Microwave Sensors for Breast Cancer Detection
Abstract
:1. Introduction
2. Electrical Properties of Tissue
2.1. Dielectric Properties of Breast Tissues
2.2. Modelling of Biological Tissue
3. Microwave Breast Imaging
3.1. Microwave Tomography
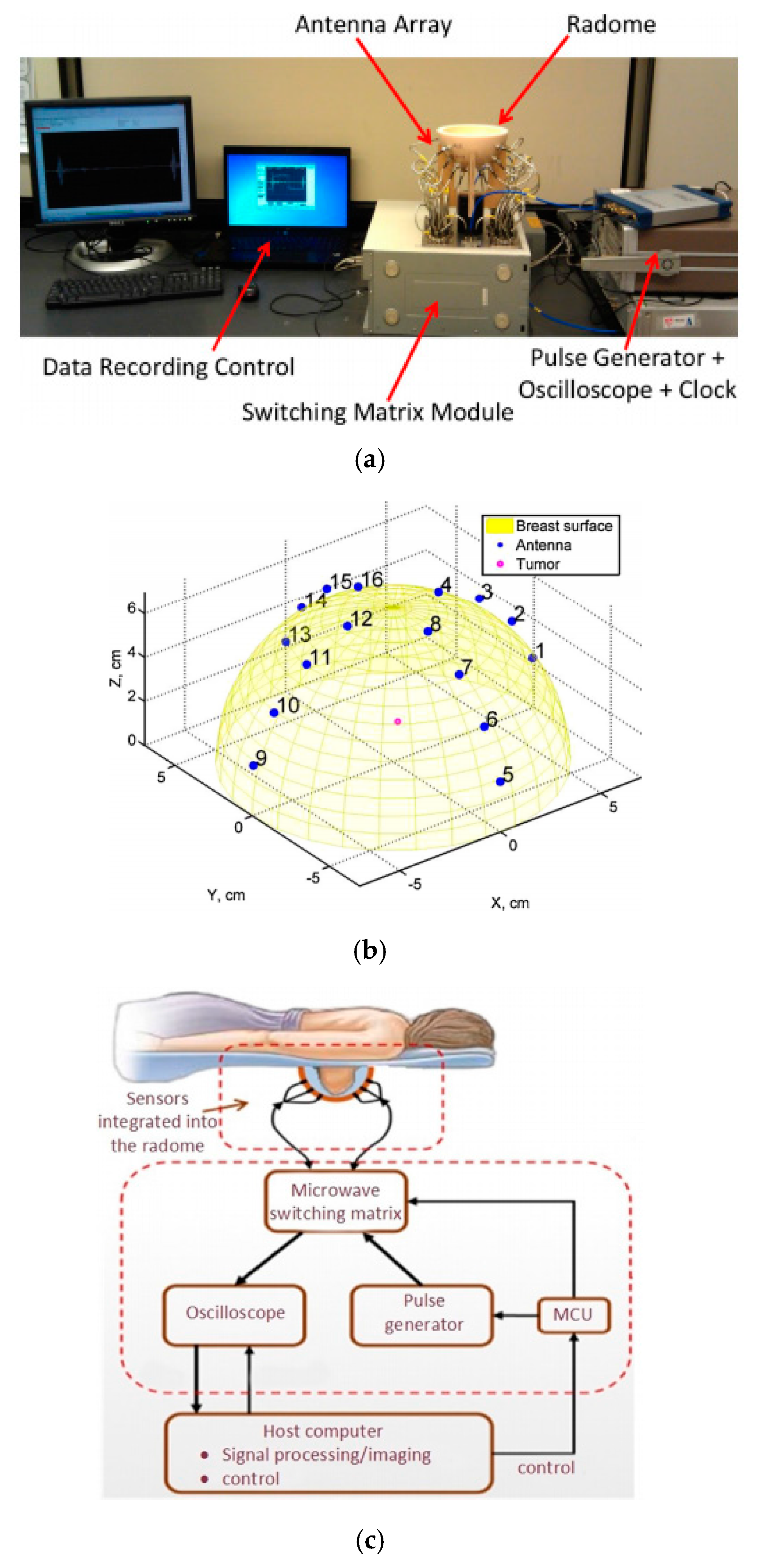
3.2. Radar Based Microwave Imaging
4. RF Sensors for Biomedical Applications
4.1. Microwave Sensors for Microwave Breast Imaging Systems
4.2. RF Biosensors for Cancer Biomarker Detection
5. Challenges and Future Works
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Fontana, R.J.; Sherry, F.; Conjeevaram, H.S.; Su, G.L.; Lok, A.S. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J. Hepatol. 2015, 42, 218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, X.; Sheng, L.; Xu, S.; Dong, L.; Liu, L. Perioperative chemotherapy more of a benefit for overall survival than adjuvant chemotherapy for operable gastric cancer: An updated meta-analysis. Sci. Rep. 2015, 5, 12850. [Google Scholar] [CrossRef] [PubMed]
- Mcguire, S. World Cancer report 2014. Geneva, Switzerland: World health organization, international agency for research on cancer, who press, 2015. Adv. Nutr. 2016, 7, 418. [Google Scholar] [CrossRef] [PubMed]
- Mohebian, M.R.; Marateb, H.R.; Mansourian, M.; Mañanas, M.A.; Mokarian, F. A hybrid computer-aided-diagnosis system for prediction of breast cancer recurrence (HPBCR) using optimized ensemble learning. Comput. Struct. Biotechnol. J. 2017, 15, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.W.F.; Li, J.; Sparapani, R.A.; Laud, P.W.; Nattinger, A.B. The interplay between hospital and surgeon factors and the use of sentinel lymph node biopsy for breast cancer. Medicine 2016, 95, e4392. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.J.; Carrasco, A.; Culp, S.H.; Matin, S.F.; Tamboli, P.; Tannir, N.M.; Wood, C.G. Limitations of preoperative biopsy in patients with metastatic renal cell carcinoma: Comparison to surgical pathology in 405 cases. BJU Int. 2013, 189, 1692. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectr. 2017, 91, 15. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Qudsia, S.; Urooj, S.; Chaudry, N.; Arshad, A.; Andleeb, S. Blueprint of quartz crystal microbalance biosensor for early detection of breast cancer through salivary autoantibodies against ATP6AP1. Biosens. Bioelectr. 2015, 65, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, S.; Jamlos, M.F.; Ahmad, M.N.; Bellan, C.S.; Schreurs, D. Nanostructured materials with plasmonic nanobiosensors for early cancer detection: A past and future prospect. Biosens. Bioelectr. 2017, 100, 361. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.K.; Kumar, S.; Doval, D.C.; Malhotra, B.D. Development of biosensor for non-invasive oral cancer detection. Eur. J. Cancer 2017, 72, S138–S139. [Google Scholar] [CrossRef]
