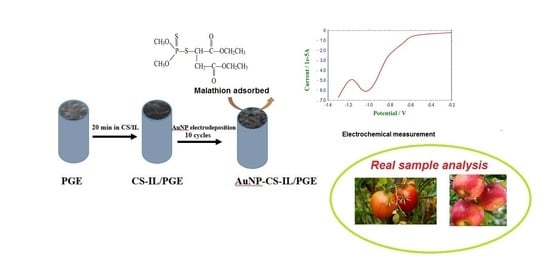
Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals and Reagents
2.3. Preparation of Modified Electrodes
2.4. Real Samples
3. Results and Discussion
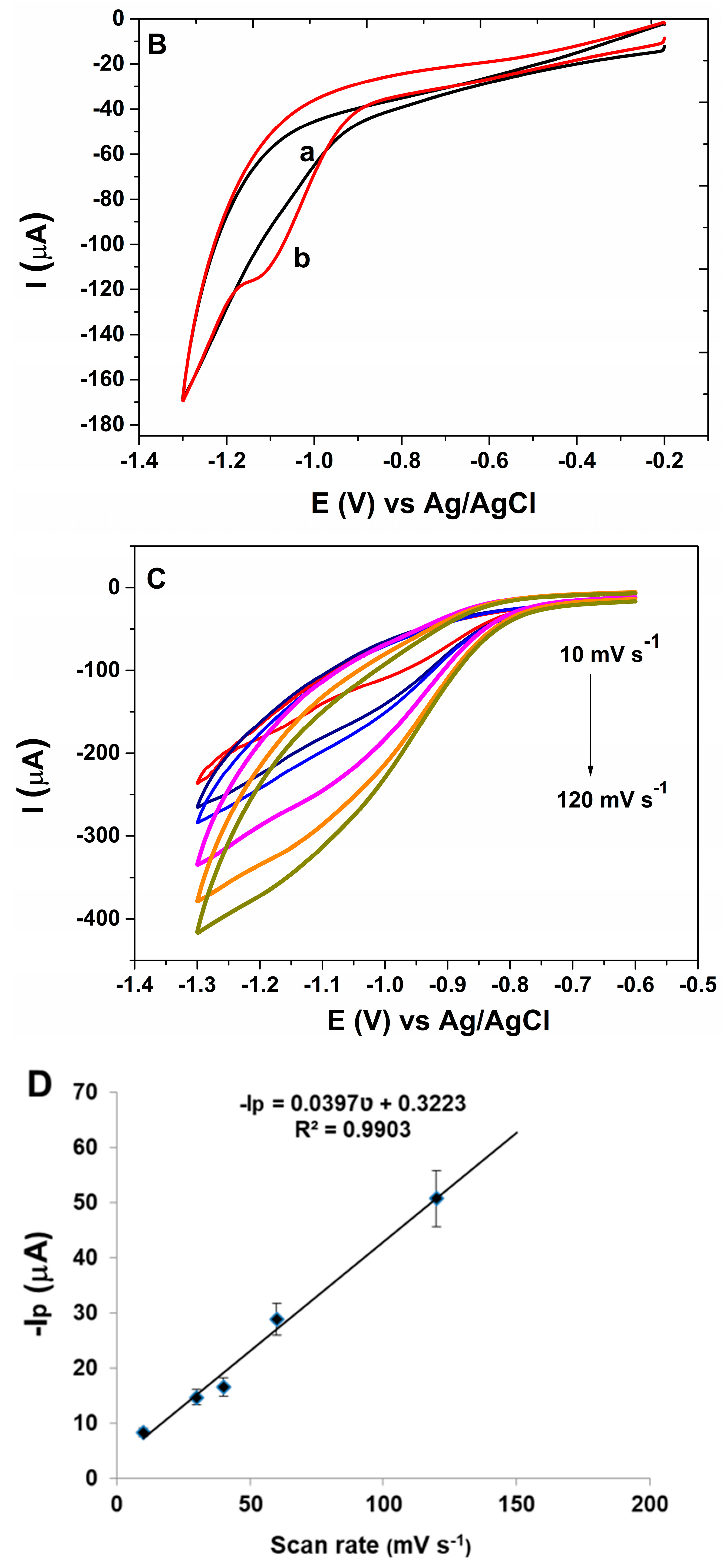
3.1. Morphological and Electrochemical Characterization of AuNP-CS-IL/PGE
3.2. Electrochemical Behavior of Malathion on AuNP-CS-IL/PGE
3.3. Analytical Performance of AuNP-CS-IL/PGE towards MLT Determination
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, D.; Yu, D.; Zhao, W.; Yang, Q.; Kajiura, H.; Li, Y.; Zhou, T.; Shi, G. A molecularly imprinted polymer based on functionalized multiwalled carbon nanotubes for the electrochemical detection of parathion-methyl. Analyst 2012, 137, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Fildes, K.; Astheimer, L.B.; Buttemer, W.A. The Effect of Acute Fenitrothion exposure on a variety of physiological indices, including avian areobic metabolism during exercise and cold exposure. Environ. Toxicol. Chem. 2009, 28, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; El-Raey, R.; Hefnawy, A.; Ibrahim, H.; Soliman, M.; Abdel-Fattah, T.M. Electrochemical sensor based on polyaniline nanofibers/single wall carbon nanotubes composite for detection of malathion. Synth. Met. 2014, 190, 13–19. [Google Scholar] [CrossRef]
- Raghu, P.; Reddy, T.M.; Reddaiah, K.; Swamy, B.E.K.; Sreedhar, M. Acetylcholinesterase based biosensor for monitoring of malathion and acephate in food samples: A voltammetric study. Food Chem. 2014, 142, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.R.; Qazi, I.A. Modified colorimetric method for the determination of malathion. Analyst 1979, 104, 1129–1134. [Google Scholar] [CrossRef]
- Quintás, G.; Garrigues, S.; De La Guardia, M. FT-Raman spectrometry determination of Malathion in pesticide formulations. Talanta 2004, 63, 345–350. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Álvarez-Llamas, G.; Puerta, Á.; Blanco, E.; Sanz-Medel, A.; Marina, M.L. Enantiomeric separation of organophosphorus pesticides by capillary electrophoresis: Application to the determination of malathion in water samples after preconcentration by off-line solid-phase extraction. Anal. Chim. Acta 2005, 543, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Abu-Qare, A.W.; Abou-Donia, M.B. Simultaneous determination of malathion, permethrin, DEET (N,N-diethyl-m-toluamide), and their metabolites in rat plasma and urine using high performance liquid chromatography. J. Pharm. Biomed. Anal. 2001, 26, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Bavcon, M.; Trebše, P.; Zupančič-Kralj, L. Investigations