A Regenerative Immunoaffinity Layer Based on the Outer Membrane of Z-Domains Autodisplaying E. coli for Immunoassays and Immunosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Culture of E. coli Cells with Autodisplayed Z-Domains and the Isolation of the OM
2.3. Regenerative Assays Based on E. coli Cells and Their Outer Membrane
2.4. SPR Measurement
3. Results and Discussion
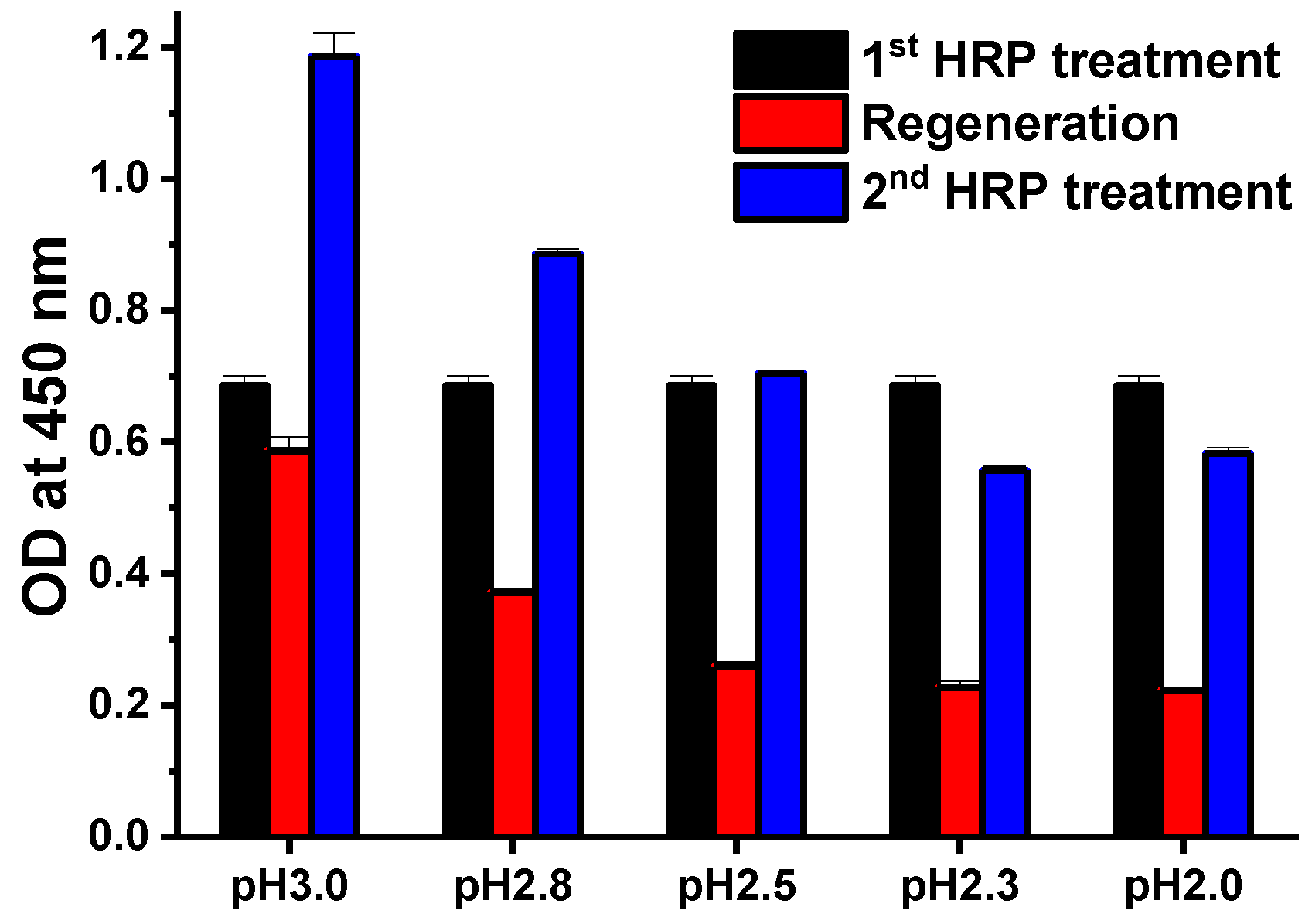
3.1. Optimization of Regenerative Assays
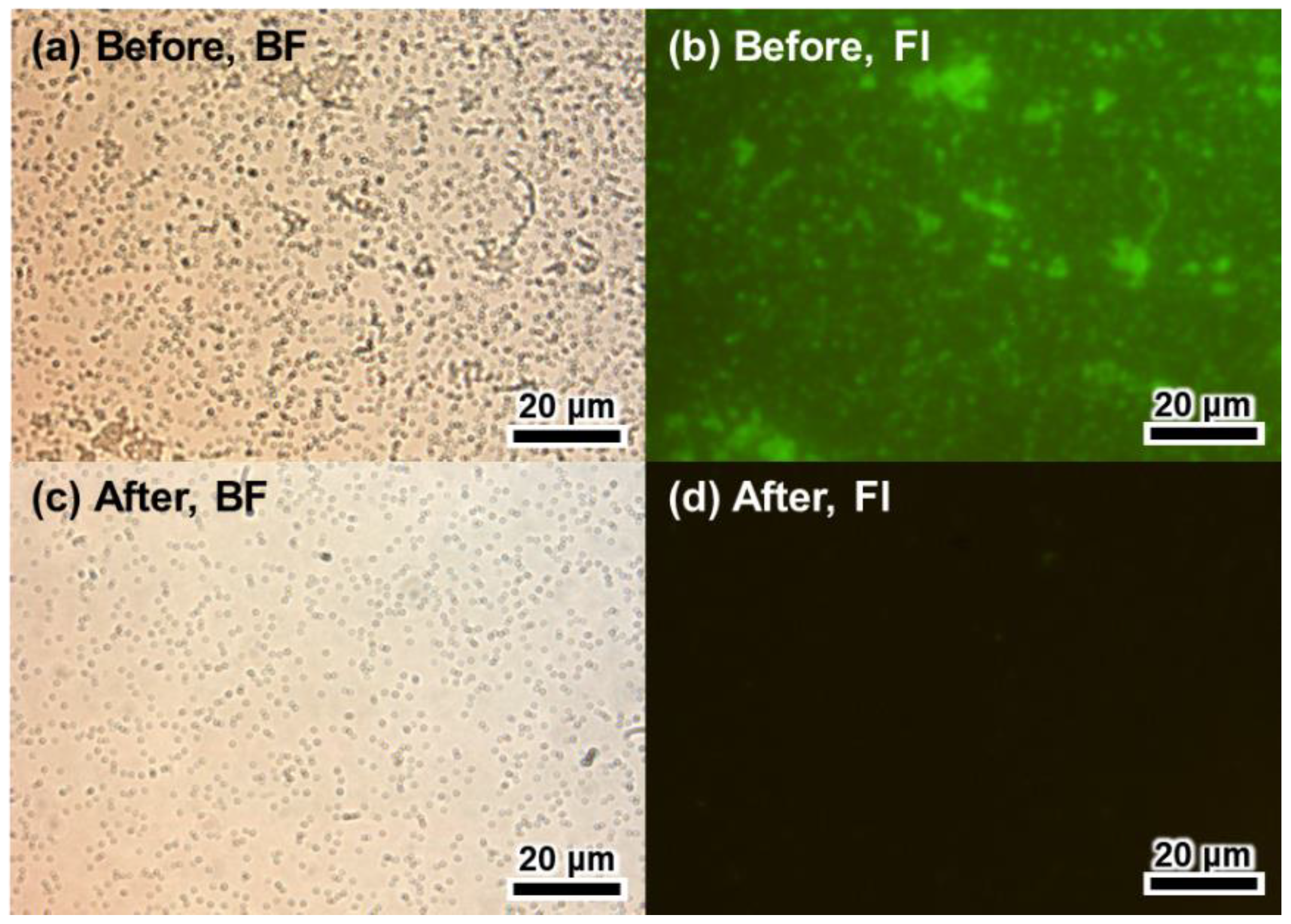
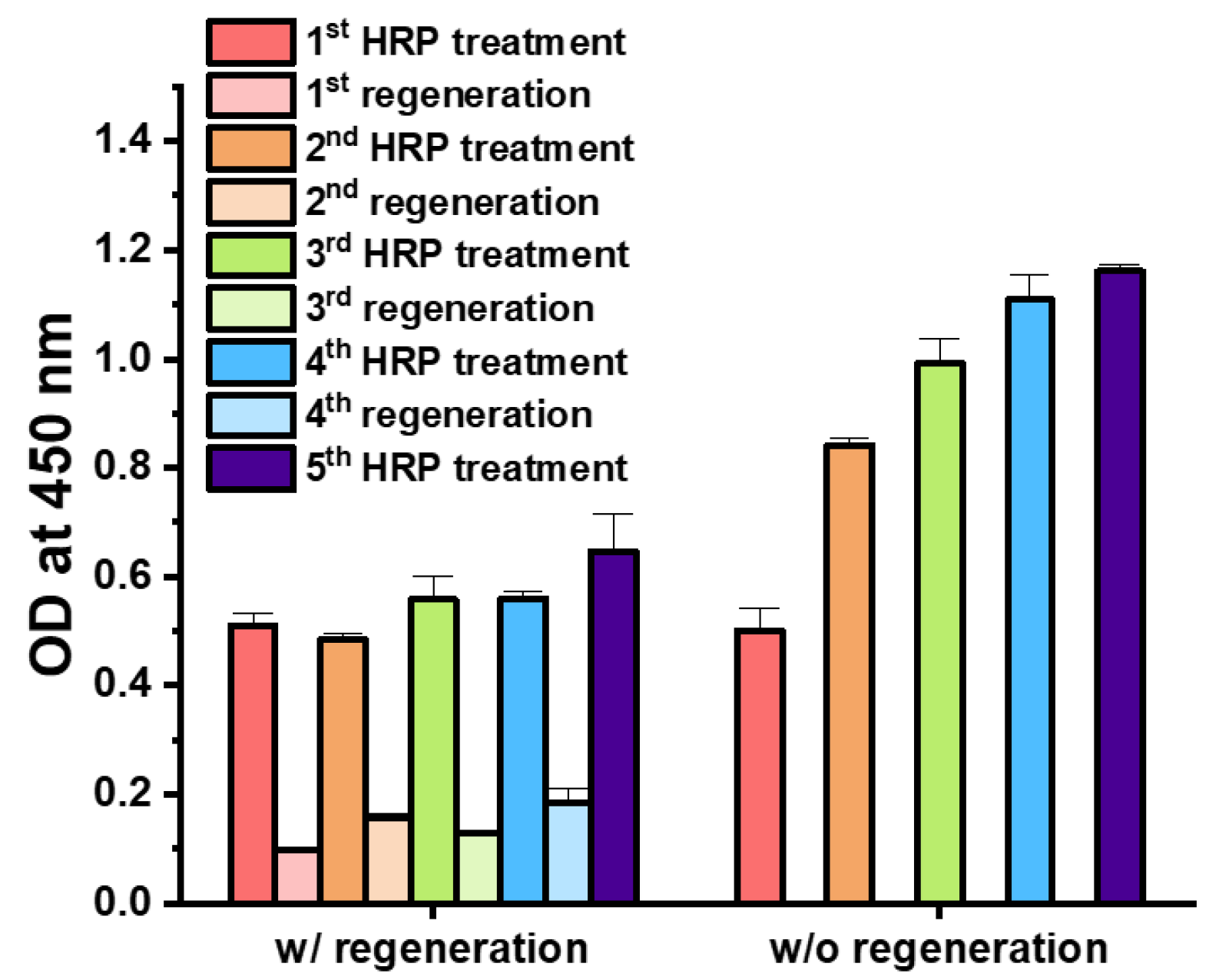
3.2. Regenerative Immunoassay Based on Z-Domains Autodisplaying E. coli
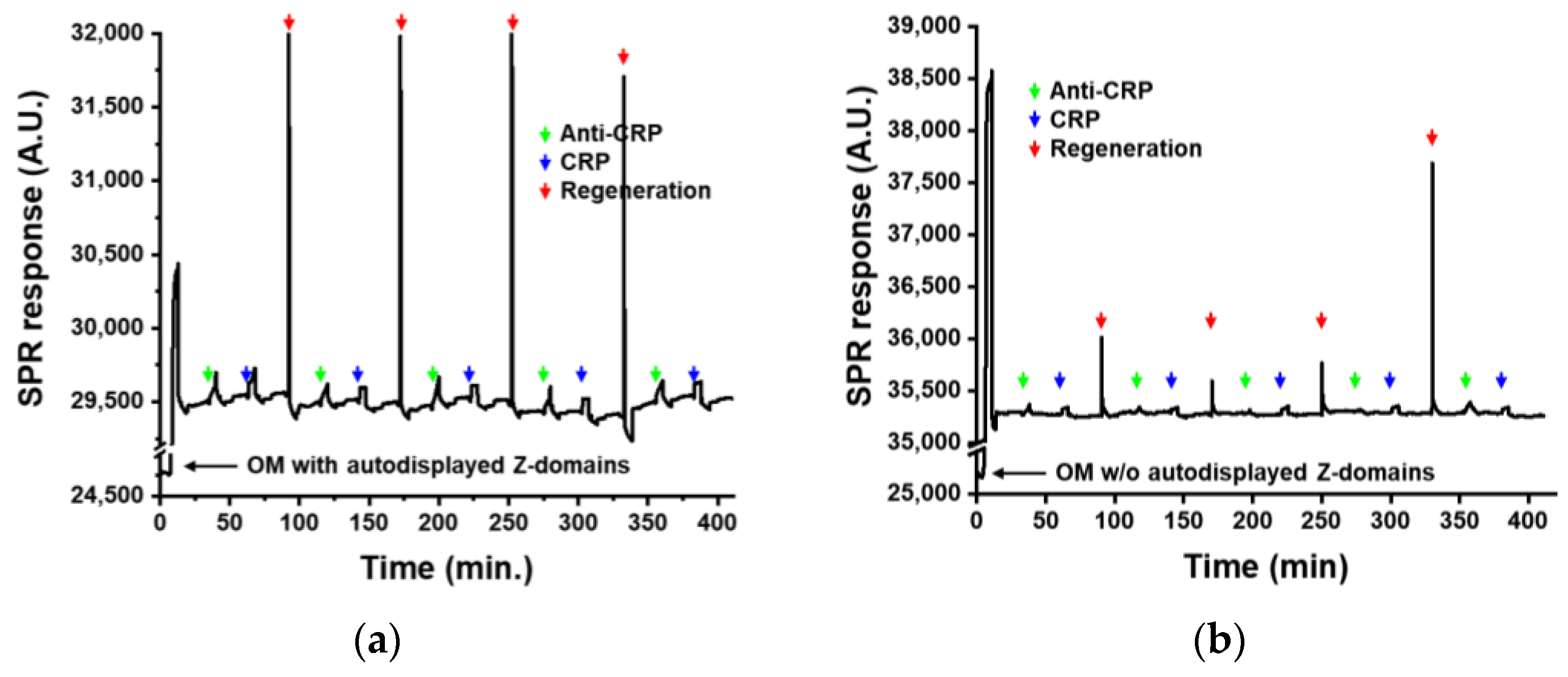
3.3. Regenerative Immunoaffinity Layer for SPR Biosensor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conroy, P.J.; Hearty, S.; Leonard, P.; O’Kennedy, R.J. Antibody production, design and use for biosensor-based applications. Semin. Cell Dev. Biol. 2009, 20, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Goode, J.A.; Rushworth, J.V.; Millner, P.A. Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2015, 31, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors—Principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Wild, D. The Immunoassay Handbook, 2nd ed.; Nature: London, UK, 2001. [Google Scholar]
