High-Resolution Shear Wave Imaging of the Human Cornea Using a Dual-Element Transducer
Abstract
:1. Introduction
2. Materials and Methods
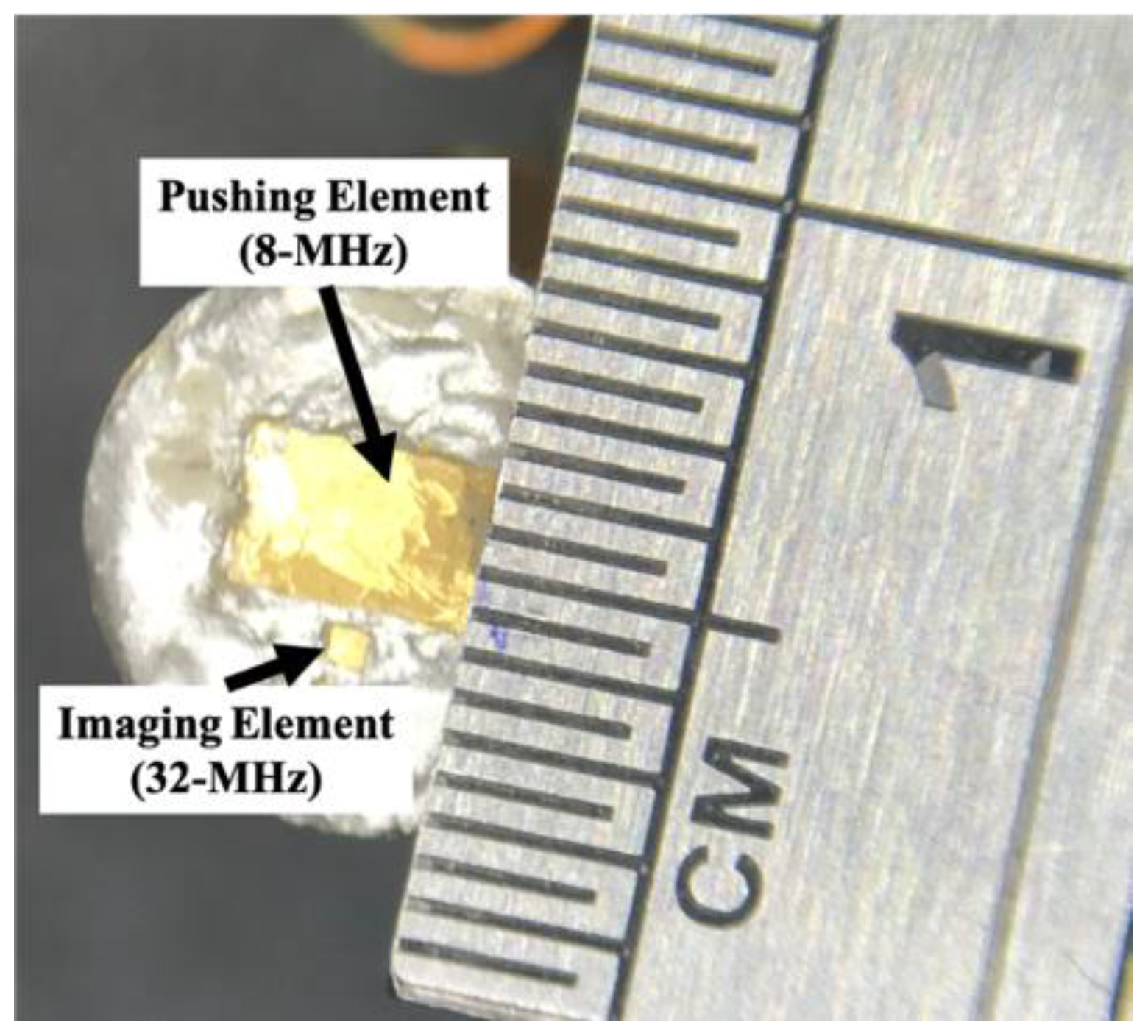
2.1. Dual-Element Transducer
2.2. Experimental Setup for HR-SWI
2.3. Timing Diagram
2.4. Scanning Scheme
2.5. Lamb Wave Empirical Young’s Modulus Estimation
2.6. Performace of HR-SWI
2.7. Gelatin Phantom
2.8. Human Corneal Samples
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elsheikh, A.; Wang, D.; Pye, D. Determination of the modulus of elasticity of the human cornea. J. Refract. Surg. 2007, 23, 808–818. [Google Scholar] [PubMed]
- Nordan, L.T. Keratoconus: Diagnosis and treatment. Int. Ophthalmol. Clin. 1997, 37, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.J. Aetiology of spheroidal degeneration of the cornea in Labrador. Br. J. Ophthalmol. 1981, 65, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.P. Photokeratitis and other phototoxic effects on the cornea and conjunctiva. Int. J. Toxicol. 2002, 21, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, T.; Loesel, F.H.; Kurtz, R.M.; Horvath, C.; Bille, J.F.; Mourou, G. Corneal refractive surgery with femtosecond lasers. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 902–910. [Google Scholar] [CrossRef]
- Moren, H.; Malmsjo, M.; Mortensen, J.; Ohrstrom, A. Riboflavin and ultraviolet a collagen crosslinking of the cornea for the treatment of keratitis. Cornea 2010, 29, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Ameri, H.; DeBoer, C.; Rowley, A.P.; Xu, X.; Sun, L.; Wang, S.H.; Humayun, M.S.; Shung, K.K. Evaluation of lens hardness in cataract surgery using high-frequency ultrasonic parameters in vitro. Ultrasound Med. Biol. 2007, 33, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, R.; Tsui, P.H.; Zhou, Q.; Humayun, M.S.; Shung, K.K. Measurements of attenuation coefficient for evaluating the hardness of a cataract lens by a high-frequency ultrasonic needle transducer. Phys. Med. Biol. 2009, 54, 5981–5994. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.C.; Zhou, Q.; Ameri, H.; Wu, D.W.; Sun, L.; Wang, S.H.; Humayun, M.S.; Shung, K.K. Determining the acoustic properties of the lens using a high-frequency ultrasonic needle transducer. Ultrasound Med. Biol. 2007, 33, 1971–1977. [Google Scholar] [CrossRef]
- Tsui, P.H.; Huang, C.C.; Chang, C.C.; Wang, S.H.; Shung, K.K. Feasibility study of using high-frequency ultrasonic Nakagami imaging for characterizing the cataract lens in vitro. Phys. Med. Biol. 2007, 52, 6413–6425. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, J.; Singh, M.; Wu, C.; Liu, C.H.; Raghunathan, R.; Aglyamov, S.R.; Vantipalli, S.; Twa, M.D.; Larin, K.V. Optical coherence elastography assessment of corneal viscoelasticity with a modified Rayleigh-Lamb wave model. J. Mech. Behav. Biomed. Mater. 2017, 66, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Larin, K.V. Noncontact depth-resolved micro-scale optical coherence elastography of the cornea. Biomed. Opt. Express 2014, 5, 3807–3821. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, J.; Singh, M.; Wu, C.; Liu, C.H.; Wang, S.; Idugboe, R.; Raghunathan, R.; Sudheendran, N.; Aglyamov, S.R.; et al. Quantitative methods for reconstructing tissue biomechanical properties in optical coherence elastography: A comparison study. Phys. Med. Biol. 2015, 60, 3531–3547. [Google Scholar] [CrossRef]