of the determination and transformations of diazinon and malathion under environmental conditions using gas chromatography coupled with a flame ionisation detector. Chemosphere 2003, 50, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.; Turkoglu, V.; Kivrak, A. Electrochemical detection of malathion pesticide using acetylcholinesterase biosensor based on glassy carbon electrode modified with conducting polymer film. Environ. Sci. Pollut. Res. 2016, 23, 12343–12351. [Google Scholar] [CrossRef]
- Zhao, H.; Ji, X.; Wang, B.; Wang, N.; Li, X.; Ni, R.; Ren, J. An ultra-sensitive acetylcholinesterase biosensor based on reduced graphene oxide-Au nanoparticles-β-cyclodextrin/Prussian blue-chitosan nanocomposites for organophosphorus pesticides detection. Biosens. Bioelectron. 2015, 65, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Narang, J.; Jain, U. Amperometric acetylcholinesterase biosensor for pesticides monitoring utilising iron oxide nanoparticles and poly(indole-5-carboxylic acid). J. Exp. Nanosci. 2016, 11, 111–122. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Wang, M.; Zhang, D.; Chen, J. Electrochemical nonenzymatic sensor based on CoO decorated reduced graphene oxide for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. Food Chem. 2014, 151, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.; Ganesh, V. Selective and sensitive electrochemical detection of methyl parathion using chemically modified overhead projector sheets as flexible electrodes. Sens. Actuators B Chem. 2016, 227, 169–177. [Google Scholar] [CrossRef]
- Huo, D.; Li, Q.; Zhang, Y.; Hou, C.; Lei, Y. A highly efficient organophosphorus pesticides sensor based on CuO nanowires-SWCNTs hybrid nanocomposite. Sens. Actuators B Chem. 2014, 199, 410–417. [Google Scholar] [CrossRef]
- Soomro, R.A.; Hallam, K.R.; Ibupoto, Z.H.; Tahira, A.; Sherazi, S.T.H.; Sirajjuddin; Memon, S.S.; Willander, M. Amino acid assisted growth of CuO nanostructures and their potential application in electrochemical sensing of organophosphate pesticide. Electrochim. Acta 2016, 190, 972–979. [Google Scholar] [CrossRef]
- Vidal, L.; Riekkola, M.L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Cokeliler, D.; Gunasekaran, S. Reduced Graphene Oxide/Carbon Nanotube/Gold Nanoparticles Nanocomposite Functionalized Screen-Printed Electrode for Sensitive Electrochemical Detection of Endocrine Disruptor Bisphenol A. Electroanalysis 2015, 27, 2527–2536. [Google Scholar] [CrossRef]