- Lu, B.; Smyth, M.R.; O’Kennedy, R. Oriented immobilization of antibodies and its applications in immunoassays and immunosensors. Analyst 1996, 121, 29R–32R. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.P.; Jacoby, M.A.; Ligler, F.S.; King, K.D. Effectiveness of protein A for antibody immobilization for a fiber optic biosensor. Biosens. Bioelectron. 1997, 12, 329–336. [Google Scholar] [CrossRef]
- Bae, Y.M.; Oh, B.K.; Lee, W.; Lee, W.H.; Choi, J.W. Study on orientation of immunoglobulin G on protein G layer. Biosens. Bioelectron. 2005, 21, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Park, J.M.; Bernhardt, R.; Pyun, J.C. Immunosensor with a controlled orientation of antibodies by using NeutrAvidin-protein A complex at immunoaffinity layer. J. Biotechnol. 2006, 126, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H. A simple and real-time sensing of human serum albumin using antibody-modified CNT-FET. BioChip J. 2017, 11, 116–120. [Google Scholar] [CrossRef]
- Owaku, K.; Goto, M.; Ikariyama, Y.; Aizawa, M. Protein A Langmuir-Blodgett film for antibody immobilization and its use in optical immunosensing. Anal. Chem. 1995, 67, 1613–1616. [Google Scholar] [CrossRef]
- Park, M.; Jose, J.; Pyun, J.C. SPR biosensor by using E. coli outer membrane layer with autodisplayed Z-domains. Sens. Actuators B Chem. 2011, 154, 82–88. [Google Scholar] [CrossRef]