- Ambroziński, Ł.; Song, S.; Yoon, S.J.; Pelivanov, I.; Li, D.; Gao, L.; Shen, T.T.; Wang, R.K.; O’Donnell, M. Acoustic micro-tapping for non-contact 4D imaging of tissue elasticity. Sci. Rep. 2015, 6, 38967. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Ma, T.; Shih, C.C.; Heur, M.; Jun, Z.; Shung, K.K.; Varma, R.; Humayun, M.; Zhou, Q. Ultrasonic Micro-Elastography to Assess Biomechanical Properties of the Cornea. IEEE Trans. Biomed. Eng. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef]
- Huang, C.C.; Sun, L.; Dailey, S.H.; Wang, S.H.; Shung, K.K. High frequency ultrasonic characterization of human vocal fold tissue. J. Acoust. Soc. Am. 2007, 122, 1827. [Google Scholar] [CrossRef]
- Huang, C.C. High-frequency attenuation and backscatter measurements of rat blood between 30 and 60 MHz. Phy.s Med. Biol. 2010, 55, 5801–5815. [Google Scholar] [CrossRef]
- Shung, K.K. Diagnostic Ultrasound: Imaging and Blood Flow Measurements; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-1138892880. [Google Scholar]
- Hollman, K.W.; Emelianov, S.Y.; Neiss, J.H.; Jotyan, G.; Spooner, G.J.R.; Juhasz, T.; Kurtz, R.M.; O’Donnell, M. Strain imaging of corneal tissue with an ultrasound elasticity microscope. Cornea 2002, 21, 68–73. [Google Scholar] [CrossRef]
- Nightingale, K.R.; Palmeri, M.L.; Nightingale, R.W.; Trahey, G.E. On the feasibility of remote palpation using acoustic radiation force. J. Acoust. Soc. Am. 2001, 110, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M.; Wold, L.E.; Alizad, A.; Greenleaf, J.F. Vibro-acoustic tissue mammography. IEEE Trans. Med. Imaging 2002, 21, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleke, C.; Konofagou, E.E. Harmonic motion imaging for focused ultrasound (HMIFU): A fully integrated technique for sonication and monitoring of thermal ablation in tissues. Phys. Med. Biol. 2008, 53, 1773–1793. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Mcaleavey, S.A.; Fong, K.L.; Trahey, G.E.; Nightingale, K.R. Dynamic mechanical response of elastic spherical inclusions to impulsive acoustic radiation force excitation. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2006, 53, 2065–2079. [Google Scholar] [CrossRef] [Green Version]
- Sarvazyan, A.P.; Rudenko, O.V.; Swanson, S.D.; Fowlkes, J.B.; Emelianov, S.Y. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 1998, 24, 1419–1435. [Google Scholar] [CrossRef]
- Urs, R.; Lloyd, H.O.; Silverman, R. Acoustic radiation force for noninvasive evaluation of corneal biomechanical changes induced by cross-linking therapy. J. Ultrasound Med. 2014, 33, 1417–1426. [Google Scholar] [CrossRef]