- Wang, J.; Kawde, A.-N.; Sahlin, E. Renewable pencil electrodes for highly sensitive stripping potentiometric measurements of DNA and RNA. Analyst 2000, 125, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Yardım, Y.; Şentürk, Z. Electrochemical evaluation and adsorptive stripping voltammetric determination of capsaicin or dihydrocapsaicin on a disposable pencil graphite electrode. Talanta 2013, 112, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Eksin, E.; Muti, M.; Erdem, A. Chitosan/Ionic Liquid Composite Electrode for Electrochemical Monitoring of the Surface-Confined Interaction between Mitomycin C and DNA. Electroanalysis 2013, 25. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf. B Biointerfaces 2004, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Farjami, F. Electrodeposition of gold-platinum alloy nanoparticles on ionic liquid-chitosan composite film and its application in fabricating an amperometric cholesterol biosensor. Biosens. Bioelectron. 2011, 26, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- Angerstein-Kozlowska, H.; Conway, B.E.; Hamelin, A.; Stoicoviciu, L. Elementary steps of electrochemical oxidation of single-crystal planes of Au—I. Chemical basis of processes involving geometry of anions and the electrode surfaces. Electrochim. Acta 1986, 31, 1051–1061. [Google Scholar] [CrossRef]
- Hezard, T.; Fajerwerg, K.; Evrard, D.; Collière, V.; Behra, P.; Gros, P. Influence of the gold nanoparticles electrodeposition method on Hg(II) trace electrochemical detection. Electrochim. Acta 2012, 73, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, S.; Nogami, M.; Ikeda, S. Novel Electrochemical Interfaces with a Tunable Kinetic Barrier by Self-Assembling Organically Modified Silica Gel and Gold Nanoparticles. Langmuir 2000, 17, 1–4. [Google Scholar] [CrossRef]
- Chauhan, N.; Narang, J.; Pundir, C.S. Immobilization of rat brain acetylcholinesterase on porous gold-nanoparticle-CaCO3 hybrid material modified Au electrode for detection of organophosphorous insecticides. Int. J. Biol. Macromol. 2011, 49, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.K. Influence of selected surfactants on voltammetric determination of Fenitrothion and Mlatahion. Ecol. Chem. Eng. S 2008, 15, 263–272. [Google Scholar]
- Cekirdek, P.; Solak, A.O.; Karakuş, M.; Aydin, A.; Yılmaz, H. Investigation of Electrochemical Behavior of Some Dithiophosphonates in Acetonitrile on the Platinum and Gold Electrodes. Electroanalysis 2006, 18, 2314–2323. [Google Scholar] [CrossRef]