- Jose, J.; Park, M.; Pyun, J.C. E. coli outer membrane with autodisplayed Z-domain as a molecular recognition layer of SPR biosensor. Biosens. Bioelectron. 2010, 25, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jose, J.; Pyun, J.C. Hypersensitive immunoassay by using Escherichia coli outer membrane with autodisplayed Z-domains. Enzyme Microb. Technol. 2010, 46, 309–314. [Google Scholar] [CrossRef]
- Björk, I.; Petersson, B.Å.; Sjöquist, J. Some physicochemical properties of protein A from Staphylococcus aureus. Eur. J. Biochem. 1972, 29, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, R.; Thorén-Tolling, K.; Sjöquist, J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J. Immunol. Methods 1983, 62, 1–13. [Google Scholar] [CrossRef]
- Löwenadler, B.; Jansson, B.; Paleus, S.; Houngren, E.; Nilsson, B.; Moks, T.; Palm, G.; Josephson, S.; Philipson, L.; Uhlén, M. A gene fusion system for generating antibodies against short peptides. Gene 1987, 58, 87–97. [Google Scholar] [CrossRef]
- Iijima, M.; Kadoya, H.; Hatahira, S.; Hiramatsu, S.; Jung, G.; Martin, A.; Quinn, J.; Jung, J.; Jeong, S.Y.; Choi, E.K. Nanocapsules incorporating IgG Fc-binding domain derived from Staphylococcus aureus protein A for displaying IgGs on immunosensor chips. Biomaterials 2011, 32, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Jahnig, F.; Meyer, T.F. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol. Microbiol. 1995, 18, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I. R.; Cappello, R.; Nataro, J. P. Autotransporter proteins, evolution and redefining protein secretion. Trends Microbiol. 2000, 8, 529–532. [Google Scholar] [CrossRef]