- Devillers, M.; Ahmad, L.; Korri-Youssoufi, H.; Salmon, L. Carbohydrate-based electrochemical biosensor for detection of a cancer biomarker in human plasma. Biosens. Bioelectr. 2017, 96, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Catuogno, S.; Esposito, C.L.; Quintavalle, C.; Cerchia, L.; Condorelli, G.; Franciscis, V.D. Recent advance in biosensors for microRNAs detection in cancer. Cancers 2011, 3, 1877. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Sung, M.S.; Vahdat, L.T.; Shah, M.A.; Santana, S.M.; Altavilla, G.; Kirbyd, B.J.; Giannakakou, P. Isolation of breast cancer and gastric cancer circulating tumor cells by use of an anti HER2-based microfluidic device. Lab Chip 2014, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Hyun, E.; Seo, J.; Blundell, C.; Kim, H.C.; Lee, E.; Lee, S.; Moon, A.; Moon, W.K.; Huh, D. A microengineered pathophysiological model of early-stage breast cancer. Lab Chip 2015, 15, 3350. [Google Scholar] [CrossRef] [PubMed]
- Tsopela, A.; Laborde, A.; Salvagnac, L.; Ventalon, V.; Bedelpereira, E.; Séguy, I.; Temple-Boyer, P.; Juneau, P.; Izquierdo, R.; Launay, J. Development of a lab-on-chip electrochemical biosensor for water quality analysis based on microalgal photosynthesis. Biosens. Bioelectr. 2016, 79, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.W.; Beveridge, R.A.; Muss, H.; Fritsche, H.A.; Hortobagyi, G.; Theriault, R.; Kiang, D.; Kennedy, B.J.; Evelegh, M. Use of Truquant BR radioimmunoassay for early detection of breast cancer recurrence in patients with stage ii and stage iii disease. J. Clin. Oncol. 1997, 15, 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Pertschuk, L.P.; Axiotis, C.A. Steroid hormone receptor immunohistochemistry in breast cancer: Past, present, and future. Breast J. 2015, 5, 3–12. [Google Scholar] [CrossRef]
- Nugent, A.; Mcdermott, E.; Duffy, K.; O’Higgins, N.; Fennelly, J.J.; Duffy, M.J. Enzyme-linked immunosorbent assay of c-erbb-2 oncoprotein in breast cancer. Clin. Chem. 1992, 38, 1471–1474. [Google Scholar] [PubMed]
- Chourb, S. Enhanced Immuno-Detection of Breast Cancer Biomarkers: Shed Extracellular Domain of HER-2/neu and CA15-3. Master’s Thesis, University of Massachusetts Lowell, Lowell, MA, USA, 2010. [Google Scholar]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef] [PubMed]
- Sushma, S.J.; Kumar, S.C.P. Advancement in research techniques on medical imaging processing for breast cancer detection. Int. J. Electr. Comput. Eng. 2015, 6, 717–724. [Google Scholar]
- Morrow, M.; Waters, J.; Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 2011, 378, 1804–1811. [Google Scholar] [CrossRef]
- Jones, E.F.; Ray, K.M.; Li, W.; Seo, Y.; Franc, B.L.; Chien, A.J.; Esserman, L.J.; Pampaloni, M.H.; Joe, B.N.; Hylton, N.M. Dedicated breast positron emission tomography for the evaluation of early response to neoadjuvant chemotherapy in breast cancer. Clin. Breast Cancer 2017, 17, e155. [Google Scholar] [CrossRef] [PubMed]
- Magna, G.; Casti, P.; Jayaraman, S.V.; Salmeri, M.; Mencattini, A.; Martinelli, E.; Di Natale, C. Identification of mammography anomalies for breast cancer detection by an ensemble of classification models based on artificial immune system. Knowl. Syst. 2016, 101, 60–70. [Google Scholar] [CrossRef]
- Patel, B.K.; Garza, S.A.; Eversman, S.; Lopezalvarez, Y.; Kosiorek, H.; Pockaj, B.A. Assessing tumor extent on contrast-enhanced spectral mammography versus full-field digital mammography and ultrasound. Clin. Imag. 2017, 46, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hellquist, B.N.; Czene, K.; Hjälm, A.; Nyström, L.; Jonsson, H. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years with a high or low risk of breast cancer: Socioeconomic status, parity, and age at birth of first child. Cancer 2012, 118, 1170–1171. [Google Scholar] [CrossRef]