- Shih, C.C.; Huang, C.C.; Zhou, Q.; Shung, K.K. High-resolution acoustic-radiation-force-impulse imaging for assessing corneal sclerosis. IEEE Trans. Med. Imaging 2013, 32, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Touboul, D.; Gennisson, J.L.; Bercoff, J.; Fink, M. High-Resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Trans. Med. Imaging 2009, 28, 1881–1893. [Google Scholar] [CrossRef]
- Silverman, R.H. High-resolution ultrasound imaging of the eye–a review. Clin. Exp. Ophthalmol. 2009, 37, 54–67. [Google Scholar] [CrossRef]
- Food and Drug Administration. Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers; US Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health: Rockville, MD, USA, 2008.
- Palmeri, M.L.; Wang, M.H.; Dahi, J.J.; Frinkley, K.D.; Nightingale, K.R. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med. Biol. 2008, 34, 546–558. [Google Scholar] [CrossRef]
- Song, P.; Macdonald, M.C.; Behler, R.H.; Lanning, J.D.; Wang, M.H.; Urban, M.W.; Manduca, A.; Zhao, H.; Callstrom, M.R.; Alizad, A.; et al. Two-dimensional shear wave elastography on conventional ultrasound scanners with time-aligned sequential tracking (TAST) and comb-push ultrasound shear elastography (CUSE). IEEE Trans. Ultrason. Ferroelect. Freq. Control 2015, 62, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Rouze, N.C.; Wang, M.H.; Palmeri, M.L.; Nightingale, K.R. Parameters affecting the resolution and accuracy of 2-D quantitative shear wave images. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2012, 59, 1729–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.C.; Shih, M.H.; Chang, K.F.; Huang, J.M.; Shin, J.M.; Lin, W.C. Characterizing clinical isolates of Acanthamoeba castellanii with high resistance to polyhexamethylene biguanide in Taiwan. J. Microbiol. Immunol. Infect. 2015, 50, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, P.Y.; Shih, C.C. Estimating the viscoelastic modulus of a thrombus using an ultrasonic shear wave approach. Med. Phys. 2013, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Shih, C.C.; Liu, T.Y.; Lee, P.Y. Assessing the viscoelastic properties of thrombus using a solid-sphere-based instantaneous force approach. Ultrasound Med. Biol. 2011, 37, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.R.; Palmeri, M.L.; Gregg, T. Analysis of contrast in images generated with transient acoustic radiation force. Ultrasound Med. Biol. 2006, 32, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mikula, E.; Winkler, M.; Juhasz, T.; Brown, D.J.; Shoa, R.; Tran, S.; Jester, J.V. Axial mechanical and structural characterization of keratoconus corneas. Exp. Eye Res. 2018, 175, 14–19. [Google Scholar] [CrossRef]
- Hollender, P.J.; Rosenzweig, S.J.; Nightingale, K.R.; Trahey, G.E. Single- and multiple-track-location shear wave and acoustic radiation force impulse imaging: Matched comparison of contrast, contrast-to-noise ratio and resolution. Ultrasound Med. Biol. 2015, 41, 1043–1057. [Google Scholar] [CrossRef]
- Nenadic, I.Z.; Urban, M.W.; Aristizabal, S.; Mitchell, S.A.; Humphrey, T.C.; Greenleaf, J.F. On Lamb and Rayleigh wave convergence in viscoelastic tissues. Phys. Med. Biol. 2011, 56, 6723–6738. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.C.; Qian, X.; Ma, T.; Han, Z.; Huang, C.C.; Zhou, Q.; Shung, K.K. Quantitative assessment of thin-layer tissue viscoelastic properties using ultrasonic micro-elastography with lamb wave model. IEEE Trans. Med. Imaging 2018, 37, 1887–1898. [Google Scholar] [CrossRef]