- Jose, J.; Meyer, T.F. The autodisplay story, from discovery to biotechnical and biomedical applications. Microbiol. Mol. Biol. Rev. 2007, 71, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Pyun, J.C.; Jose, J. Orientation and density control of proteins on solid matters by outer membrane coating: Analytical and diagnostic applications. J. Pharm. Biomed. Anal. 2018, 147, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Maas, R.M.; Teese, M.G. Autodisplay of enzymes—Molecular basis and perspectives. J. Biotechnol. 2012, 161, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, E.; Weiss, S.; Winterer, E.; Maas, R.; Heinzle, E.; Jose, J. Esterase autodisplay: Enzyme engineering and whole-cell activity determination in microplates with pH sensors. Appl. Environ. Microbiol. 2008, 74, 4782–4791. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.D.; Hannemann, F.; Teese, M.G.; Bernhardt, R.; Jose, J. Autodisplay of functional CYP106A2 in Escherichia coli. J. Biotechnol. 2012, 161, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Park, M.; Pyun, J.C. Highly sensitive immunoassay based on E. coli with autodisplayed Z-domain. Anal. Chim. Acta 2010, 667, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Bong, J.H.; Yoo, G.; Jose, J.; Kang, M.J.; Pyun, J.C. Optimization of a FACS based-immunoassay using E. coli autodisplaying Z-domains. BioChip J. 2013, 7, 173–179. [Google Scholar] [CrossRef]