- Onega, T.; Goldman, L.E.; Walker, R.L.; Miglioretti, D.L.; Buist, D.S.; Taplin, S.; Geller, B.M.; Hill, D.A.; Smith-Bindman, R. Facility mammography volume in relation to breast cancer screening outcomes. J. Med. Screen. 2016, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Artis, F.; Dubuc, D.; Fournie, J.J.; Poupot, M.; Grenier, K. Microwave biosensor dedicated to the dielectric spectroscopy of a single alive biological cell in its culture medium. In Proceedings of the 2013 IEEE MTT-S International Microwave Symposium Digest (IMS), Seattle, WA, USA, 2–7 June 2013. [Google Scholar]
- Zhou, Y.; Li, C.; Tang, L.; Ma, L.; Wang, Q.; Liu, Q. A permanent bar pattern distributed target for microwave image resolution analysis. IEEE Geosci. Remot. Sens. Lett. 2017, 14, 164–168. [Google Scholar] [CrossRef]
- Alwan, M.S.S.; Katbay, Z. Investigation of tumor using an antenna scanning system. IEEE Microwave Symp. 2014, 171, 1401–1406. [Google Scholar]
- Zhao, X.; Zhuang, H.; Yoon, S.C.; Dong, Y.; Wang, W.; Zhao, W. Electrical impedance spectroscopy for quality assessment of meat and fish: A review on basic principles, measurement methods, and recent advances. J. Food Qual. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Foster, K.R.; Schwan, H.P. Dielectric properties of tissues and biological materials: A critical review. CRIT Rev. Biomed. Eng. 1989, 17, 25. [Google Scholar] [PubMed]
- Martellosio, A.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L.; Bellomi, M.; et al. Dielectric properties characterization from 0.5 to 50 GHz of breast cancer tissues. IEEE Trans. Microw. Theory 2017, 65, 1–14. [Google Scholar]
- Bharati, S.; Rishi, P.; Tripathi, S.K.; Koul, A. Changes in the electrical properties at an early stage of mouse liver carcinogenesis. Bioelectromagnetics 2013, 34, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T.R.; Okoniewski, M.; Fear, E.C.; Mew, D.; Banks, B.; Ogilvie, T. A preliminary study of the electrical properties of healthy and diseased lymph nodes. In Proceedings of the IEEE International Symposium on Antenna Technology and Applied Electromagnetics & the American Electromagnetics Conference, Ottawa, ON, Canada, 5–8 July 2010; pp. 1–3. [Google Scholar]
- Grant, J.P.; Clarke, R.N.; Symm, G.T.; Spyrou, N.M. In vivo dielectric properties of human skin from 50 MHz to 2.0 GHz. Phys. Med. Biol. 1988, 33, 607. [Google Scholar] [CrossRef] [PubMed]
- Irastorza, R.M.; Blangino, E.; Carlevaro, C.M.; Vericat, F. Modeling of the dielectric properties of trabecular bone samples at microwave frequency. Med. Biol. Eng. Comput. 2014, 52, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lue, W.M.; Boyden, P.A. Abnormal electrical properties of myocytes from chronically infarcted canine heart. alterations in Vmax and the transient outward current. Circulation 1992, 85, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Schepps, J.L.; Foster, K.R. The UHF and microwave dielectric properties of normal and tumour tissues: variation in dielectric properties with tissue water content. Phys. Med. Biol. 1980, 25, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Ward, E.R.; Story, B. A review of dielectric properties of normal and malignant breast tissue. In Proceedings of the IEEE SoutheastCon, Columbia, SC, USA, 5–7 April 2002; pp. 457–462. [Google Scholar]