- Last, J.A.; Thomasy, S.M.; Croasdale, C.R.; Russell, P.; Murphy, C.J. Compliance profile of the human cornea as measured by atomic force microscopy. Micron 2012, 43, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.; Chai, D.; Kriling, S.; Nien, C.J.; Brown, D.J.; Jester, B.; Juhasz, T.; Jester, J.V. Nonlinear optical macroscopic assessment of 3-D corneal collagen organization and axial biomechanics. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8818–8827. [Google Scholar] [CrossRef]
- Uchio, E.; Ohno, S.; Kudoh, J.; Aoki, K.; Kisielewicz, L.T. Simulation model of an eyeball based on finite element analysis on a supercomputer. Br. J. Ophthalmol. 1999, 83, 1106–1111. [Google Scholar] [CrossRef] [Green Version]
- Mikula, E.; Hollman, K.; Chai, D.; Jester, J.V.; Juhasz, T. Measurement of corneal elasticity with an acoustic radiation force elasticity microscope. Ultrasound Med. Biol. 2014, 40, 1671–1679. [Google Scholar] [CrossRef]
- Dias, J.M.; Ziebarth, N.M. Anterior and posterior corneal stroma elasticity assessed using nanoindentation. Exp. Eye Res. 2013, 115, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Orssengo, G.J.; Pye, D.C. Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bull. Math. Biol. 1999, 61, 551–572. [Google Scholar] [CrossRef]
- Cartwright, N.E.K.; Tyrer, J.R.; Marshall, J. Age-related differences in the elasticity of the human cornea. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4324–4329. [Google Scholar] [CrossRef]





| Methodology | Shear Modulus (kPa) | IOP (mmHg) | Reference |
|---|---|---|---|
| Inflation test | 2 55.6–123.9 | No | [40] |
| Atomic force microscopy | 2 1.79–27.39 | No | [41] |
| Nanoindentation | 10.21–269.01 | No | [42] |
| 1 ARFEM | 0.5–1.67 | 20 | [43] |
| Needle indentation | 0.75–1.75 | 50 | [44] |
| The present study | 3.71–19.33 | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Shih, C.-C.; Lin, W.-C.; Ma, T.; Zhou, Q.; Shung, K.K.; Huang, C.-C. High-Resolution Shear Wave Imaging of the Human Cornea Using a Dual-Element Transducer. Sensors 2018, 18, 4244. https://doi.org/10.3390/s18124244
Chen P-Y, Shih C-C, Lin W-C, Ma T, Zhou Q, Shung KK, Huang C-C. High-Resolution Shear Wave Imaging of the Human Cornea Using a Dual-Element Transducer. Sensors. 2018; 18(12):4244. https://doi.org/10.3390/s18124244
Chicago/Turabian StyleChen, Pei-Yu, Cho-Chiang Shih, Wei-Chen Lin, Teng Ma, Qifa Zhou, K. Kirk Shung, and Chih-Chung Huang. 2018. "High-Resolution Shear Wave Imaging of the Human Cornea Using a Dual-Element Transducer" Sensors 18, no. 12: 4244. https://doi.org/10.3390/s18124244
APA StyleChen, P. -Y., Shih, C. -C., Lin, W. -C., Ma, T., Zhou, Q., Shung, K. K., & Huang, C. -C. (2018). High-Resolution Shear Wave Imaging of the Human Cornea Using a Dual-Element Transducer. Sensors, 18(12), 4244. https://doi.org/10.3390/s18124244