- Park, M.; Bong, J.H.; Yoo, G.; Jose, J.; Kang, M.J.; Pyun, J.C. Flow cytometric immunoassay using E. coli with autodisplayed Z-domains. Enzyme Microb. Technol. 2013, 53, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Yoo, G.; Bong, J.H.; Jose, J.; Kang, M.J.; Pyun, J.C. Isolation and characterization of the outer membrane of Escherichia coli with autodisplayed Z-domains. Biochim. Biophys. Acta 2015, 1848, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.P.; Weller, M.G.; Duveneck, G.L.; Ehrat, M.; Widmer, H.M. Fiber-optic evanescent wave biosensor for the detection of oligonucleotides. Anal. Chem. 1996, 68, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Asanov, A.N.; Wilson, W.W.; Oldham, P.B. Regenerable biosensor platform: A total internal reflection fluorescence cell with electrochemical control. Anal. Chem. 1998, 70, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Michalzik, M.; Wendler, J.; Rabe, J.; Büttgenbach, S.; Bilitewski, U. Development and application of a miniaturised quartz crystal microbalance (QCM) as immunosensor for bone morphogenetic protein-2. Sens. Actuators B Chem. 2005, 105, 508–515. [Google Scholar] [CrossRef]
- Queirós, R.B.; de-Los-Santos-Álvarez, N.; Noronha, J.; Sales, M.G.F. A label-free DNA aptamer-based impedance biosensor for the detection of E. coli outer membrane proteins. Sens. Actuators B Chem. 2013, 181, 766–772. [Google Scholar] [CrossRef]