- Pethig, R. Dielectric properties of biological materials: Biophysical and medical applications. IEEE Trans. Electr. Insul. 1984, 19, 453–474. [Google Scholar] [CrossRef]
- Lazebnik, M.; Okoniewski, M.; Booske, J.H.; Hagness, S.C. Highly accurate Debye models for normal and malignant breast tissue dielectric properties at microwave frequencies. IEEE Microw. Wirel. Compon. 2007, 17, 822–824. [Google Scholar] [CrossRef]
- Joines, W.T.; Zhang, Y.; Li, C.; Jirtle, R.L. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med. Phys. 1994, 21, 547. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Popovic, D.; Mccartney, L.; Watkins, C.; Lindstrom, M.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2017, 52, 2637–2656. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.S.; Mishra, R.K.; Swarup, A.; Thomas, J.M. Dielectric properties of normal & malignant human breast tissues at radiowave & microwave frequencies. Indian J. Biochem. Biol. 1984, 21, 76–79. [Google Scholar]
- Swarup, A.; Stuchly, S.S.; Surowiec, A. Dielectric properties of mouse MCA1 fibrosarcoma at different stages of development. Bioelectromagnetics 1991, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, A.J.; Stuchly, S.S.; Barr, J.R.; Swarup, A. Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans. Biomed. Eng. 1988, 35, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tezuka, Y.; Saito, K.; Ito, K. Dielectric properties and water contents of coagulated biological tissue by microwave heating. IEICE Comex. 2015, 4, 105–110. [Google Scholar] [CrossRef]
- Cruciani, S.; Santis, V.D.; Feliziani, M.; Maradei, F. Cole-Cole vs Debye models for the assessment of electromagnetic fields inside biological tissues produced by wideband EMF sources. In Proceedings of the IEEE Asia-Pacific Symposium on Electroman. Comp. (APEMC), Singapore, 21–24 May 2012; pp. 685–688. [Google Scholar]
- Said, T.; Varadan, V.V. Variation of Cole-Cole model parameters with the complex permittivity of biological tissues. In Proceedings of the IEEE MTT-S International Microwave Symposium Digest (MTT'09), Boston, MA, USA, 7–12 June 2009; pp. 1445–1448. [Google Scholar]
- Mustafa, S.; Abbosh, A.M.; Nguyen, P.T. Modeling human head tissues using fourth-order Debye model in convolution-based three-dimensional finite-difference time-domain. IEEE Trans. Antenna Propag. 2014, 62, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Chu, X.; Dilmaghani, R.; Ghavami, M. Low-complexity Cole-Cole expression for modelling human biological tissues in (FD)2TD method. Electron. Lett. 2017, 43, 143–144. [Google Scholar] [CrossRef]
- Zastrow, E.; Davis, S.K.; Lazebnik, M.; Kelcz, F.; Veen, B.D.V.; Hagness, S.C. Development of anatomically realistic numerical breast phantoms with accurate dielectric properties for modeling microwave interactions with the human breast. IEEE Trans. Bio-Med Eng. 2008, 55, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bond, E.J.; Veen, B.D.V.; Hagness, S.C. An overview of ultra-wideband microwave imaging via space-time beamforming for early-stage breast-cancer detection. IEEE Antenna Propag. M. 2005, 47, 19–34. [Google Scholar]