- Du, D.; Liu, J.; Zhang, X.; Cui, X.; Lin, Y. One-step electrochemical deposition of a graphene-ZrO2 nanocomposite: Preparation, characterization and application for detection of organophosphorus agents. J. Mater. Chem. 2011, 21, 8032–8037. [Google Scholar] [CrossRef]
- Overbury, S.H.; Schwartz, V.; Mullins, D.R.; Yan, W.; Dai, S. Evaluation of the Au size effect: CO oxidation catalyzed by Au/TiO2. J. Catal. 2006, 241, 56–65. [Google Scholar] [CrossRef]
- Wang, T.; Reid, R.C.; Minteer, S.D. A Paper-based Mitochondrial Electrochemical Biosensor for Pesticide Detection. Electroanalysis 2016, 28, 854–859. [Google Scholar] [CrossRef]








| Electrode | MLT Linear Range | Electrochemical Technique | LOD | Reference |
|---|---|---|---|---|
| AChE–AuNPs–CaCO3–Au–SiSG | 0.1–100 nM | CV | 0.1 nM | [27] |
| AChE-Fe3O4NP-MWCNTs/Au | 0.1–40 nM | Amperometry | 0.1 nM | [12] |
| AChE–SiSG–CPE | 0.07–1.3 ppm | DPV | 0.058 ppm (0.174 nM) | [4] |
| CS/AChE/PB-CS/ERGO-AuNPs-β-CD/GCE | 7.98–2 × 103 pg mL−1 | Amperometry | 4.14 pg mL−1 | [11] |
| Poly(TTP)/AChE/GCE | 9.99–99.01 nM | CV | 4.08 nM | [10] |
| CuO NWs–SWCNTs/GCE | 0.3–1.4 nM | DPV | 0.3 nM | [15] |
| Gly-CuO/GCE/nafion | 1–12 nM | DPV | 0.1 nM | [16] |
| PANI-Nanofibers-SWCNTs Graphite Electrode | 2 × 10−7–14 × 10−7 mol L−1 | DPV | 2 × 10−7 mol L−1 | [3] |
| Mitochondria modified paper based electrodes | 20–60 nM | CV | 20 nM | [32] |
| AuNP-CS-IL/PGE | 0.89–5.94 nM and 5.94–44.6 nM | SWV | 0.68 nM | Present work |
| Interfering Species | Current Ratio a |
|---|---|
| K+ | 1.020 |
| Na+ | 0.990 |
| Bi3+ | 0.900 |
| SO42− | 1.101 |
| NO3− | 0.900 |
| Cl− | 0.950 |
| Fenitrothion | 0.986 |
| Sample | Malathion Added (nM) | Malathion Found (nM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| Tomato | 0.893 | 1.02 | 114.2 | 7.9 |
| 11.01 | 10.1 | 91.7 | 10.5 | |
| Apple | 0.893 | 0.814 | 91.1 | 2.3 |
| 1.488 | 1.570 | 105.5 | 10.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolat, G.; Abaci, S. Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite. Sensors 2018, 18, 773. https://doi.org/10.3390/s18030773
Bolat G, Abaci S. Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite. Sensors. 2018; 18(3):773. https://doi.org/10.3390/s18030773
Chicago/Turabian StyleBolat, Gulcin, and Serdar Abaci. 2018. "Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite" Sensors 18, no. 3: 773. https://doi.org/10.3390/s18030773
APA StyleBolat, G., & Abaci, S. (2018). Non-Enzymatic Electrochemical Sensing of Malathion Pesticide in Tomato and Apple Samples Based on Gold Nanoparticles-Chitosan-Ionic Liquid Hybrid Nanocomposite. Sensors, 18(3), 773. https://doi.org/10.3390/s18030773