef]




| Materials | Sensitivity | Response Time | Recovery Time | Ref. |
|---|---|---|---|---|
| PI a/MWCNT (2.0 wt %) | 0.0018 (20–90% RH) | 5 s | - | [16] |
| MWCNTs | 0.0028(11–75.5% RH) | 16 s | 8 s | [25] |
| MWCNT network | 0.0056 (25–95% RH) | 3 s | 25 s | [26] |
| PEI b/MWCNT (Layer-by-layer) | 0.0099 (5–85% RH) | 2 s | 30 s | [27] |
| a-C c film/n-Si | 0.0238 (11–95% RH) | ~3 min | ~4 min | [28] |
| KC d-MWCNT-G e | 0.025 (20–80% RH) | 50 s | 100 s | [29] |
| BCB f, 4024-40, Dow Chemical | 0.0250 (50–90% RH) | 0.5 s | 4.5 s | [30] |
| Carbon Nanosheets | 0.033 (11–40% RH) | 30 s | 90 s | [31] |
| Carbon Nanoplumes | 0.117 (5–65% RH) | 8 s | 9.7 s | ours |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, S.; Peng, X.; Wang, L.; Duan, S.; Chu, J.; Jia, P. Highly Sensitive Humidity Sensor Based on Oblique Carbon Nanoplumes. Sensors 2018, 18, 3407. https://doi.org/10.3390/s18103407
Qiao S, Peng X, Wang L, Duan S, Chu J, Jia P. Highly Sensitive Humidity Sensor Based on Oblique Carbon Nanoplumes. Sensors. 2018; 18(10):3407. https://doi.org/10.3390/s18103407
Chicago/Turabian StyleQiao, Siqi, Xiaoyan Peng, Lidan Wang, Shukai Duan, Jin Chu, and Pengfei Jia. 2018. "Highly Sensitive Humidity Sensor Based on Oblique Carbon Nanoplumes" Sensors 18, no. 10: 3407. https://doi.org/10.3390/s18103407