- Meaney, P.M.; Golnabi, A.H.; Epstein, N.R.; Geimer, S.D.; Fanning, M.W.; Weaver, J.B.; Paulsen, K.D. Integration of microwave tomography with magnetic resonance for improved breast imaging. Med. Phys. 2013, 40, 103101. [Google Scholar] [CrossRef] [PubMed]
- Rubaek, T.; Fhager, A.; Jensen, P.D.; Mohr, J.J.; Persson, M. Microwave imaging for breast cancer detection: Comparison of tomographic imaging algorithms using single-frequency and time-domain data. In Proceedings of the General Assembly and Scientific Symposium, Istanbul, Turkey, 13–20 August 2011; pp. 1–4. [Google Scholar]
- Hawley, M.S.; Broquetas, A.; Jofre, L.; Bolomey, J.C.; Gaboriaud, G. Microwave imaging of tissue blood content changes. J. Biomed. Eng. 1991, 13, 197–202. [Google Scholar] [CrossRef]
- Epstein, N.R.; Meaney, P.M.; Paulsen, K.D. 3D parallel-detection microwave tomography for clinical breast imaging. Rev. Sci. Instrum. 2014, 85, 124704–124712. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.M.; Fanning, M.W.; Raynolds, T.; Fox, C.J.; Fang, Q.; Kogel, C.A.; Poplack, S.P.; Paulsena, K.D. Initial clinical experience with microwave breast imaging in women with normal mammography. Acad. Radiol. 2007, 14, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Bucci, O.M.; Bellizzi, G.; Borgia, A.; Costanzo, S.; Crocco, L.; Massa, G.D.; Scapaticci, R. Experimental framework for magnetic nanoparticles enhanced breast cancer microwave imaging. IEEE Access 2017, 5, 1. [Google Scholar] [CrossRef]
- Bevacqua, M.T.; Scapaticci, R. A compressive sensing approach for 3D breast cancer microwave imaging with magnetic nanoparticles as contrast agent. IEEE Trans. Med. Imaging 2016, 35, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Z.; Zhu, X.; Lu, Y.; Wang, B.; Nie, Z.; Liu, Q. Block based compressive sensing method of microwave induced thermoacoustic tomography for breast tumor detection. J. Appl. Phys. 2017, 122, 024702. [Google Scholar] [CrossRef]
- Florestapia, D.; Rodriguez, D.; Solis, M.; Kopotun, N.; Latif, S.; Maizlish, O.; Fu, L.; Gui, Y.; Hu, C.; Pistorius, S. Experimental feasibility of multistatic holography for breast microwave radar image reconstruction. Med. Phys. 2016, 43, 4674. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; Caruso, M.; Khan, M.S.; Bevilacqua, A.; Capobianco, A.D.; Neviani, A. An integrated microwave imaging radar with planar antennas for breast cancer detection. IEEE Trans. Microw. Theory 2013, 61, 2108–2118. [Google Scholar] [CrossRef]
- Tapia, D.F.; Pistorius, S. We-g-211-01: Breast microwave radar image reconstruction using circular holography: Initial results on preclinical datasets. Med. Phys. 2011, 38, 3835. [Google Scholar] [CrossRef]
- Fear, E.C.; Li, X.; Hagness, S.C.; Stuchly, M.A. Confocal microwave imaging for breast cancer detection: localization of tumors in three dimensions. IEEE Trans. Bio-Med Eng. 2002, 49, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Tong, K.F.; Al-Armaghany, A.; Leung, T.S. A feasiblity study of elastography based confocal microwave imaging technique for breast cancer detection. Optik. Int. J. Light Electr. Opt. 2017, 144, 108–144. [Google Scholar] [CrossRef]