- Mattos, A.; Freitas, T.; Silva, V.; Dutra, R. A dual quartz crystal microbalance for human cardiac troponin T in real time detection. Sens. Actuators B Chem. 2012, 161, 439–446. [Google Scholar] [CrossRef]
- Xu, M.; Luo, X.; Davis, J.J. The label free picomolar detection of insulin in blood serum. Biosens. Bioelectron. 2013, 39, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Baker, Z.; Harrison, R.W.; Miller, B.F. Action of synthetic detergents on the metabolism of bacteria. J. Exp. Med. 1941, 73, 249–271. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, D.; Pyun, J.-C.; Jose, J.; Park, M. A Regenerative Immunoaffinity Layer Based on the Outer Membrane of Z-Domains Autodisplaying E. coli for Immunoassays and Immunosensors. Sensors 2018, 18, 4030. https://doi.org/10.3390/s18114030
Jeon D, Pyun J-C, Jose J, Park M. A Regenerative Immunoaffinity Layer Based on the Outer Membrane of Z-Domains Autodisplaying E. coli for Immunoassays and Immunosensors. Sensors. 2018; 18(11):4030. https://doi.org/10.3390/s18114030
Chicago/Turabian StyleJeon, Daseul, Jae-Chul Pyun, Joachim Jose, and Min Park. 2018. "A Regenerative Immunoaffinity Layer Based on the Outer Membrane of Z-Domains Autodisplaying E. coli for Immunoassays and Immunosensors" Sensors 18, no. 11: 4030. https://doi.org/10.3390/s18114030