- Bond, E.J.; Li, X.; Hagness, S.C.; Van Veen, B.D. Microwave imaging via space-time beamforming for early detection of breast cancer. J. Electromagnet. Wave 2003, 17, 357–381. [Google Scholar] [CrossRef]
- Li, X.; Davis, S.K.; Hagness, S.C.; van der Weide, D.W.; Van Veen, B.D. Microwave imaging via space-time beamforming: experimental investigation of tumor detection in multilayer breast phantoms. IEEE Trans. Microw. Theory 2004, 52, 1856–1865. [Google Scholar] [CrossRef]
- Wang, L.; Simpkin, R.; Al-Jumaily, A.M. Holographic microwave imaging for medical applications. J. Biomed. Sci. Eng. 2013, 6, 823–833. [Google Scholar] [CrossRef]
- Elsdon, M.; Leach, M.; Skobelev, S.; Smith, D. Microwave Holographic Imaging of Breast Cancer. In Proceedings of the IEEE International Symposium on Microwave, Antenna, Propagation and EMC Technologies for Wireless Communications, Hangzhou, China, 16–17 August 2007; pp. 966–969. [Google Scholar]
- Karamfard, S.S.; Asl, B.M. 2-Stage Delay-Multiply-and-Sum Beamforming for Breast Cancer Detection Using Microwave Imaging. In Proceedings of the Iranian Conference on Electrical Engineering (ICEE 2017), Tehran, Iran, 2–4 May 2017; pp. 101–106. [Google Scholar]
- Khosrowshahli, E.; Jeremić, A. Bayesian estimation of tumours in breasts using microwave imaging. Antimicrob. Agents Chemother. 2010, 52, 1670–1676. [Google Scholar]
- Elsdon, M.; Smith, D.; Leach, M.; Foti, S.J. Experimental investigation of breast tumor imaging using indirect microwave holography. Microw. Opt. Technol. Lett. 2006, 48, 480–482. [Google Scholar] [CrossRef]
- Wang, L.; Al-Jumaily, A.M.; Simpkin, R. Imaging of 3-D dielectric objects using far-field holographic microwave imaging technique. Prog. Electromagn. Res. B 2014, 61, 135–147. [Google Scholar] [CrossRef]
- Rahman, A.; Islam, M.T.; Singh, M.J.; Kibria, S.; Akhtaruzzaman, M. Electromagnetic performances analysis of an ultra-wideband and flexible material antenna in microwave breast imaging: to implement a wearable medical bra. Sci. Rep. 2016, 6, 38906. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, N.K. Microwave imaging for breast cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Latif, S.; Flores-Tapia, D.; Pistorius, S.; Shafai, L. A planar ultrawideband elliptical monopole antenna with reflector for breast microwave imaging. Microw. Opt. Technol. Lett. 2014, 56, 808–813. [Google Scholar] [CrossRef]
- Manohar, M.; Kshetrimayum, R.S.; Gogoi, A.K. A compact printed triangular monopole antenna for ultra-wideband applicatio. Microw. Opt. Technol. Lett. 2014, 56, 1155–1159. [Google Scholar] [CrossRef]
- Kanj, H.; Popovic, M. A novel ultra-compact broadband antenna for microwave breast tumor detection. Prog. Electromagn. Res. 2008, 86, 169–198. [Google Scholar] [CrossRef]
- Bahramiabarghouei, H.; Porter, E.; Santorelli, A.; Gosselin, B.; Popovic, M.; Rusch, L.A. Flexible 16 antenna array for microwave breast cancer detection. IEEE Trans. Bio-Med Eng. 2015, 62, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, E.; Asili, M.; Chen, P.; Demirci, U.; Younan, N. Flexible microwave antenna applicator for chemothermotherapy of the breast. In Proceedings of the EAI International Conference on Wireless Mobile Communication and Healthcare, Athens, Greece, 3–5 November 2014; pp. 1778–1781. [Google Scholar]
- Porter, E.; Bahrami, H.; Santorelli, A.; Gosselin, B.; Rusch, L.; Popovich, M. A wearable microwave antenna array for time-domain breast tumor screening. IEEE Trans. Med. Imaging. 2016, 35, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Agarwal, M.; Ghane, P.; Varahramyan, K. Flexible microstrip antenna for skin contact application. Int. J. Antenna Propag. 2012, 911–940. [Google Scholar] [CrossRef]
- Nelson, S.O. Near-field measurements of dielectric properties of granular materials with microstrip antennas for microwave-sensing applications. Res. Nondestruct. Eval. 2006, 17, 1–16. [Google Scholar]
- Shannon, C.J.; Fear, E.C.; Okoniewski, M. Dielectric-filled slotline bowtie antenna for breast cancer detection. Electron. Lett. 2005, 41, 388–390. [Google Scholar] [CrossRef]
- Nilavalan, R.; Craddock, I.J.; Preece, A.; Leendertz, J.; Benjamin, R. Wideband microstrip patch antenna design for breast cancer tumour detection. IET Microw. Antenna Propag. 2007, 1, 277–281. [Google Scholar] [CrossRef]
- Shenouda, M.H.; Fear, E.C. Design of dielectric immersed tapered slotline antenna for radar-based microwave breast imaging. Microw. Opt. Technol. Lett. 2009, 51, 633–638. [Google Scholar] [CrossRef]
- Bourqui, J.; Okoniewski, M.; Fear, E.C. Balanced antipodal Vivaldi antenna with dielectric director for near-field microwave imaging. IEEE Trans. Antenna Propag. 2010, 58, 2318–2326. [Google Scholar] [CrossRef]
- Gibbins, D.; Klemm, M.; Craddock, I.J.; Leendertz, J.A.; Preece, A.; Benjamin, R. A comparison of a wide-slot and a stacked patch antenna for the purpose of breast cancer detection. IEEE Trans. Antenna Propag. 2010, 58, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fathy, A.E.; Mahfouz, M.R. Novel compact tapered microstrip slot antenna for microwave breast imaging. In Proceedings of the IEEE International Symposium on Antennas and Propagation (APSURSI), Spokane, WA, USA, 3–8 July 2011; pp. 2119–2122. [Google Scholar]
- Chan, H.S.; Abd-Alhameed, R.A.; Chung, S.W.J.; Zhou, D. The design of a resistively loaded bowtie antenna for applications in breast cancer detection systems. IEEE Trans. Antenna Propag. 2012, 60, 2526–2530. [Google Scholar]
- John, S.; Mark, H.; Paul, C.; Mahta, M. A preclinical system prototype for focused microwave thermal therapy of the breast. IEEE Trans. Bio-Med. Eng. 2012, 59, 2431–2438. [Google Scholar]
- Wang, L.; Simpkin, R.; Al-Jumaily, A.M. Open-ended waveguide antenna for microwave breast cancer detection. In Proceedings of the IEEE International Workshop on Electromagnetics (iWEM 2013), Kowloon, China, 1–3 August 2013; pp. 65–68. [Google Scholar]
- Nepote, M.S.; Herrera, D.R.; Tapia, D.F.; Latif, S.; Pistorius, S. A comparison study between horn and Vivaldi antennas for 1.5–6 GHz breast microwave radar imaging. In Proceedings of the European Conference on IEEE Antennas and Propagation, The Hague, The Netherlands, 6–11 April 2014; pp. 59–62. [Google Scholar]
- Ahadi, M. Square monopole antenna for microwave imaging, design and characterisation. IET Microw. Antenna Propag. 2014, 10, 1–9. [Google Scholar] [CrossRef]
- Kahar, M.; Ray, A.; Sarkar, D.; Sarkar, P.P. An UWB microstrip monopole antenna for breast tumor detection. Microw. Opt. Technol. Lett. 2015, 57, 49–54. [Google Scholar] [CrossRef]
- Karli, R.; Ammor, H.; Virdee, B.S. Early detection of breast tumors using UWB microstrip antenna imaging. Microw. Opt. Technol. Lett. 2016, 58, 2101–2106. [Google Scholar] [CrossRef]
- Li, H.; Xiao, S.; Guo, Y.X. Broadband circularly polarized implantable antenna for biomedical applications. Electron. Lett. 2016, 52, 504–506. [Google Scholar] [CrossRef]
- Li, Y.; Porter, E.; Santorelli, A.; Popović, M.; Coates, M. Microwave breast cancer detection via cost-sensitive ensemble classifiers: phantom and patient investigation. Biomed. Signal Process. 2017, 31, 366–376. [Google Scholar] [CrossRef]
- Ting, J.; Oloumi, D.; Rambabu, K. A miniaturized broadband bow-tie antenna with improved cross-polarization performance. Int. J. Electron. Commun. 2017, 78, 173–180. [Google Scholar] [CrossRef]
- Guha, S.; Jamal, F.I.; Wenger, C. A review on passive and integrated near-field microwave biosensors. Biosensors 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.H.; Moon, H.S.; Jang, I.S.; Choi, J.S.; Yook, J.G.; Jung, H. A planar split-ring resonator-based microwave biosensor for label-free detection of biomolecules. Sens. Actuators B Chem. 2012, 169, 26–31. [Google Scholar] [CrossRef]
- Yang, C.H.; Kuo, L.S.; Chen, P.H. Development of a multilayered polymeric DNA biosensor using radio frequency technology with gold and magnetic nanoparticles. Biosens. Bioelectr. 2012, 31, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, H.J.; Lee, J.H.; Jung, H.I.; Yook, J.G. A highly sensitive and label free biosensing platform for wireless sensor node system. Biosens. Bioelectr. 2013, 50, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Camli, B.; Kusakci, E.; Lafci, B.; Salman, S.; Torun, H.; Yalcinkaya, A. Cost-effective, microstrip antenna driven ring resonator microwave biosensor for biospecific detection of glucose. IEEE J. Sel. Top. Quant. 2017, 23, 1. [Google Scholar] [CrossRef]
- Garrett, J.D.; Fear, E.C. Average dielectric property analysis of complex breast tissue with microwave transmission measurements. Sensors 2015, 15, 1199. [Google Scholar] [CrossRef] [PubMed]
- Tselev, A.; Velmurugan, J.; Ievlev, A.V.; Kalinin, S.V.; Kolmakov, A. Seeing through walls at the nanoscale: microwave microscopy of enclosed objects and processes in liquids. ACS Nano 2016, 10, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Kurrant, D.; Bourqui, J.; Curtis, C.; Fear, E. Evaluation of 3D acquisition surfaces for radar-based microwave breast imaging. IEEE Trans. Antenna Propag. 2015, 63, 4910–4920. [Google Scholar] [CrossRef]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L. Microwave Sensors for Breast Cancer Detection. Sensors 2018, 18, 655. https://doi.org/10.3390/s18020655
Wang L. Microwave Sensors for Breast Cancer Detection. Sensors. 2018; 18(2):655. https://doi.org/10.3390/s18020655
Chicago/Turabian StyleWang, Lulu. 2018. "Microwave Sensors for Breast Cancer Detection" Sensors 18, no. 2: 655. https://doi.org/10.3390/s18020655
APA StyleWang, L. (2018). Microwave Sensors for Breast Cancer Detection. Sensors, 18(2), 655. https://doi.org/10.3390/s18020655




